-
马尾松Pinus massoniana广泛分布于中国南部和东南部,种植面积超过200万 hm2,具有重要的经济价值和生态功能[1]。近年来,马尾松林植物多样性较低,受病虫害严重,大面积马尾松逐渐被砍伐或被其他树种自然替代,从而转变为阔叶林以提升森林的生态效益[2]。然而,阔叶林和马尾松林的凋落物数量和质量、根系性状、土壤微生物等均存在较大差异[1, 3],影响土壤碳循环过程,造成不同林分固碳能力差异显著。土壤团聚体和土壤有机碳之间关系密切,两者相互促进。有研究表明:土壤有机碳可以作为一种胶结剂参与大团聚体的形成过程,而土壤团聚体产生的空间隔离则会对有机碳产生物理保护作用,进而提高有机碳的稳定性[4−6]。土壤有机碳和土壤团聚体与菌根真菌关系密切,而林分转变引起的重要变化之一,是与根系共生的菌根真菌类型的改变[7],但是目前关于林分转变导致的优势菌根类型变化如何影响土壤团聚体特性及稳定性的影响还不清楚。
外生菌根(ectomycorrhiza,ECM)真菌和丛枝菌根(arbuscular mycorrhiza,AM)真菌是重要的陆地植物共生真菌,能够与陆地80%以上的植物形成菌根共生体[8],它们对植物生长、植物群落和生态系统过程的影响已经被广泛研究[8−9]。菌根能够通过根外菌丝缠绕土壤颗粒从而形成团聚体,进而影响土壤结构[6, 10−13]。THORNTON等[14]研究发现:ECM真菌对土壤团聚体具有促进作用。相比于ECM,AM根外菌丝数量较少[6],因此对土壤颗粒的缠绕聚集作用可能较弱。除了菌丝的缠绕作用,菌丝代谢物是土壤团聚体的重要黏合剂。研究表明:ECM真菌菌丝分泌的疏水化合物是重要的土壤团聚体的黏合剂[14],AM真菌则通过其细胞壁组分球囊霉素相关土壤蛋白(glomalin-related soil protein,GRSP)胶结团聚土壤颗粒[15],其在土壤中能稳定存在3~12 a,对土壤团聚体具有深远影响[4, 16]。QIN等[4]研究发现:阔叶林转变为毛竹Phyllostachys pubescens林过程中,AM促进了土壤大团聚体形成。但是,目前关于不同菌根类型转变对土壤团聚体组成影响的研究还较少,不同类型菌根对土壤团聚体组成及稳定性的影响研究还不够深入。
土壤团聚体主要通过对微生物的空间隔离,从物理层面保护土壤有机碳[5]。微生物则主要通过分泌胞外水解酶获取土壤中的碳、氮、磷等养分[17]。有研究表明:水解酶活性与有机质的分解与矿化过程具有显著相关性[18]。例如,β-葡萄糖苷酶和β-纤维二糖苷酶可以将土壤有机质中的纤维素和半纤维素分解为纤维素二糖、果糖等低分子量糖[19]。因此,通过酶活性可以体现微生物对团聚体中有机碳的利用与转化过程。菌根真菌对水解酶活性的影响已被广泛报道[20],在不同养分条件下,菌根真菌可能促进或抑制微生物分泌水解酶[2, 7],但是不同类型菌根对团聚体中水解酶活性的影响还不清楚。
马尾松林是典型的ECM优势林,而阔叶林为AM优势林。本研究以中国亚热带2种典型林分马尾松林和阔叶林为对象,通过湿筛分离土壤团聚体,测定2种林分不同粒级的土壤团聚体有机碳质量分数、麦角固醇质量分数、GRSP以及土壤水解酶活性,进而探讨不同菌根类型对土壤团聚体组成和特征的影响及其可能的作用机制,为评估不同菌根类型对亚热带森林土壤碳汇的影响提供参考依据。
-
研究区位于浙江省建德市杨村桥镇十里埠村、白云亭以及玉泉寺后山(29°54′~29°58′N,119°43′~119°48′E)。该区位于浙江省西部,温暖湿润,四季分明,雨热同期,属于亚热带北缘季风区,年均气温为17.4 ℃,年均降水量为1 600.0 mm,年均日照总时数为1 760.0 h。土壤类型为红壤,植被类型主要有常绿阔叶林、落叶阔叶林、针叶林以及针阔混交林。本研究选择亚热带森林演替过程中的初级和终级阶段,分别为马尾松林和阔叶林。
阔叶林主要树种有白栎Quercus fabri、枫香Liquidambar formosana、栲树Castanopsis fargesii,林下植被有连蕊茶Camellia cuspidata、芒萁Dicranopteris dichotoma等。马尾松林下植被主要为芒萁等蕨类植物。
-
于2021年8月在建德市杨村桥镇十里埠村、白云亭以及玉泉寺后山分别建立样地,样地之间分别间隔8~10 km,每个样地均包括相互接壤的马尾松林和阔叶林样地。根据建德市林业局记录的信息,所选阔叶林的地点原本均生长马尾松,在20~25 a前部分马尾松林砍伐后被阔叶林替代。在每个样地的马尾松林和阔叶林中分别选择3个样方(10 m×10 m),每个样方之间距离为20 m以上,利用五点取样法采集0~20 cm表层原状土壤(3个样地共计18个样方),保存于塑料盒内置于冰上带回实验室分析。
-
土壤团聚体的筛分按照ELLIOTT[21]的方法,通过土壤团聚体分析仪分离出不同粒级的水稳性团聚体,主要包括直径(d)>250 μm的大团聚体、d为53~250 μm的微团聚体以及d<53 μm的粉黏粒。选择2000、250、53 μm的筛子(由上往下),形成套筛,每个样品称取25 g,置于最上层筛子中,去除根和石头,将套筛放置在水桶中,水桶中装去离子水至一定高度。筛子浸入水中放置30 min,后开启土壤团聚体分析仪,上下振荡10 min,振幅3~4 cm。最后依次获得不同粒径的团聚体,将筛子上的团聚体在50 ℃烘干,记录每个样品的各粒径团聚体的质量。重复上述操作,将样品冻干,用于团聚体性质和酶活性测定。
平均质量直径(mean weight diameter, DMW)作为水稳性团聚体稳定性的指标[22],计算公式为DMW=${\displaystyle \sum _{i=1}^{n}X{W}_{i}}$。其中:X是团聚体每个部分的平均直径,Wi是i团聚体在整个样本中所占的比例,n是团聚体分级的数量(n=3)。
-
土壤各粒级团聚体有机碳采用重铬酸钾-浓硫酸外加热法测定,全氮采用凯式定氮法测定[23]。此外,测定5种土壤酶活性,分别为与微生物碳获取相关的β-葡萄糖苷酶(β-glucosidase,BG)、β-纤维二糖苷酶(β-D-cellobiosidase,CEL),与微生物氮获取相关的β-N-乙酰氨基葡萄糖苷酶(N-acetyl-β-glucosaminidase,NAG)、亮氨酸氨基肽酶(leucineaminopeptidase,LEU)以及与微生物磷获取相关的酸性磷酸酶(phosphatase,PHOS)。土壤酶活性测定参照CHEN等[18]荧光微孔板检测法,称取2 g鲜土于离心管中,加入30 mL pH 5.0的乙酸钠缓冲液,在25 ℃、180 r·min−1振荡30 min,用磁力搅拌器搅拌1 min,吸取200 μL土壤悬液于96孔板中,立即加入底物,于25 ℃黑暗培养3 h,在365 nm激发波长和450 nm发射波长下进行荧光定量,计算酶活性。5种酶活性的单位均为nmol·g−1·h−1。
使用酶活性向量角(vector angle, VA)和向量长度(vector length, VL)[24]评估不同土壤团聚体粒级中微生物碳、氮、磷代谢限制,计算公式为:VA=Degrees[ATAN2(x, y)],VL=$ \sqrt{{x}^{2}+{y}^{2}} $。其中:x为碳磷获取酶的比值,y为碳氮获取酶的比值,x用(BG+CEL)/(BG+CEL+PHOS)表示,y用(BG+CEL)/(BG+CEL+NAG+LEU)表示。土壤向量角表示微生物受到相对氮或磷限制,向量角越大,受到磷限制越大,向量角越小,受到氮限制更强;向量长度越大表示微生物受到碳限制越大。Degrees为将弧度转换成角度函数,ATAN2表示将根据给定的x轴及y轴坐标,返回正切值。
-
AM真菌生物量以球囊霉素相关土壤蛋白表示,分别测定易提取态球囊霉素相关土壤蛋白(easily extractable glomalin-related soil protein, EE-GRSP)和总球囊霉素相关土壤蛋白(total glomalin-related soil protein, T-GRSP)[22]。称取1 g土壤,用柠檬酸钠溶液提取EE-GRSP和T-GRSP,EE-GRSP在高温高压下提取1次,T-GRSP多次提取,直至浸提液无色透明。以牛血清蛋白为标线,采用考马斯亮蓝法显色,在分光光度计上测定。
根据AWAD等[25]使用的方法,首先测定鲜土中的麦角固醇质量分数,然后称取30 g鲜土于塑料瓶中,将其置于25 ℃、恒定湿度的生物培养箱中培养5个月,在此期间,外生菌根真菌死亡,5个月后再次测定土壤中的麦角固醇质量分数,ECM生物量即为培养前的麦角固醇质量分数减去培养后的麦角固醇质量分数。麦角固醇提取与测定参考曹梦等[26]的方法。称取鲜土4 g,加入4 mL色谱级甲醇,涡旋振荡后离心,吸取上清液于2 mL装有50 mg N-丙基-乙二胺键合硅胶和100 mg无水硫酸镁的离心管中,涡旋后离心,取上清液过0.22 μm有机系滤膜,采用液相色谱法上机测定,同时制作麦角固醇的标准曲线。使用的色谱柱为Agilent HC-C18 (250.0 mm × 4.6 mm,5 μm),以色谱级甲醇为流动相,流速为1 mL·min−1,进样量为20 μL,柱温为25 ℃,波长为280 nm。
-
采用双因素方差分析(two-way ANOVA)比较ECM优势林和AM优势森林中不同粒级土壤团聚体之间的有机碳质量分数、胞外酶活性、GRSP和ECM真菌生物量差异。采用独立样本t检验分析ECM真菌占优势森林和AM真菌占优势森林同一粒级团聚体的占比与团聚体稳定性,运用SPSS 21.0进行分析。采用Origin 2021作图。基于随机森林的回归分析通过R语言rfPermute包进行。
-
如图1A所示:阔叶林土壤中大团聚体(d>250 μm)占比显著高于马尾松林土壤(P<0.05),而微团聚体(d为53~250 μm)和粉黏粒(d<53 μm)呈现相反趋势,其中粉黏粒占比的下降达到显著水平(P<0.05)。此外,马尾松林土壤团聚体平均质量直径显著低于阔叶林土壤(P<0.05)(图1B)。

Figure 1. Proportion of soil aggregate size and mean weight diameter in forests dominated by different mycorrhizal types
如图2所示:阔叶林土壤仅粉黏粒的土壤有机碳和全氮显著低于马尾松林土壤(P<0.05)。此外,大团聚体的有机碳和全氮质量分数均高于其他2个粒级。阔叶林土壤大团聚体的碳氮比显著低于马尾松林土壤(P<0.05)。
-
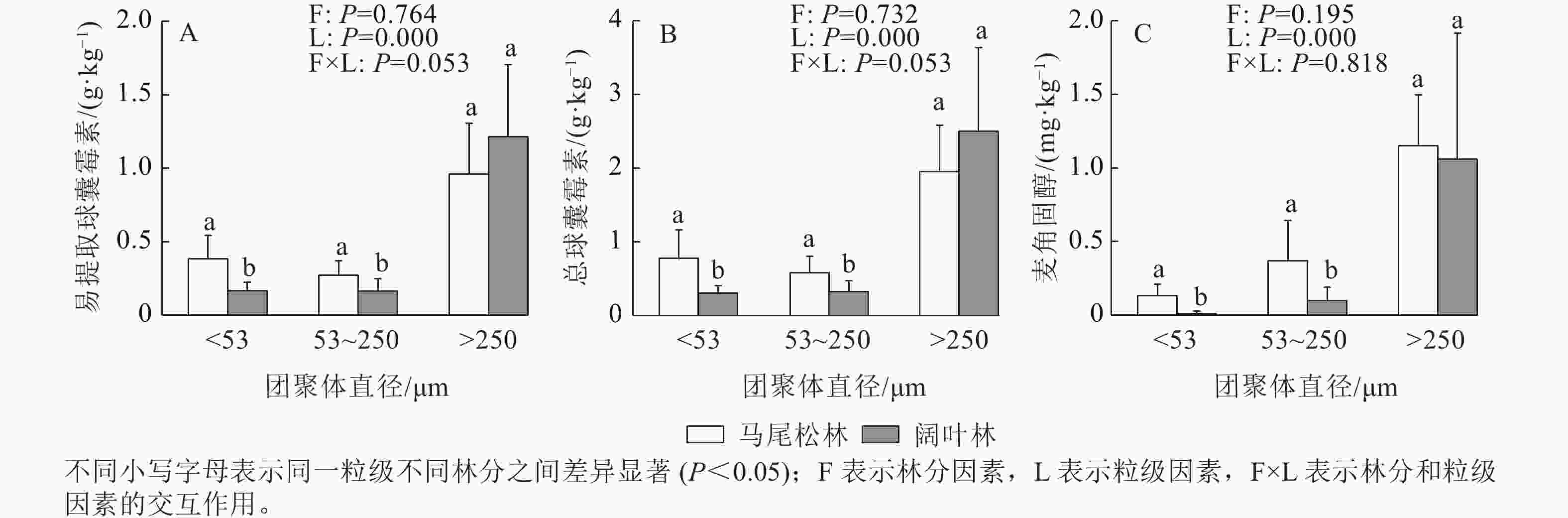
如图3所示:EE-GRSP、T-GRSP和麦角固醇均主要存在于土壤大团聚体中,大团聚中的EE-GRSP和T-GRSP呈现出阔叶林高于马尾松林的趋势,而麦角固醇呈现相反的趋势。阔叶林土壤的微团聚体和粉黏粒中的EE-GRSP、T-GRSP和麦角固醇质量分数均显著低于马尾松林土壤(P<0.05)。
-
由图4可见:阔叶林土壤3个粒级团聚体的β-葡萄糖苷酶(BG)和β-N-乙酰氨基葡萄糖苷酶(NAG)活性均低于马尾松林(P<0.05)。t检验结果表明:仅粉黏粒的β-纤维二糖苷酶(CEL)活性与微团聚体的PHOS活性在2个林分之间存在显著差异(P<0.05),但双因素分析表明:阔叶林团聚体中CEL活性低于马尾松林,PHOS活性高于马尾松林。
向量分析发现:3个粒级团聚体的向量角都大于45°,且阔叶林显著低于马尾松林(P<0.05);阔叶林土壤大团聚体的向量长度显著高于马尾松林大团聚体,微团聚体和粉黏粒的向量长度在2个林分之间差异不显著。
-
土壤大团聚体有机碳与总氮、EE-GRSP、LEU、T-GRSP、CEL和NAG紧密相关(P<0.05,图5A);微团聚体有机碳与总氮、EE-GRSP、T-GRSP、BG和ECM生物量显著相关(P<0.05,图5B);粉黏粒中的有机碳变异主要与EE-GRSP、总氮和ECM相关(P<0.05,图5C)。
-
相比于ECM优势马尾松林,AM优势阔叶林土壤大团聚体占比更高,粉黏粒占比更低,因此推测ECM有利于微团聚体和粉黏粒的产生,AM可能与土壤大团聚体的形成具有更加紧密的关系。叶思源等[27]研究表明:ECM真菌显著升高了土壤团聚体中粉黏粒比例,相应降低了大团聚体比例,该结论与本研究结果基本相同。本研究中,阔叶林土壤微团聚体和粉黏粒中ECM生物量低于马尾松林,可能在马尾松林中,ECM分泌胶结物质形成大团聚体的比例较少,导致其在微团聚体和粉黏粒中较高。随机森林分析表明:ECM生物量与微团聚体和粉黏粒中的有机碳变异显著相关,但对大团聚体有机碳没有显著贡献。可能是ECM促进大团聚体形成的能力较差,而AM在促进土壤大团聚体形成方面的作用已经被广泛报道,例如QIN等[4]研究发现:AM生物量与土壤大团聚体占比显著相关。AM真菌菌丝在土壤中广泛延伸,通过对土壤颗粒的缠绕作用,促进土壤大团聚体产生[9]。而ECM真菌在植物根系表面形成“哈氏网”,主要在根系表面聚集,通过缠绕作用形成大团聚体的能力相对较差[6]。此外,AM真菌的代谢产物GRSP是促进土壤大团聚体的重要机制之一。GRSP作为“胶水”将土壤粉黏粒和微团聚体黏结成大团聚体,导致其在大团聚体中较高,这与SITU等[22]的研究结果一致。本研究表明:土壤团聚体中大团聚体的EE-GRSP和T-GRSP质量分数明显高于微团聚体和粉黏粒,印证了GRSP对土壤大团聚体形成的重要作用。综上表明:与ECM相比,阔叶林中AM占优势,更有利于促进土壤大团聚体的形成。
本研究表明:马尾松林转变为阔叶林显著提升了土壤团聚体的平均质量直径。平均质量直径表征土壤团聚体大小分布状况,值越大表明团聚体的平均粒径团聚度越高,稳定性越强[28]。一般来说,大团聚体占比越多,土壤平均质量直径越大,土壤团聚体稳定性越高。因此,本研究中阔叶林土壤团聚体的稳定性比马尾松林更高,主要是由于阔叶林中的大团聚体比例显著高于马尾松林,增加了土壤团聚体的稳定性,其机制可能与GRSP有关。LIU等[29]研究表明:GRSP在大团聚体中显著增加,平均质量直径也随之增加,土壤团聚体稳定性增加。GRSP被认为是稳定存在的胶黏剂,可以在土壤中存在3~12 a[29],因此对土壤团聚体的稳定性具有深远影响。本研究随机森林分析表明:EE-GRSP和T-GRSP均与土壤团聚体有机碳显著相关。此外,有机质与土壤团聚体的平均质量直径存在正向相关[30−31],较高平均质量直径有利于土壤有机质质量分数的提升。因此推测AM通过提高土壤团聚体稳定性,促进有机碳固存,这与屠嘉莹等[2]的研究结果一致,阔叶林土壤的有机质质量分数显著高于马尾松林。总的来说,相比于ECM优势林,AM优势林可促进土壤大团聚体的形成,提高团聚体的稳定性,有利于土壤有机碳固存。
-
土壤团聚体通过对微生物的空间隔离,稳定土壤有机碳[5],而微生物通过分泌胞外酶获取土壤中的有机碳[17],因此水解酶活性在一定程度上体现了团聚体中有机碳的转化过程。本研究3个粒级的土壤团聚体碳氮循环相关酶活性在2种不同优势菌根类型森林中的趋势总体一致,均表现为阔叶林显著低于马尾松林。有研究表明:ECM优势马尾松林土壤整体受到强烈的氮限制[2, 6]。本研究也表明:马尾松林土壤大团聚体中的碳氮比显著高于阔叶林,一方面说明马尾松林中土壤氮有效性较低,为了植物更好生长,需要增强氮循环酶活性,从而获得更多的氮素。ECM真菌可以分泌特异性胞外酶[7, 32],进而分解有机质获取有效氮源,因此氮相关酶活性较高。然而微生物不能单一获取土壤中的氮素,而是进行对复杂有机质的共同代谢过程,因此提高了微生物碳获取相关酶活性[33]。相较于ECM,AM直接分泌胞外酶的能力十分有限[34],其更多通过与微生物的相互作用提高水解酶活性[4, 34],但是其正负影响效应受到土壤养分等因素影响。有研究报道了AM通过与微生物竞争氮,抑制胞外酶活性[7]。因此,相比于ECM,AM对水解酶活性可能存在负向影响。此外,WANG等[19]研究了不同林型对于土壤团聚体酶活性的影响,表明不同林型凋落物质量的差异是驱动土壤团聚体中酶活性变化的主要因素。ECM优势生态系统凋落物碳氮比较高,凋落物质量较低[2, 6],而AM优势生态系统中含有相对丰富的氮源,凋落物质量较高。由于本研究中马尾松林的凋落物质量较低,导致了氮循环酶活性较高,这与WANG等[19]的研究结果一致。因此,ECM优势林向AM优势林转变,通过降低水解酶活性更有利于土壤团聚体对有机碳的保护。向量长度分析表明:阔叶林土壤大团聚体中的碳限制较弱,因此微生物对获取碳源的投入更少[35],这可能是阔叶林土壤大团聚体中碳循环相关酶(BG)活性相较于马尾松林较低的原因之一。以往的研究证实微生物碳获取酶活性与有机碳损失显著相关[20],随机森林结果也显示酶活性与团聚体中有机碳变异显著相关。因此,马尾松林向阔叶林转变可能降低了团聚体碳循环相关酶活性,减少了微生物对有机碳的利用和转化。
-
马尾松林向阔叶林转变,其优势菌根类型由ECM转变为AM。同时,这个过程增加了大团聚体的占比,提高了土壤团聚体稳定性,大团聚体中有机碳呈上升趋势,微团聚体和粉黏粒中的有机碳显著降低。此外,相比于ECM优势马尾松林,AM优势阔叶林土壤团聚体中具有较低的碳氮循环相关酶活性。碳循环相关酶活性、GRSP以及ECM生物量是影响团聚体中有机碳变异的重要因子。综上,以ECM为优势的马尾松林转变为以AM为优势的阔叶林时,提高了土壤团聚体对有机碳的保护作用,降低了土壤团聚体中酶活性,有利于土壤有机碳固存。
Impact of shifts among mycorrhizal types on soil aggregate composition and characteristics
doi: 10.11833/j.issn.2095-0756.20230376
- Received Date: 2023-06-26
- Accepted Date: 2023-10-10
- Rev Recd Date: 2023-09-28
- Available Online: 2023-11-23
- Publish Date: 2023-11-23
-
Key words:
- arbuscular mycorrhiza /
- ectomycorrhiza /
- soil organic carbon /
- enzyme activities /
- glomalin-related soil protein
Abstract:
| Citation: | MA Xingcong, JIN Wenhao, TU Jiaying, et al. Impact of shifts among mycorrhizal types on soil aggregate composition and characteristics[J]. Journal of Zhejiang A&F University, 2023, 40(6): 1149-1157. DOI: 10.11833/j.issn.2095-0756.20230376 |













 DownLoad:
DownLoad:



