-
厚竹Phyllostachys edulis ‘Pachyloen’又称厚壁毛竹,是江西省特有的毛竹P. edulis变异新品种,2008年获植物新品种保护权[1],2017年被审定为国家级优良品种。厚竹因其秆壁厚度是等径毛竹的1.8~2.0倍且性状稳定而得名[2];生物学和生态学特性与毛竹相似,但生物量、竹笋中营养物质含量[1]、抗寒性[3]、氮代谢水平和利用率[4-5]、二氧化碳(CO2)和光能利用率[6-8]等均高于毛竹;作为笋用和笋材兼用竹种,其经济价值、种质价值、碳储量和固碳能力[9-10]较高,推广前景广阔。内源激素作为植物体内重要的活性物质,其质量分数和相互作用对植物的生长和发育起着非常重要的调节和控制作用[11-12]。何奇江等[13-14]、胡超宗等[15]和黄坚钦等[16]对雷竹Ph. praecox的内源激素进行了研究,郭少玲等[17]研究了60Coγ辐射对毛竹种子萌发过程中内源激素的影响,丁兴萃[18]和郑郁善等[19]研究了毛竹笋期的内源激素分布,方楷等[20]对厚竹展叶期竹秆内源激素进行了研究,张艳华等[21]研究了厚竹新竹中内源激素对繁殖方式的响应,吴兴波等[22]研究了氯霉素对毛竹幼苗色素质量分数及叶绿素荧光的影响。结果显示,内源激素对竹笋的生长发育影响较大,竹子不同器官中及竹笋不同部位的内源激素质量分数不同,人为干预措施可以改变竹子中内源激素的质量分数和分布。过去对竹子内源激素的研究多集中于某一特定时期或单一器官,主要研究单一内源激素。关于竹子孕笋成竹期内源激素的分布和动态变化,及各种内源激素的相对质量分数对竹子孕笋成竹的影响还值得深入研究。本试验通过分析厚竹从鞭芽萌动到成竹过程中不同部位内源激素的质量分数,研究厚竹孕笋成竹过程中内源激素的分布特征和动态变化规律,探索内源激素对厚竹孕笋成竹的调控作用,为进一步研究内源激素调控竹类植物生长的机理和厚竹定向培育提供理论依据。
HTML
-
采样林分:采样地位于江西农业大学竹类植物种质园内(28°45′24.1″N,115°49′50.2″E),海拔49.5 m,低丘岗地,土壤为红壤。采样林分为厚竹纯林,1995年从厚竹原产地(江西省万载县高村乡严田村,28°06′33.38″N,114°26′24.78″E)引种繁育而成,面积约为0.5 hm2,立竹胸径为4.0~7.0 cm,立竹高度为6.0~10.0 m,竹林结构合理,生长正常。
采样时间:2015年1月20日,笋芽膨大期,采集竹鞭潜伏芽、笋芽和冬笋样品。2015年3月31日,竹笋快速生长期,采集竹鞭潜伏芽和春笋样品。2015年6月10日,新竹长成期,采集新竹样品。
采样部位:潜伏芽整芽取样。已经萌动的笋芽分芽肉和芽箨取样。冬笋和春笋分笋箨及顶部、中部和基部笋肉取样,春笋增加中部和基部笋肉的内壁、外壁及笋根取样。新竹分中上部竹秆、中下部竹枝、竹叶和篼根取样。各取样对象均选择3个及以上作为重复,采集样品量约1 g·部位-1。
样品处理:野外采集样品后立即放入冰盒中,2 h内转入-85 ℃超低温冰箱中冷冻。每一时期全部样品采集后用干冰包埋送中国农业大学分析。
-
内源激素测定方法为酶联免疫吸附测定法(ELISA),每个样品平行测定3次,测定指标为吲哚-3-乙酸(IAA),赤霉素(GA3),玉米素核苷(ZR)和脱落酸(ABA)。
-
试验数据采用SPSS 17.0分析软件分析,用单因素方差分析和Duncan多重比较对厚竹孕笋成竹期鞭芽、竹笋和新竹不同部位中的内源激素质量分数和比值进行差异性分析。采用Excel 2007统计软件进行数据处理和图表制作。
1.1. 试验材料
1.2. 样品分析
1.3. 数据处理
-
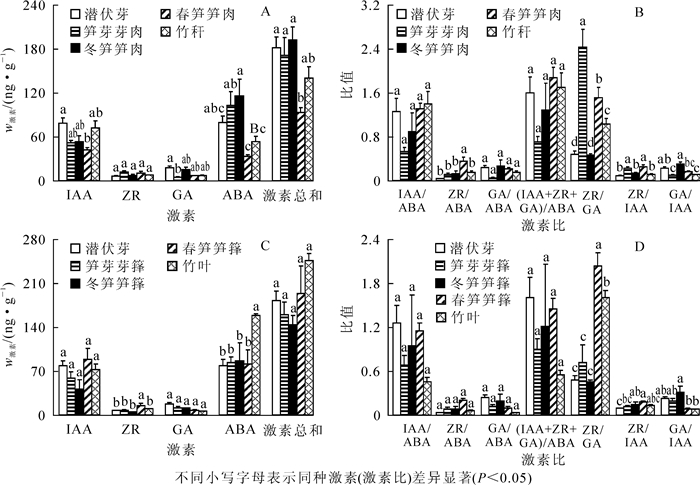
从表 1看出:笋芽膨大期,所有部位内源激素质量分数均表现为ABA>IAA>GA3>ZR,由于此时竹林处于休眠期,内源激素在不同部位间均无显著差异。竹笋快速生长期,潜伏芽和春笋的生命活动旺盛程度不同,内源激素质量分数也不相同,潜伏芽中ABA>IAA>ZR>GA3,而春笋中IAA>ABA>ZR>GA3,差异程度因部位而异。新竹抽枝展叶后,潜伏芽和竹叶中内源激素质量分数表现为ABA>IAA>ZR>GA3,竹秆中内源激素质量分数则是IAA>ABA>ZR>GA3。
时期 部位 wIAA/(ng·g-1) wZR/(ng·g-1) wGA3/(ng·g-1) wABA/(ng·g-1) wALL/(ng·g-1) 笋芽膨大期 潜伏芽 78.73 ± 7.91 Aa 6.90 ± 0.70 Ab 17.54 ± 2.76 Ab 79.32 ± 9.23 Aa 182.48 ± 14.45 A 笋芽芽箨 58.17 ± 11.15 Ab 7.19 ± 2.04 Ac 10.77 ± 2.56 Ac 84.23 ± 8.12 Aa 160.35 ± 20.12 A 笋芽芽肉 68.76 ± 9.11 Ab 6.56 ± 2.08 Ac 10.40 ± 2.83 Ac 103.01 ± 19.03 Aa 188.73 ± 23.83 A 冬笋笋箨 41.43 ± 14.38 Aab 4.84 ± 0.39 Ab 10.64 ± 0.45 Ab 86.84 ± 28.78 Aa 143.76 ± 15.57 A 冬笋笋肉 53.32 ± 8.87 Ab 7.18 ± 1.41 Ac 15.81 ± 3.29 Ac 116.44 ± 22.81 Aa 192.74 ± 18.21 A 竹笋快速生长期 潜伏芽 51.04 ± 9.36 Ba 8.79 ± 1.66 Bb 6.81 ± 1.03 Ab 70.45 ± 22.83 Aa 137.08 ± 34.71 B 春笋笋箨 89.17 ± 17.61 Aa 15.56 ± 2.74 Ab 7.61 ± 1.16 Ab 81.51 ± 22.22 Aa 193.84 ± 43.54 A 春笋笋肉 43.45 ± 2.44 Bb 8.93 ± 0.96 Bc 6.38 ± 0.34 Ac 35.30 ± 1.68 Ba 94.06 ± 4.36 C 新竹长成期 潜伏芽 67.75 ± 7.72 Aa 9.72 ± 0.83 Ab 7.07 ± 0.52 Ab 78.50 ± 14.33 Ba 163.04 ± 12.45 B 竹叶 71.90 ± 9.42 Ab 9.48 ± 1.10 Ac 5.86 ± 0.42 Ac 159.05 ± 1.75 Aa 246.28 ± 10.65 A 竹秆 72.11 ± 10.04 Aa 7.84 ± 0.28 Ab 7.66 ± 0.57 Ab 53.46 ± 7.73 Ba 141.07 ± 14.98 B 说明:同列不同大写字母表示同一发育期内差异显著;同行不同小写字母表示不同内源激素间差异显著 Table 1. Contents of endogenous hormones in shoots and new bamboos
进一步分析冬笋、春笋和新竹不同部位的内源激素。从表 2看出:不同内源激素质量分数因植株形态或部位不同而不同。冬笋所有部位均为ABA>IAA>GA3>ZR,春笋所有部位为IAA>ABA>ZR>GA3,新竹的竹枝和竹叶为ABA>IAA>ZR>GA3,而竹秆和蔸根则为IAA>ABA>ZR>GA3。
植株形态 部位 wIAA/(ng·g-1) wZR/(ng·g-1) wGA3/(ng·g-1) wABA/(ng·g-1) wALL/(ng·g-1) 冬笋 笋箨 41.43 ± 14.38 Aab 4.84 ± 0.39 Ab 10.64 ± 0.45 Ab 86.84 ± 28.78 Aa 143.76 ± 15.57 A 笋尖 38.25 ± 8.39 Aab 5.29 ± 1.04 Ab 12.00 ± 2.36 Ab 133.10 ± 51.02 Aa 188.65 ± 50.3 A 中部笋肉 54.21 ± 13.45 Aab 8.00 ± 2.78 Ab 14.66 ± 3.94 Ab 97.19 ± 41.54 Aa 174.06 ± 28.49 A 基部笋肉 67.49 ± 21.89 Ab 8.26 ± 3.53 Ac 20.76 ± 9.41 Ac 119.02 ± 39.81 Aa 215.52 ± 13.9 A 春笋 笋箨 89.17 ± 17.61 Aa 15.56 ± 2.74 Ab 7.61 ± 1.16 Ab 81.51 ± 22.22 Aa 193.84 ± 43.54 A 笋尖 44.64 ± 5.33 Ba 17.36 ± 3.35 Abc 9.00 ± 1.57 Ac 29.93 ± 4.99 Bb 100.93 ± 12.86 B 中部内壁 39.34 ± 0.84 Ba 6.66 ± 0.56 Bb 5.85 ± 0.13 Ab 38.31 ± 2.81 Ba 90.16 ± 4.14 B 中部外壁 52.62 ± 5.60 Ba 8.54 ± 0.94 Bc 6.85 ± 0.52 Ac 40.60 ± 2.39 Bb 108.62 ± 4.79 B 基部内壁 39.92 ± 2.68 Ba 7.49 ± 1.71 Bc 5.46 ± 0.18 Ac 33.01 ± 4.24 Bb 85.89 ± 8.28 B 基部外壁 44.70 ± 14.75 Ba 7.07 ± 1.63 Bb 5.46 ± 0.6 Ab 35.30 ± 8.71 Ba 92.53 ± 25.16 B 笋根 37.08 ± 6.71 Ba 6.07 ± 0.46 Bb 5.79 ± 0.89 Ab 36.82 ± 2.26 Ba 85.77 ± 6.02 B 新竹 竹叶 71.90 ± 9.42 Ab 9.48 ± 1.10 Ac 5.86 ± 0.42 Ac 159.05 ± 1.75 Aa 246.28 ± 10.65 A 竹枝 58.20 ± 10.95 Aa 8.05 ± 0.48 Ab 7.05 ± 0.58 Ab 75.80 ± 6.44 Ba 149.10 ± 8.92 B 竹秆 72.11 ± 10.04 Aa 7.84 ± 0.28 Ac 7.66 ± 0.57 Ac 53.46 ± 7.73 Cb 141.07 ± 14.98 B 蔸根 70.12 ± 11.59 Aa 8.30 ± 1.19 Ab 5.94 ± 0.62 Ab 37.88 ± 1.24 Cc 122.24 ± 11.27 B 说明:同列不同大写字母表示同一植株体中差异显著;同行不同小写字母表示不同内源激素间差异显著 Table 2. Contents of endogenous hormones in different parts of bamboo
由表 1和表 2还可看出:同种内源激素因发育时期或部位不同存在差异。笋芽膨大期,冬笋中促进生长类激素表现向基性增加;ABA则是笋尖和笋基部高,中部笋肉低,但潜伏芽、笋芽和冬笋之间及同一植株形态不同部位之间同种内源激素质量分数和内源激素总量差异均不显著。竹笋快速生长期,潜伏芽和春笋的内源激素质量分数差异显著,春笋笋箨中各内源激素质量分数显著高于笋肉,笋肉外壁中IAA,ZR和GA3呈向基性减少,但差异性未达到显著程度。ABA主要在叶片中合成,新竹叶片中ABA质量分数显著高于其他部位,并呈向基性减少。
-
激素间的相互作用对植物的生长和发育有重要的调节和控制作用[9],研究激素间交互作用对厚竹生长发育的影响意义重大。研究认为,促进生长类激素与抑制生长类激素质量分数的比值对植物生长发育有重要的调控作用[23]。由表 3可知:生长中心的转移和不同植株形态的生命活动不同,厚竹在孕竹成笋过程中各内源激素质量分数的比值有较大差异,并且不同激素间的比值差异程度不同。从表 4看出:冬笋同一部位不同内源激素比值间存在显著差异,但相同内源激素比值在不同部位之间差异性均不显著。春笋笋尖由于细胞分裂和伸长生长活动旺盛,其中ZR和GA3在内源激素中的相对质量分数显著偏高,ZR/GA3也显著偏高,说明笋尖中细胞分裂活动尤为旺盛。新竹叶片中ABA质量分数显著偏高,促进生长类激素与ABA的比值显著低于其他部位,并呈显著向基性升高;新竹中代谢活动旺盛部位中的ZR/GA3显著高于代谢活动较弱的部位,而GA3/IAA值则相反。
时期 部位 IAA/ABA ZR/ABA GA3/ABA (IAA+ZR+GA3)/ABA ZR/IAA GA3/IAA ZR/GA3 笋芽 潜伏芽 1.26 ± 0.24 Aa 0.04 ± 0.00 Ab 0.24 ± 0.03 Ab 1.60 ± 0.29 Aa 0.22 ± 0.02 Ab 0.09 ± 0.01 Bb 0.49 ± 0.06 Ab 膨大 笋芽芽箨 0.69 ± 0.12 Aa 0.09 ± 0.02 Ab 0.12 ± 0.02 Ab 0.90 ± 0.15 Ab 0.12 ± 0.01 ABb 0.19 ± 0.04 Bb 0.71 ± 0.24 Aa 期 笋芽芽肉 0.72 ± 0.18 Aa 0.07 ± 0.04 Ab 0.10 ± 0.01 Ab 0.89 ± 0.21 Aa 0.09 ± 0.02 Bb 0.16 ±0.04 Bb 0.84 ± 0.48 Aa 冬笋笋箨 0.94 ± 0.71 Aa 0.08 ± 0.04 Ac 0.19 ± 0.10 Ac 1.21 ± 0.85 Aa 0.14 ± 0.04 ABc 0.31 ± 0.08 Abc 0.45 ± 0.02 Abc 冬笋笋肉 0.90 ± 0.33 Aab 0.12 ± 0.05 Ac 0.26 ± 0.11 Abc 1.28 ± 0.49 Aa 0.14 ± 0.02 ABc 0.31 ± 0.04 Ab 0.46 ± 0.03 Ab 笋快速生长期 潜伏芽 0.79 ± 0.10 Bc 0.14 ± 0.02 Ad 0.11 ± 0.02 Bd 1.03 ± 0.13 Bb 0.17 ± 0.01 Ad 0.14 ± 0.00 ABd 1.28 ± 0.06 Ba 春笋笋箨 1.15 ± 0.11 Ac 0.20 ± 0.02 Ad 0.10 ± 0.02 Bd 1.45 ± 0.14 ABb 0.18 ± 0.01 Ad 0.09 ± 0.00 Bd 2.04 ± 0.18 Aa 春笋笋肉 1.25 ± 0.06 Ab 0.27 ± 0.04 Ac 0.19 ± 0.01 Ac 1.70 ± 0.10 Aa 0.20 ± 0.02 Ac 0.15 ± 0.01 Ac 1.37 ± 0.10 Bb 新竹长成期 潜伏芽 1.05 ± 0.28 ABa 0.06 ± 0.01 Bb 0.10 ± 0.02 ABb 1.3 ± 0.34 ABa 0.11 ± 0.01 Ab 0.15 ± 0.01 Ab 1.38 ± 0.11 ABa 竹叶 0.45 ± 0.06 Bab 0.06 ± 0.01 Bb 0.04 ± 0.00 Bb 0.55 ± 0.07 Bab 0.13 ± 0.01 Ab 0.08 ± 0.01 Ab 1.61 ± 0.09 Aa 竹秆 1.40 ± 0.23 Aab 0.15 ± 0.02 Ac 0.15 ± 0.03 Ac 1.70 ± 0.27 Aa 0.11 ± 0.01 Ac 0.11 ± 0.01 Ac 1.04 ± 0.09 Bb 说明:同列不同大写字母表示同一植株体中差异显著;同行不同小写字母表示不同内源激素比值间差异显著 Table 3. Ratios among different endogenous hormones in bamboo shoots and new bamboos during different periods
植株体 部位 IAA/ABA ZR/ABA GA3/ABA (IAA+ZR+GA3)/ABA ZR/IAA GA3/IAA ZR/GA3 冬笋 笋箨 0.94 ± 0.71 Aa 0.08 ± 0.04 Ab 0.19 ± 0.10 Ab 1.21 ± 0.85 Aa 0.14 ± 0.04 Ab 0.31 ± 0.08 Aab 0.45 ± 0.02 Aab 笋尖 0.55 ± 0.38 Aa 0.05 ± 0.02 Ab 0.12 ± 0.04 Ab 0.73 ± 0.44 Aa 0.16 ± 0.05 Ab 0.35 ± 0.10 Aab 0.44 ± 0.01 Aab 笋中部 1.05 ± 0.67 Aa 0.16 ± 0.12 Ab 0.28 ± 0.19 Ab 1.49 ± 0.98 Aa 0.14 ± 0.01 Ab 0.27 ± 0.05 Ab 0.54 ± 0.07 Aab 笋基部 1.10 ± 0.80 Aa 0.15 ± 0.12 Ab 0.38 ± 0.31 Aab 1.63 ± 1.23 Aa 0.12 ± 0.02 Ab 0.29 ± 0.05 Ab 0.41 ± 0.02 Aab 春笋 笋箨 1.15 ± 0.11 Ac 0.20 ± 0.02 Bd 0.10 ± 0.02 Bd 1.45 ± 0.14 Ab 0.18 ± 0.01 Bd 0.09 ± 0.00 Cd 2.04 ± 0.18 Aa 笋尖 1.56 ± 0.23 Ab 0.62 ± 0.15 Ac 0.30 ± 0.03 Ac 2.48 ± 0.40 Aa 0.38 ± 0.04 Ac 0.20 ± 0.02 Ac 1.98 ± 0.36 Aab 中部内壁 1.04 ± 0.06 Ab 0.17 ± 0.01 Bc 0.15 ± 0.01 Bc 1.36 ± 0.07 Aa 0.17 ± 0.01 Bc 0.15 ± 0.00 ABc 1.14 ± 0.09 Bb 中部外壁 1.32 ± 0.22 Aa 0.21 ± 0.04 Bb 0.17 ± 0.02 Bb 1.71 ± 0.28 Aa 0.16 ± 0.01 Bb 0.13 ± 0.01 Bb 1.25 ± 0.12 ABa 基部内壁 1.23 ± 0.10 Aa 0.22 ± 0.03 Bb 0.17 ± 0.02 Bb 1.62 ± 0.10 Aa 0.18 ± 0.03 Bb 0.14 ± 0.01 Bb 1.37 ± 0.30 ABa 基部外壁 1.23 ± 0.16 Aa 0.21 ± 0.04 Bb 0.16 ± 0.02 Bb 1.60 ± 0.18 Aa 0.17 ± 0.02 Bb 0.14 ± 0.04 Bb 1.30 ± 0.27 ABa 笋根 1.04 ± 0.25 Aa 0.17 ± 0.02 Bb 0.16 ± 0.03 Bb 1.37 ± 0.31 Aa 0.17 ± 0.03 Bb 0.16 ± 0.00 ABb 1.09 ± 0.15 Ba 新竹 竹叶 0.45 ± 0.06 Cb 0.06 ± 0.01 Cc 0.04 ± 0.00 Cc 0.55 ± 0.07 Cb 0.13 ± 0.01 Ac 0.08 ± 0.01 Bc 1.61 ± 0.09 Aa 竹枝 0.80 ± 0.21 BCa 0.11 ± 0.02 BCb 0.10 ± 0.02 Bb 1.00 ± 0.25 BCa 0.15 ± 0.02 Ab 0.13 ± 0.02 Ab 1.15 ± 0.04 BCa 竹秆 1.40 ± 0.23 BAab 0.15 ± 0.02 Bc 0.15 ± 0.03 ABc 1.70 ± 0.27 Ba 0.11 ± 0.01 Ac 0.11 ± 0.01 ABc 1.04 ± 0.09 Cb 蔸根 1.87 ± 0.36 Aba 0.22 ± 0.03 Ac 0.16 ± 0.01 Ac 2.24 ± 0.37 Aa 0.12 ± 0.02 Ac 0.09 ± 0.01 Bc 1.39 ± 0.07 Bb 说明:同列不同大写字母表示同一植株体中差异显著;同行不同小写字母表示不同内源激素比值间差异显著 Table 4. Ratios of different endogenous hormones in different bamboo parts
-
潜伏芽是指竹鞭上未分化发育成笋或鞭的细小鞭芽,与已萌发的笋芽或鞭芽相比,生长极为缓慢或不生长。对不同生长期潜伏芽中的内源激素质量分数和比值进行比较可知,笋芽膨大期潜伏芽中GA3的质量分数显著高于竹笋快速生长期和新竹长成期;除GA3外,其他内源激素的绝对量变化不大(图 1A)。不同内源激素相对质量分数却有显著变化,且动态变化规律不同。由图 1B可知:ZR/ABA先增加后降低,不同生长时期差异显著;ZR/GA3和ZR/IAA随成笋进程显著降低。由此证实从笋芽膨大期到快速生长期,潜伏芽内GA3相对量增多,为打破潜伏芽休眠进而分化为笋芽做好准备;而竹笋快速生长期,竹笋内ZR相对量提高,有助于促进细胞分裂。
-
图 2为厚竹不同形态下不同部位的内源激素质量分数及比值的变化。潜伏芽分化为笋芽,芽肉中GA3质量分数显著下降(图 2A),GA3/IAA显著下降但ZR/GA3和ZR/IAA显著升高(图 2B),说明潜伏芽至笋芽的分化以细胞分裂为主。冬笋为了适应低温环境并有利于打破休眠,从笋芽芽肉发育后,冬笋笋肉中GA3/IAA显著升高,而ZR/GA和ZR/IAA显著降低。进入竹笋快速生长期,春笋笋肉ABA质量分数出现显著降低(图 2A),GA3/IAA显著降低而ZR/ABA,ZR/IAA和ZR/GA3均显著升高(图 2B),可以认为此时竹笋生长以细胞数量增加为主。新竹抽枝展叶后,竹林的生长中心转入地下,新竹的细胞分裂活动基本停止,故竹秆中ABA质量分数显著增加,促进细胞分裂和伸长生长的ZR和GA3的相对质量分数显著降低。潜伏芽至笋芽芽箨、冬笋笋箨、春笋笋箨、竹叶的内源激素的动态变化趋势与笋肉相似(图 2C,图 2D),竹笋快速生长期,笋(秆)箨生长迅速,脱落也快,笋箨中ZR,IAA和ABA均保持较高水平。
综合图 1和图 2可知:内源激素对笋芽分化和竹笋生长具有重要调控作用。较高的IAA和较低的ABA有利于竹笋的分化和生长,较高的GA3有利于打破潜伏芽和冬笋的休眠,促进笋芽分化和冬笋萌动,而较高的ZR有利于促进竹笋生长。ZR/ABA,ZR/IAA,ZR/GA3及GA3/IAA比单一内源激素的绝对量更能反映厚竹孕笋成竹过程的变化,说明内源激素间的交互作用对厚竹的孕笋成竹有显著的调控作用。
2.1. 厚竹竹笋和新竹内源激素质量分数与分布特征
2.2. 厚竹内源激素比值分析
2.3. 厚竹孕笋成竹过程中内源激素动态变化
2.3.1. 潜伏芽内源激素动态变化
2.3.2. 厚竹孕笋成竹过程中内源激素动态变化
-
笋芽膨大期,竹林处于休眠状态,冬笋及鞭芽内抑制生长类激素(如ABA)质量分数显著高于促进生长类激素,抑制了笋芽和冬笋的快速生长,以应对低温环境。竹笋进入快速生长期后,促进细胞分裂的激素显著增加,抑制生长类激素显著减少,此时可见笋尖ZR质量分数明显增加,并且显著高于竹笋的中部和基部;ABA质量分数显著降低,GA3质量分数在竹笋各部位差异不显著,说明进入速生期后,竹笋生长以细胞分裂为主。新竹长成后,竹叶中的ZR和GA3质量分数显著低于笋尖,提示厚竹抽枝展叶后,地上系统的细胞分裂和生长基本停止,地下竹鞭已经成为竹林系统的生长中心。
春笋笋肉中促进生长类激素的相对质量分数显著高于潜伏芽,特别是笋尖内ZR/IAA,ZR/ABA,GA3/IAA及GA3/ABA显著高于其他植株体或部位,新竹长成后,竹林系统生长中心转入地下,新竹中促进生长类激素与抑制生长类激素的比值呈显著向基性递增,生命活动相对旺盛的叶片和蔸根中的GA3/IAA显著低于竹秆和竹枝,但ZR/GA3则刚好相反,表明内源激素的相对质量分数对厚竹生长发育有显著影响,较高比例的ZR有利于碳水化合物的合成和器官的生长,促进竹笋和及篼根生长,而较高比例的GA3则有利于打破潜伏芽休眠,促进物质的转化及纤维素和木质素的形成,说明内源激素间的协调或拮抗作用对厚竹孕笋成竹有重要的调控作用,但其作用机理还有待于深入研究。
郑郁善等[19]研究发现,内源激素在毛竹春笋中的质量分数表现为激动素(KT)>赤霉素(GA3)>IAA,在新竹叶片中表现为GA3>IAA>KT;王波等[23]发现无论笋或新竹叶片,均表现为GA3质量分数显著高于IAA;方楷等[24]研究发现:毛竹竹笋和新竹各个部位中,均表现为GA3质量分数显著高于玉米素(ZT)。本研究发现:厚竹春笋和新竹各个部位,均表现为IAA>ZR>GA3,其中IAA质量分数显著高于ZR和GA3。厚竹中促进细胞壁形成和蛋白质合成的IAA及促进细胞分裂的ZR质量分数高,促进细胞伸长生长的GA3质量分数相对较少,有利于竹壁的横向增长,可能是造成厚竹竹壁变厚的原因之一。
厚竹中不同内源激素的质量分数存在较大差异,且不同部位的差异程度不同,从潜伏芽到新竹抽枝展叶,内源激素的质量分数及比值均发生显著变化。潜伏芽和冬笋中GA3质量分数偏高,春笋中ZR质量分数偏高,说明不同的内源激素对厚竹生长发育的调控作用不同,较高的GA3质量分数有利于促进笋芽分化和冬笋萌动,较高的ZR质量分数则会促进细胞分裂,有利于促进竹笋的生长。在厚竹孕笋成竹过程中,ZR/GA3,ZR/IAA和GA3/IAA变化显著,且与厚竹的生长发育活动关系密切,表明ZR,GA3和IAA之间的协调或拮抗作用对厚竹的孕笋成竹有重要的调控功能。












 DownLoad:
DownLoad: