-
冬青属Ilex植物多常绿,树冠优美,果实通常红色光亮,长期宿存,是良好的庭园观赏和城市绿化树种,拥有巨大的园林应用潜力。冬青属种子具有种胚后熟的特性[1],常规播种繁殖生长缓慢,且后代性状易发生分离。嫁接繁殖步骤繁琐,操作技术不易掌握,管理要求严格。相比之下,扦插繁殖操作简单,繁殖系数较高,还能保持植物的优良性状。然而,冬青属植物中的很多种类扦插生根困难、成活率低,严重制约了该属植物的推广和应用[2]。金晓玲等[3]研究了34种杂交冬青的生态适应性和扦插成活率后发现:不同品种的冬青扦插成活率存在较大差异,其中光滑冬青Ilex glabra系列栽培品种扦插成活率较高(90.5%~100.0%),美洲冬青I. verticillata系列栽培品种较低(47.5%~64.3%)。生产上常常使用植物生长调节剂处理插穗以获得较高的生根率。胡曼筠等[4]研究发现:经500 mg·L−1 钾盐吲哚丁酸(KIBA)处理的华中枸骨I. centrochinensis生根率最高,雌雄株分别为83.33%和87.50%,比对照明显提高了20.83%。冬青‘长叶阿尔塔’I. × altaclerensis ‘Belgica Aurea’为冬青属常绿小乔木,是欧洲冬青I. aquifolium和加那利冬青I. perado的园艺杂交种,均为雌株[5]。该植物茎绿色,具黄色条纹;叶边缘金黄色不规则,中央有灰绿色斑纹;入秋红果累累,经冬不落,是优良的观干、观叶、观果树种。此外,该树种耐修剪,适应性强,亮丽的色彩很适合与其他彩叶植物搭配种植,极具园林应用前景。但‘长叶阿尔塔’扦插生根较为困难,经萘乙酸(NAA)、吲哚乙酸(IAA)、吲哚丁酸(IBA)、GGR6生根粉处理后生根率均不到40%,生根持续时间较长,生根机制尚不清楚[6]。本研究考察了植物生长调节剂种类和质量浓度、处理方法、基质类型对插穗生根的影响,并从形态解剖学角度探讨了插穗不定根的发生及发育过程,旨在揭示‘长叶阿尔塔’插穗的生根机制,为冬青属植物扦插繁殖技术提供理论基础。
-
材料取自宁波高新农业技术实验园区苗圃。从10 a以上生长健壮、无病虫害且无机械损伤的‘长叶阿尔塔’嫁接苗母株上采集当年生半木质化枝条为插穗。插穗长度为8~10 cm,上切口平剪,下切口45° 斜剪,保留3~5个芽及顶端2片1/2成熟叶。本研究在南京林业大学园林实验中心温室的扦插床上进行。扦插前基质均经过消毒处理,插床配置间歇自动喷雾装置,保持扦插环境相对空气湿度为90%,插穗上方3 m处覆盖50%遮阳网。扦插时温室内温度为20~25 ℃。
-
以植物生长调节剂种类和质量浓度、处理方法、基质类型为试验因素,每个因素下设3个水平,采用3因素3水平正交试验设计(表1),试验共9个处理,每处理30根插穗,重复3次。
处理号 植物生长调节剂 处理方法 基质类型[V(草
炭)∶V(蛭
石)∶V(珍珠岩)]种类 质量浓度/
(mg∙L−1)T1 ABT1生根粉 300 浸泡1 h 3∶3∶4 T2 ABT1生根粉 500 浸泡1 h 4∶3∶3 T3 ABT1生根粉 1 000 速蘸10 s 4∶2∶4 T4 NAA 300 浸泡1 h 4∶3∶3 T5 NAA 500 浸泡1 h 4∶2∶4 T6 NAA 1 000 速蘸10 s 3∶3∶4 T7 IBA 300 浸泡1 h 4∶2∶4 T8 IBA 500 浸泡1 h 3∶3∶4 T9 IBA 1 000 速蘸10 s 4∶3∶3 Table 1. L9(34) orthogonal experimental design for cutting of I. × altaclerensis ‘Belgica Aurea’
-
春季扦插80 d后统计相关生根指标。观测指标包括:插穗存活率(%)、生根率(%)、生根数量(单个处理的单株平均不定根数量,条)、最长不定根长(单个处理的单株平均最长不定根长,cm)、平均不定根长(单个处理的单株平均根长,cm),并对插条生根部位和特征进行观察记录。计算插穗存活率=存活插穗数/总插穗数×100%;生根率=生根插穗数/总插穗数×100%;根系效果指数=(平均根长×根系数量)/总插穗数[7]。数据采用Excel 2003整理,并用SPSS 24.0软件进行方差分析及多重比较。
-
插穗扦插前用1 000 mg·L−1的NAA溶液速蘸插穗10 s,扦插基质为珍珠岩,每处理30根插穗,重复3次。自扦插当天开始取样,以后每隔14 d取样1次,每次随机取3根插穗,共取样6次(0 、14、28、42、56、70 d)。观察扦插生根过程中插穗基部形态变化,愈伤组织和不定根的发生情况,并拍照记录,拍照记录后的插穗用于后期解剖学观察。
-
观察‘长叶阿尔塔’插穗茎段的横切面结构及其在扦插过程中的变化;通过扫描电镜、透射电镜观察扦插过程中各类型愈伤组织的表面形态及内部细胞结构的变化。①石蜡切片。参照周乃富等[8]的方法,对插穗基部1 cm左右的茎段进行切片,并用OLYMPUS显微镜观察并拍照,分析插穗内部不定根的发生发育过程。②扫描电镜。参照FOWKE等[9]的方法,对插穗基部愈伤组织的结构进行扫描电镜观察并拍照(Quanta 200)。③透射电镜。参照WU等[10]的方法,对新鲜愈伤组织材料(0.5 cm3)进行透射电镜观察并拍照(JEM 1400)。
-
由表2可知:不同处理‘长叶阿尔塔’插穗存活率、生根率、最长根长、根系效果指数4个指标差异极显著(P<0.01)。其中,T9处理插穗存活率达63.98%、生根率达48.83%、最长根长达5.15 cm、根系效果指数达4.90,均极显著高于其他处理(P<0.01)。从表3可知:植物生长调节剂的种类和质量浓度、处理方法水平3的生根率极显著高于水平1与水平2(P<0.01);基质类型水平1与水平2的生根率极显著高于水平3(P<0.01)。综合表2和表3可知:‘长叶阿尔塔’插穗经1 000 mg·L−1 IBA溶液速蘸10 s,扦插在V(草炭)∶V(蛭石)∶V(珍珠岩)=3∶3∶4或4∶3∶3的基质中可获得较高的生根率。
处理号 插穗存活率/% 生根率/% 最长根长/cm 根系效果指数 T1 22.11±2.25 Bbc 12.74±1.37 Dd 2.69±0.70 Bbc 1.92±0.14 Bbc T2 11.37±1.20 De 8.68±0.71 DEde 1.31±0.70 BCc 1.04±0.19 BCc T3 34.18±3.14 Bc 23.62±2.47 Cc 2.65±0.75 Bbc 2.06±0.16 Bbc T4 5.42±4.72 Def 4.25±3.68 Ee 1.71±1.61 BCbc 1.07±0.96 BCc T5 0.00±0.00 Ef 0.00±0.00 Ee 0.00±0.00 Cc 0.00±0.00 Cc T6 41.61±2.67 Bb 36.09±2.94 Bb 3.09±1.20 ABb 2.55±1.07 Bb T7 16.16±3.12 Ce 8.88±1.70 DEde 2.66±0.46 Bbc 1.81±0.71 Bbc T8 23.67±3.63 Cd 12.74±2.21 Dd 2.91±0.84 ABbc 2.33±0.66 Bbc T9 63.98±6.71 Aa 48.83±7.35 Aa 5.15±1.02 Aa 4.90±0.87 Aa 说明:同列不同小写字母表示差异显著(P<0.05),同列不同大写字母表示差异极显著(P<0.01) Table 2. Effects of different treatments on rooting of I.× altaclerensis ‘Belgica Aurea’
水平 植物生长调节剂 处理方法 基质类型 1 15.01±1.51 Bb 8.62±1.51 Bb 20.52±1.51 Aa 2 13.45±1.51 Bb 7.14±1.51 Bb 20.59±1.51 Aa 3 23.48±1.51 Aa 36.18±1.51 Aa 10.83±1.51 Bb 说明:同列不同小写字母表示差异显著(P<0.05),同列不
同大写字母表示差异极显著(P<0.01)Table 3. Multiple comparison of rooting rate on different fators of orthogonal test
-
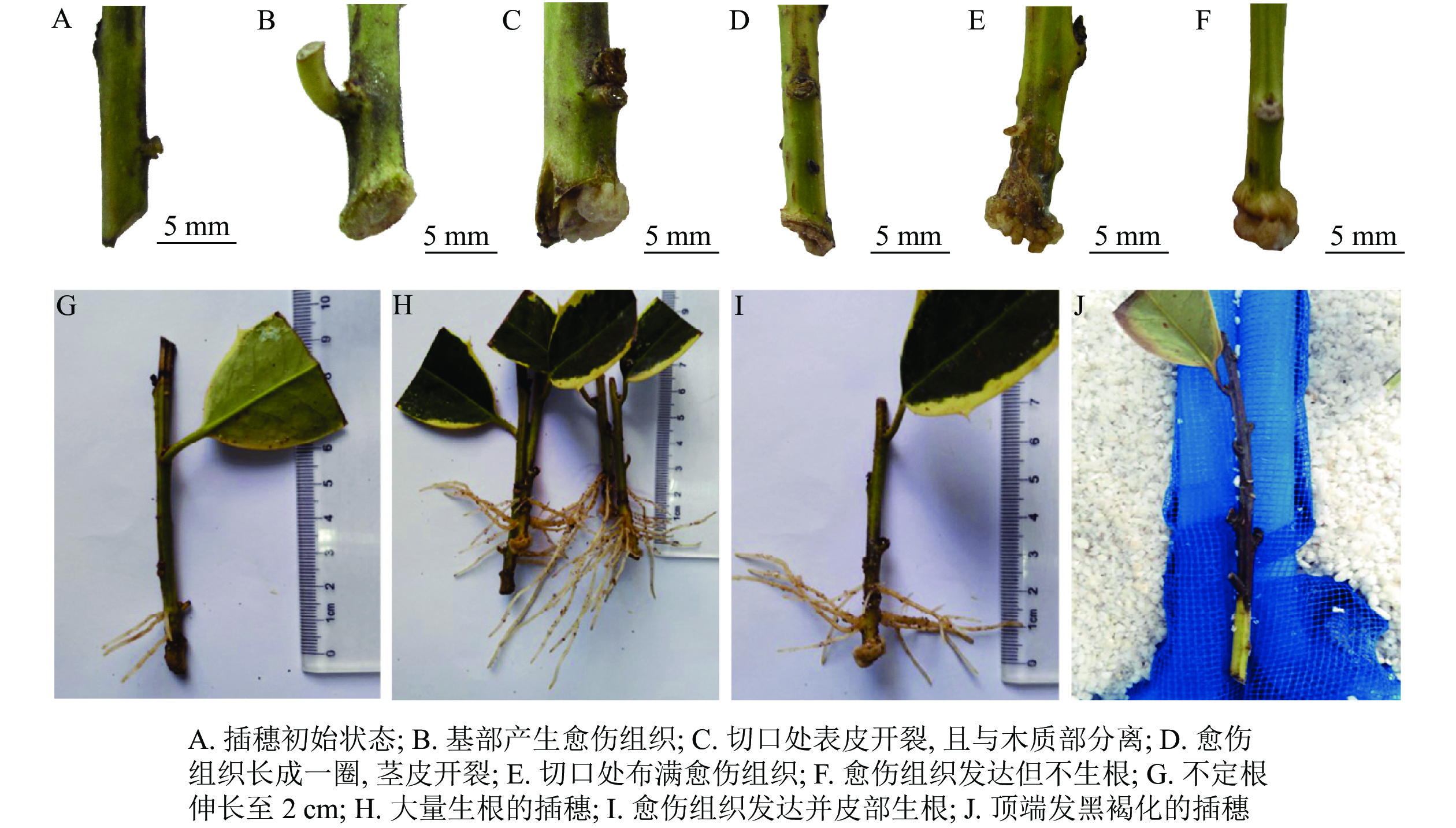
与扦插前(图1A)相比,扦插14 d时,插穗切口边缘能够观察到少量浅绿色的愈伤组织(图1B);扦插28 d时,插穗切口处表皮开裂,并与木质化部分分离(图1C);扦插42 d时,插穗切口上方1 cm左右的部位出现条状开裂,此时愈伤组织较多,沿着表皮与木质化部分的界限呈环状分布(图1D);扦插56 d时,大部分插穗切口处均形成点状、浅黄色的愈伤组织,少量不定根突破皮层,开始皮外伸长生长(图1E);扦插70 d时,多数插穗基部均能明显观察到不定根,其生长部位在距插穗下切口上部2 cm内,有些不定根可生长至2 cm左右(图1G);扦插90 d时(不在取样周期内,仅用于外部形态观察),有大量不定根形成,其长度超过5 cm(图1H)。同时,扦插过程中也发现有些插穗基部形成发达的愈伤组织,将切口全部包住(图1F),但并未观察到愈伤组织内长出不定根(图1I)。此外,有些插穗既不长出愈伤组织,也没有形成不定根,也不死亡,出现“假活”现象;也有部分插穗自顶端开始发黑褐化,最终死亡(图1J)。
-
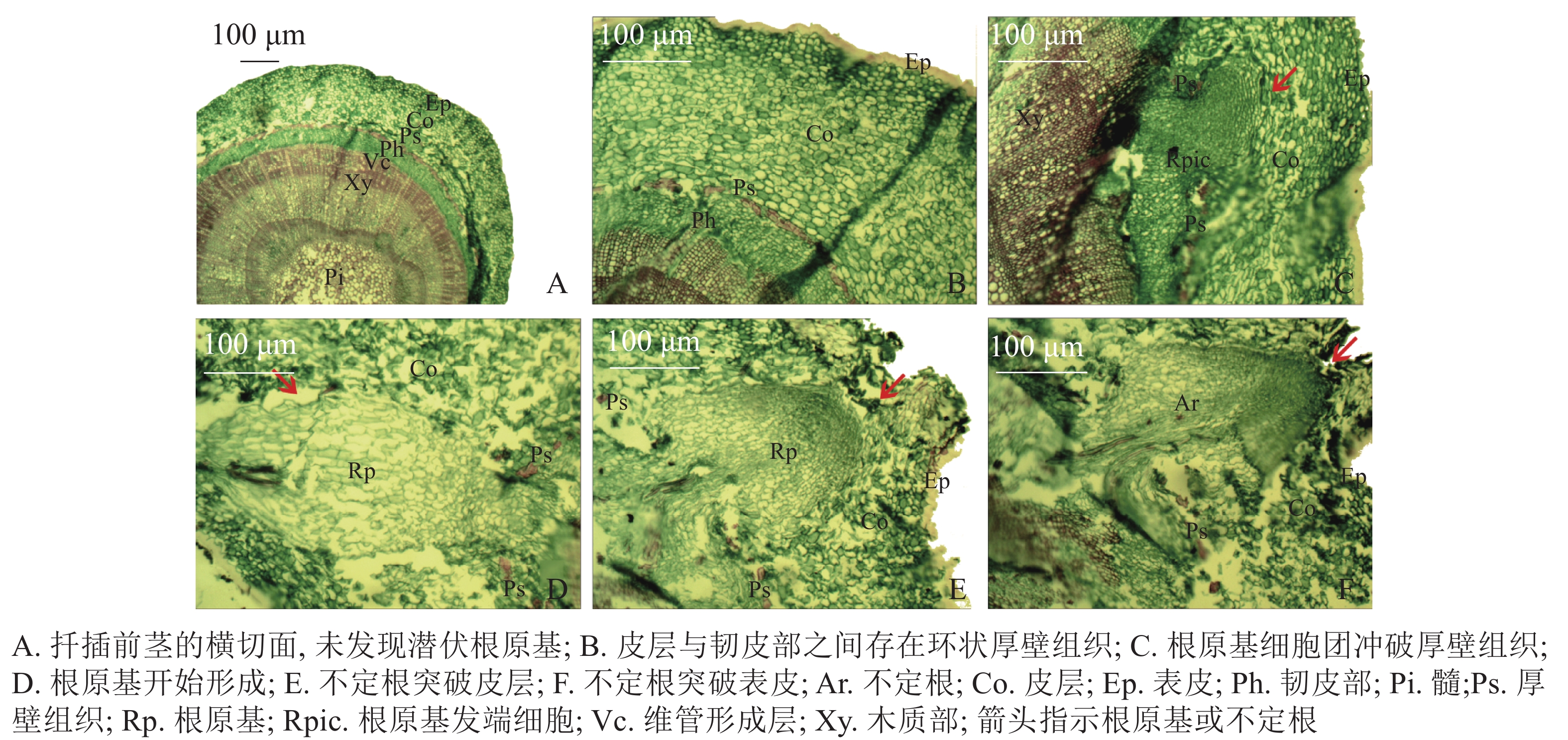
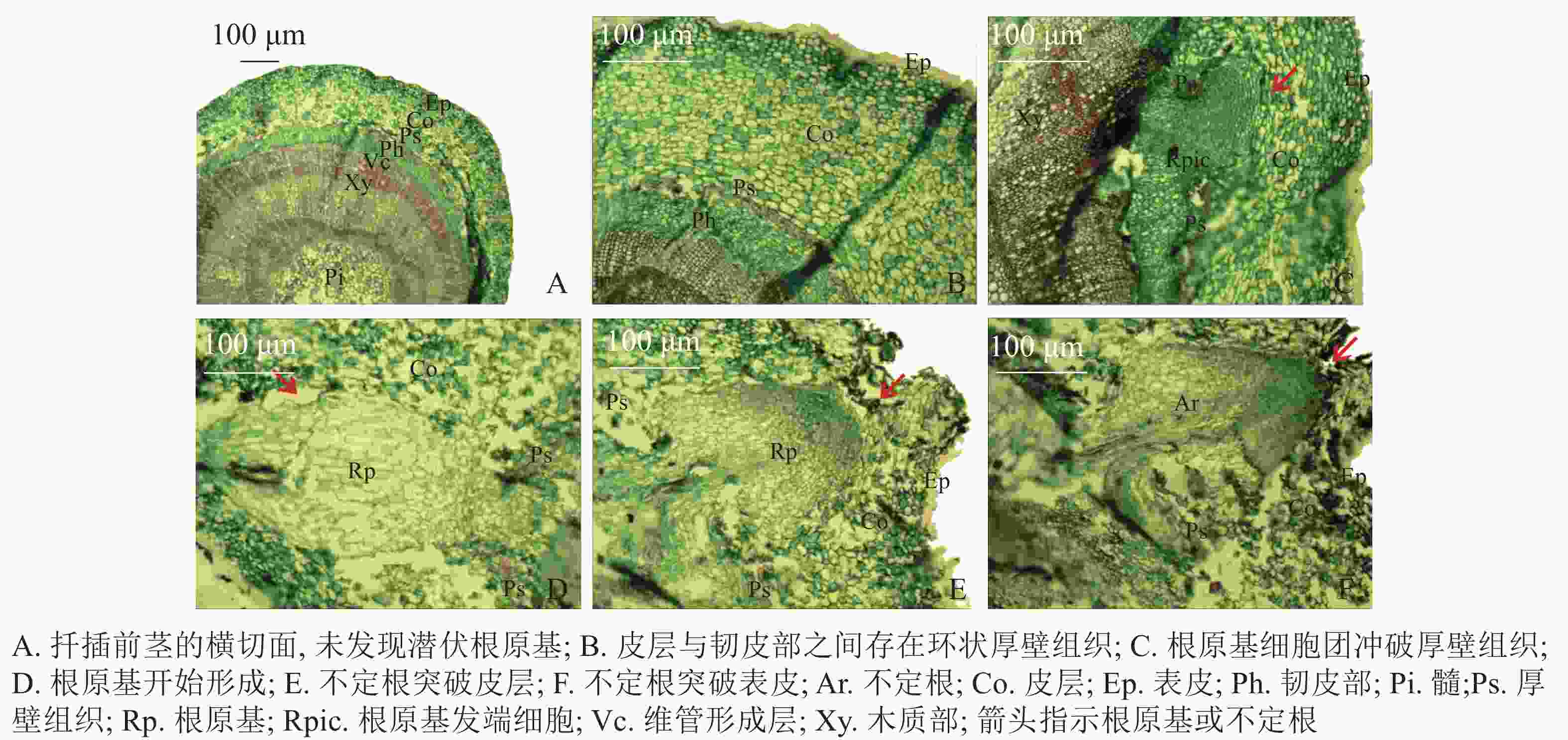
扦插前‘长叶阿尔塔’嫩枝插穗的横切面由表皮(Ep)、皮层(Co)、维管柱3部分组成(图2A)。维管柱包括韧皮部(Ph)、维管形成层(Vc)、木质部(Xy)、髓(Pi)等部分;在皮层与韧皮部之间存在由一层或多层纤维细胞组成的环状厚壁组织(Ps),呈连续或不连续的环状排列,被染成红色(图2B)。试验中大量切片观察并未发现插穗茎段横切面内存在潜伏根原基,表明‘长叶阿尔塔’的根原基是在扦插后诱导产生的。
-
根据解剖观察结果,‘长叶阿尔塔’插穗生根过程可划分为3个时期,即形成层细胞活跃期、不定根原基形成期和不定根形成期。①形成层细胞活跃期:由于植物生长调节剂的诱导,形成层细胞在28 d左右开始旺盛分裂,连续平周分裂产生胞质浓、细胞核大、染色深的薄壁细胞,并有向外扩张的趋势。②不定根原基形成期:根诱导42 d左右,髓射线正对的形成层细胞分裂最旺盛,在髓射线加宽部位和紧靠韧皮部的部位形成一团大小相当、细胞核较大、核仁明显、细胞质较浓的根原基细胞团(图2C)。随后,根原基细胞团不断分裂冲破连续的厚壁组织,并向皮层方向生长,突破皮层的根原基细胞团受到挤压分化形成楔形的根原基(图2D)。③不定根形成期:根原基形成后 (约56 d),朝向表皮一端的细胞团转化为不定根的顶端分生组织,顶端分生组织细胞不断分裂、生长,逐渐突破皮层细胞和表皮(图2E);同时,位于不定根根尖后端的细胞从外向内逐渐分化形成根的维管系统,最终与茎的维管系统相连形成幼根(图2F)。
-
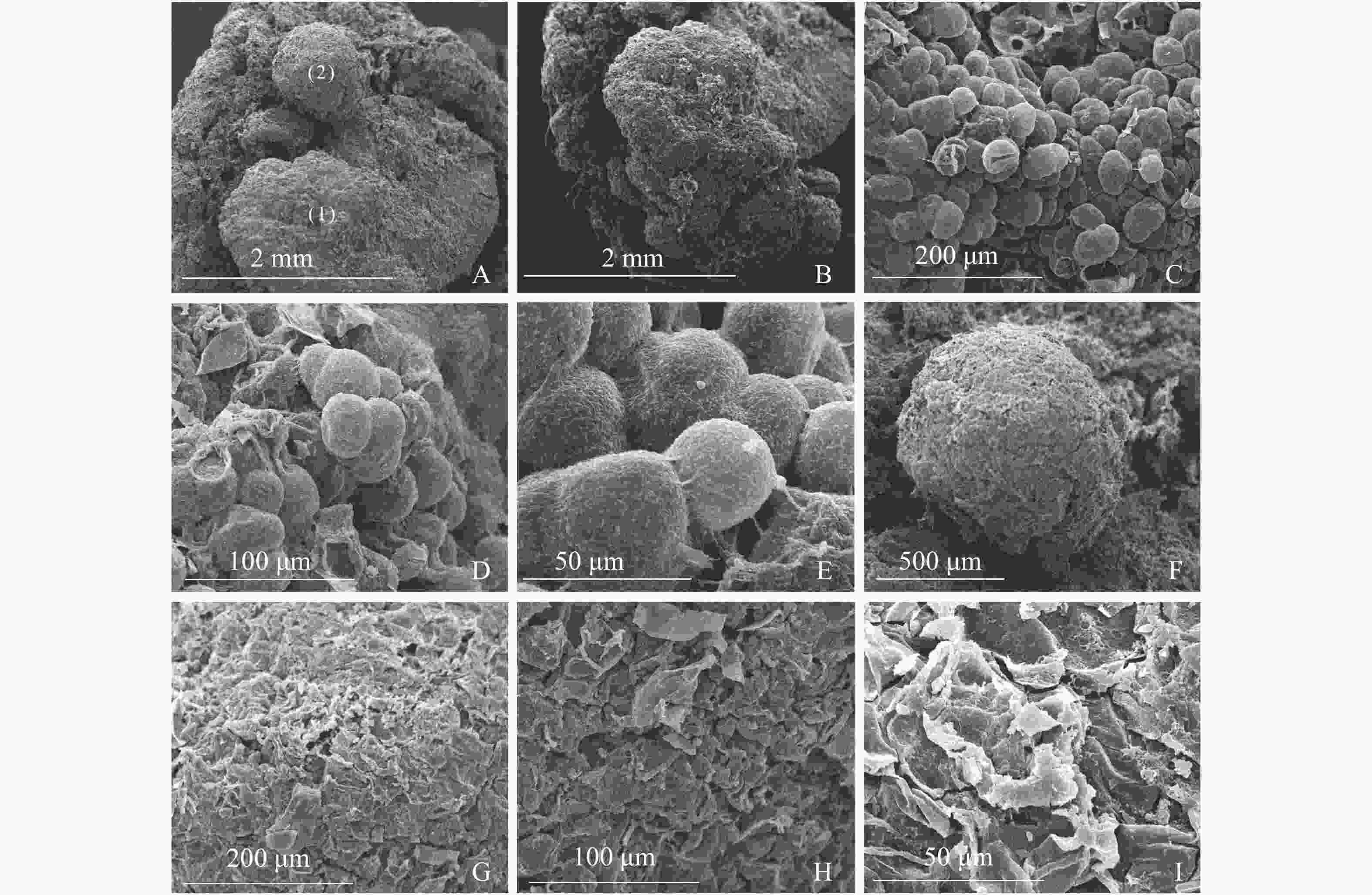
图3A显示同一插穗上2种不同类型的愈伤组织,①为白色、透明、块状的愈伤组织,②为浅黄色、不透明、点状的愈伤组织。白色块状愈伤组织表面有很多凸出且大小均一的球形细胞,细胞间间隙较小,为胚性愈伤组织细胞(图3B和图3C),多以细胞团的形式存在(图3D),表面黏液较多,且带有少量絮状附着物(图3E)。从图3F发现:浅黄色点状愈伤组织表面粗糙,细胞大多死亡破裂(图3G),细胞表面有附着物(图3H),在死亡破裂的细胞之间存在间隙,表面存在凹陷(图3I)。
-
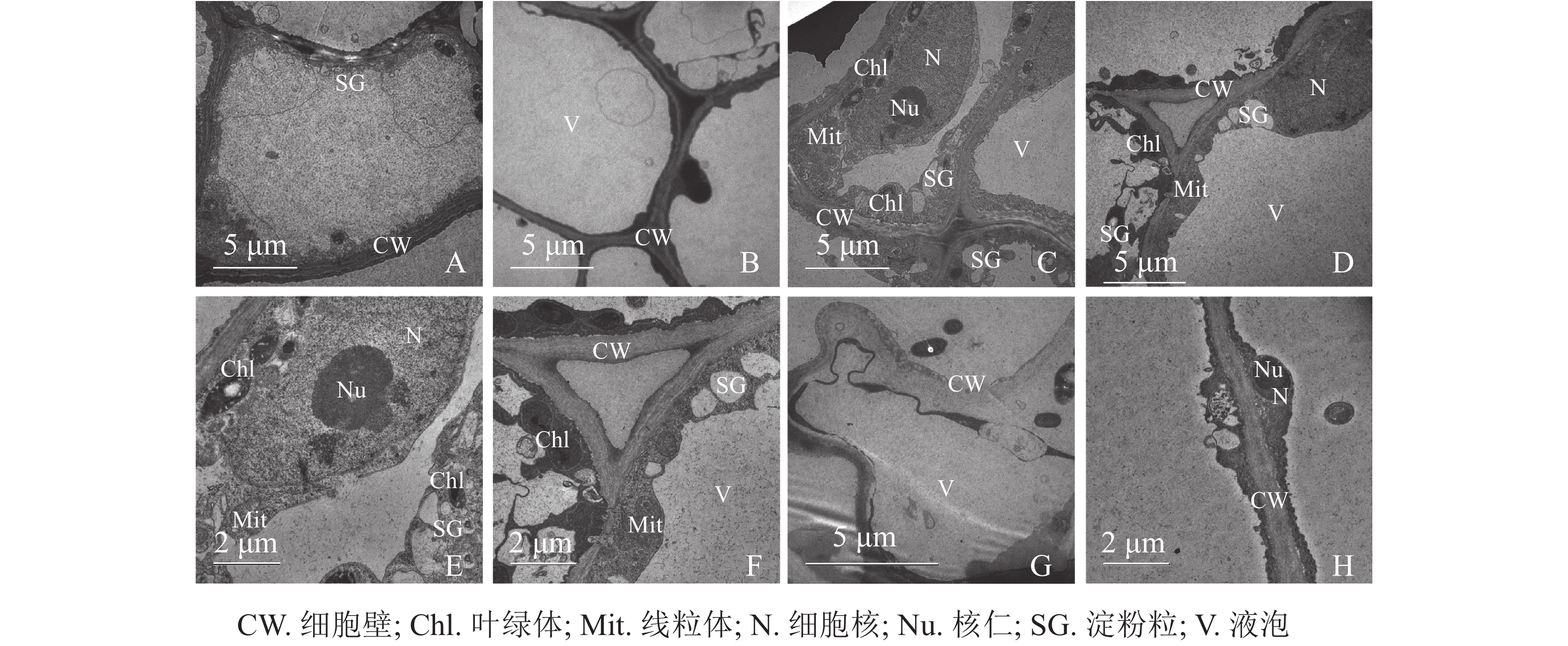
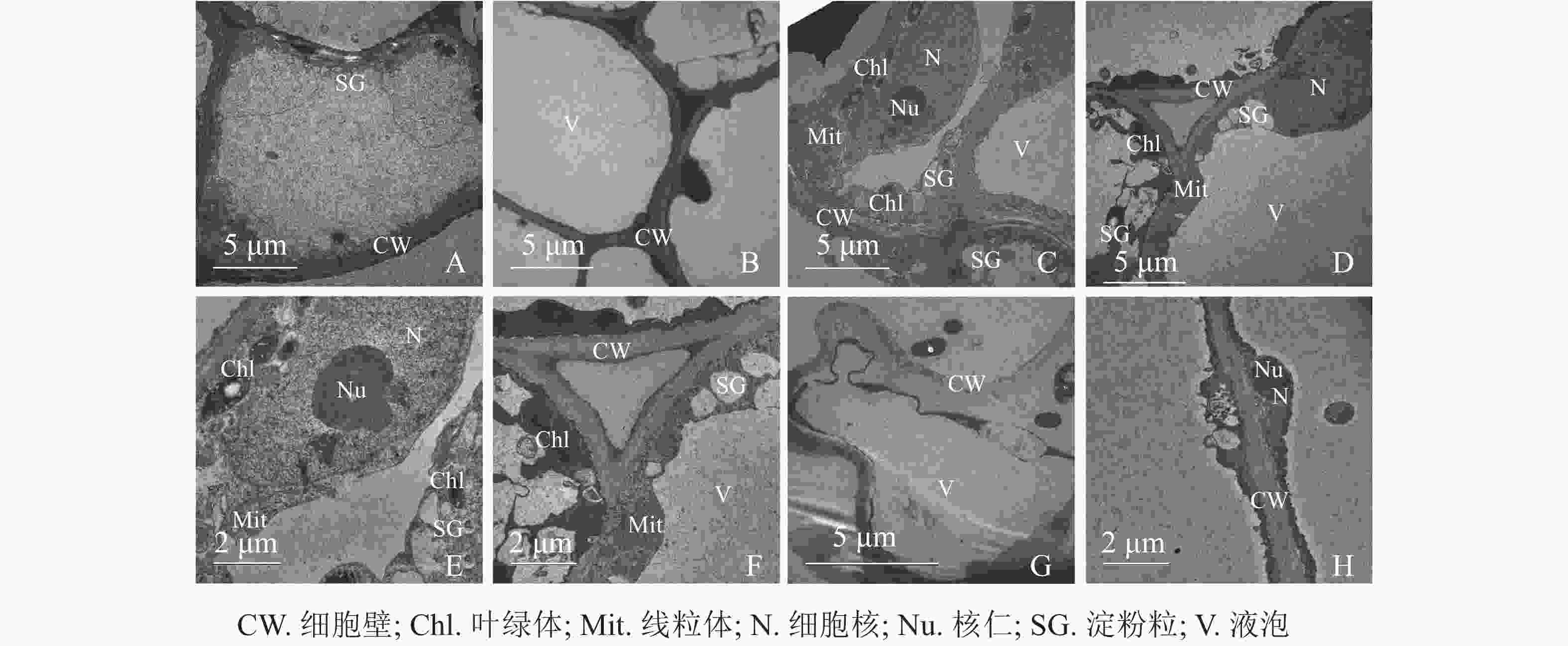
同一愈伤组织中既有胚性愈伤组织细胞也有非胚性愈伤组织细胞。胚性愈伤组织细胞质浓厚、细胞器较明显(图4A);非胚性愈伤组织细胞质稀薄,有明显的中央大液泡,几乎观察不到细胞器(图4B)。胚性愈伤组织细胞核大,核仁明显,在靠近细胞膜的胞质区域里有较多的淀粉粒和线粒体,还可观察到内含淀粉粒的叶绿体。淀粉可以为插穗生根提供充足的营养,线粒体较多说明其呼吸作用较强,代谢旺盛(图4C、4D、4E、4F)。同时,仍有部分胚性愈伤组织细胞出现轻微的质壁分离现象(图4G),这可能是老化愈伤组织中的衰老细胞。非胚性愈伤组织细胞的细胞质受到液泡挤压,细胞核等仅分布于细胞壁附近,但仍可观察到明显的核仁(图4H)。
-
选择最优的植物生长调节剂种类和质量浓度、处理时间等因素组合可以有效提高植物扦插生根率和生根数[11]。研究[12]表明:植物生长调节剂可以促进插穗基部细胞的分生与分化,加速插穗内可溶性糖、淀粉及可溶性蛋白的水解和代谢,使下切口成为营养物质的中心吸收区域;使用植物生长调节剂还可以提高插穗内过氧化物酶(POD)、多酚氧化酶(PPO)、吲哚乙酸氧化酶(IAAO)的活性,调节内源激素水平,活化形成层,促进根原基的形成[13-16]。IBA在促进难生根树种的生根及改善根系品质方面取得了较好的效果[17-18]。本研究中,IBA促进插穗生根效果显著优于ABT1和NAA,这可能是由于IBA被氧化分解的速度慢、传导扩散性能差,作用于插穗基部的时间长,有利于促进不定根的发生[19]。本试验最佳处理的生根率仅为48.83%,今后可结合其生根过程的激素调控、酶活性变化等方面对‘长叶阿尔塔’的生根机制开展更深入探究。
-
根据不定根在插穗上的形成部位不同,木本植物插穗生根类型分为皮部生根型、愈伤组织生根型和混合生根型[20]。本研究发现:‘长叶阿尔塔’插穗生根部位多在插穗切口上方2 cm左右的皮部,切口处无愈伤组织或仅有少量愈伤组织,也有少数插穗基部的愈伤组织发达,但仍然从切口上方的皮部形成多条不定根,与金建邦等[21]对欧洲鹅耳枥Carpinus betulus扦插不定根的发生发育研究结果类似。同时,观察‘长叶阿尔塔’插穗茎段的横切面切片后,并未发现潜伏根原基的存在,推测不定根是从扦插后诱导产生的诱生根原基发育而来,与山木通Clematis finetiana[22]、红花槭Acer rubrum[23]等的根原基来源相同。因此,‘长叶阿尔塔’插穗生根类型属于皮部诱导生根型。
-
插穗茎段结构是影响不定根发生、发育的“解剖学原因”[24]。对于难生根树种而言,插穗内部的机械组织(包括厚角组织与厚壁组织2类)是根原基形成和发育的阻碍因子[25-26]。树木扦插生根的难易程度与皮层和韧皮部之间的厚壁组织关系密切。多数难生根树种厚壁组织连续且呈环状,如珙桐Davidia involucrata[27]等。若插穗皮层中没有这种组织,或虽有但并不连续,则插穗生根相对容易,如喜树Camptotheca acuminata[28]等。本研究发现‘长叶阿尔塔’1年生插穗茎段皮层与韧皮部之间,存在1~2层由纤维细胞组成的环状厚壁组织,呈连续或不连续状,可能与插穗的发育程度有关;进一步观察发现仅少数根原基能突破连续的环状厚壁组织。此外,‘长叶阿尔塔’插穗生根需经历较长时间,春季56 d左右才能观察到突破表皮的不定根。由于生根进程缓慢,在此过程中插穗新叶、芽的生长消耗了大量的养分,且较长的时间容易导致插穗因病原菌侵害而褐化死亡,降低生根率。因此,为提高插穗生根率,应选择1年生、木质化程度较弱的枝条进行扦插,以减少厚壁组织对插穗生根率的影响。
-
扦插后,通常会在插穗基部表皮或表皮与木质化部分交接处形成愈伤组织,可由插穗茎段中的皮层、韧皮部、维管形成层、髓等多个部位的细胞快速分裂而来。插穗愈伤组织与不定根发生发育的关系可概括为以下3种:①愈伤组织的形成是不定根生长发育的物质基础。此类愈伤组织中可以分化形成根原基细胞,在一定条件下可发育形成不定根[29],如洒金柏Platycladus orientalis[30]等多数针叶树种均属于此类。②愈伤组织的产生与不定根形成无直接因果关系、彼此独立[31]。这类愈伤组织通常不能形成根原基,只能分化形成独立的维管束、输导组织等,在插穗与基质之间无机盐、水分等物质交换过程中起着中介作用,如灰毡毛忍冬Lonicera macranthoides[32]等属于此类。③愈伤组织的产生不利于不定根的发生、发育[33]。如高度发达的愈伤组织抑制了白桦Betula platyphylla嫩枝插穗根原基细胞的分化,进而阻碍了不定根的形成[34]。本研究发现:‘长叶阿尔塔’插穗愈伤组织产生与不定根发生、发育彼此独立,适度分化的愈伤组织能够保护切口免受外界病菌侵入,防止插穗内有效物质的流失,还可以充当水分等物质交换的桥梁。但过度分化的愈伤组织会占用插穗内部的营养物质,抑制不定根的形成。
-
冬青‘长叶阿尔塔’插穗经1 000 mg·L−1 IBA溶液速蘸10 s,扦插在V(草炭)∶V(蛭石)∶V(珍珠岩)=3∶3∶4或4∶3∶3的基质中可获得较高的生根率,其插穗生根类型属于皮部诱导生根型,根原基起源于髓射线与维管形成层交叉处,环状厚壁组织是阻碍其插穗生根的机械原因。‘长叶阿尔塔’插穗愈伤组织中并未观察到根原基发端细胞,其产生与不定根发生、发育彼此独立,可分为胚性愈伤组织和非胚性愈伤组织,胚性愈伤组织多为白色,其表面细胞体积较小且排列紧密,常成团分布,细胞核大质浓,细胞器丰富;非胚性愈伤组织细胞多为黄色,其表面细胞大多死亡破裂,空泡化明显,几乎没有细胞器。
Root formation and anatomical structure of Ilex × altaclerensis ‘Belgica Aurea’ stem cuttings
doi: 10.11833/j.issn.2095-0756.20210283
- Received Date: 2021-04-12
- Rev Recd Date: 2021-10-18
- Available Online: 2022-03-25
- Publish Date: 2022-03-25
-
Key words:
- Ilex × altaclerensis ‘Belgica Aurea’ /
- cutting /
- plant growth regulator /
- adventitious root /
- callus /
- anatomy
Abstract:
| Citation: | ZHU Xiaoyu, TONG Wanwan, ZHAO Chu, TIAN Ru’nan. Root formation and anatomical structure of Ilex × altaclerensis ‘Belgica Aurea’ stem cuttings[J]. Journal of Zhejiang A&F University, 2022, 39(2): 347-355. doi: 10.11833/j.issn.2095-0756.20210283 |






 DownLoad:
DownLoad: