-
绿色植物通过光合作用固定二氧化碳(CO2)并转变成有机物质的过程被称为植物碳汇[1−2],是降低大气中CO2浓度最主要的途径。但受到植物呼吸消耗、微生物分解和环境条件变化的影响,绝大部分被固定的碳都无法长期稳定存在[1]。植硅体(phytolith)是植物在生长过程中,通过根系吸收的无定型硅酸[Si(OH)4]经维管束输送后在植物细胞内腔或细胞间隙中形成的硅包碳化合物,几乎存在于所有植物体中,在竹亚科Bambusoideae植物中植硅体尤其丰富[3−4]。植硅体碳是在植硅体形成过程中被封存于植硅体内的有机碳[5],随植物体死亡分解进入土壤后,可以稳定存在数千年甚至上万年之久[6−7],成为陆地土壤长期碳封存的重要机制之一。这种长期的生物地球化学碳封存形式被认为在减少大气CO2,缓解温室效应方面具有很大的潜力[8]。
毛竹Phyllostachys edulis已被证明是一种植硅体碳汇能力很强的植物[6−8],在中国广泛分布。在毛竹的经营管理过程中一直有施用氮肥的习惯,氮肥的施用直接促进毛竹的光合作用,进而提高单位面积毛竹笋材产量。研究表明:在毛竹的生长过程中除了氮素以外,对硅也有很强的富集能力[9−10],而毛竹体内硅、植硅体及植硅体碳的质量分数具有极显著的相关关系[11]。氮施用后虽然增强了毛竹光合能力[12],但对硅的吸收利用以及毛竹植硅体碳汇能力的影响还需进一步探索。
本研究以毛竹为研究对象,开展氮、硅二因素三水平栽培试验,采集毛竹不同器官,并测定不同器官硅、植硅体和植硅体碳质量分数,以明确不同器官对外源氮、硅添加的响应,揭示外源氮、硅添加对毛竹植硅体碳汇的影响机理,为提升中国竹林生态系统植硅体碳汇能力提供参考。
-
本研究设计为氮、硅二因素三水平盆栽试验。具体设计见表1。
处理 尿素/
(mg·kg−1)硅酸钠/
(mg·kg−1)处理 尿素/
(mg·kg−1)硅酸钠/
(mg·kg−1)处理 尿素/
(mg·kg−1)硅酸钠/
(mg·kg−1)N0Si0 0 0 N1Si0 250 0 N2Si0 500 0 N0Si1 0 75 N1Si1 250 75 N2Si1 500 75 N0Si2 0 150 N1Si2 250 150 N2Si2 500 150 说明:氮和硅用量按照生产上常规施肥量,即尿素750和225 kg·hm−2,有效土层深度30 cm,容重1.01 g·cm−3计算。 Table 1. Experimental design and N, Si application rate
试验盆栽栽培于福州市晋安区新店镇福建省林业科学研究院苗圃(26°09′05″N,119°17′03″E,海拔为103.8 m)。试验栽培容器长、宽、高分别为30、20、30 cm,距容器底部2 cm侧面设直径为0.5 cm的小孔,保证排水通气。栽培用土壤来源于福州市闽侯县三叠井森林公园自然分布毛竹林。挖取土壤前清除地表植被和凋落物,挖取0~30 cm土层土壤,挑除石子、动植物残体后于干燥通风处晾干,过2 mm筛后备用。土壤容重为1.01 g·cm−3,土壤有机质为20.3 g·kg−1,土壤pH为5.51,土壤碱解氮、有效磷、速效钾、有效硅质量分数分别为245.00、2.93、116.60、54.10 mg·kg−1。
试验用毛竹苗来源于四川峨眉廷富育苗有限公司培育的毛竹1年生袋装实生幼苗,种源为广西桂林。栽培时竹苗呈丛状分生,4~5株·丛−1,蔸部具完整根鞭,苗高30 cm左右,生长健壮,无病虫害。栽培之前将每片竹叶剪去60%,以降低蒸腾速率,增加成活率。
每盆置风干土10.0 kg,浅栽生长均匀的幼苗4株,浇透水,至容器侧面小孔有水溢出,再取10.0 kg风干土均匀铺盖于湿土之上,保湿保水防结块,置于通风防雨透光玻璃温室中培养。栽培期间17:00浇水1次,浇水量为当地前10 a平均日降雨量。
-
竹苗生长的过程中定时定量浇水,分别收集不同处理凋落物,及时烘干储存。毛竹苗生长2 a后,采用全株采集法采集每盆毛竹样品,清洗干净后分不同器官烘干储存备用。
-
不同处理和不同器官毛竹样品在分析测定前进行粉碎(<0.5 mm)。样品植硅体的提取采用微波消解法,之后用0.800 0 mol · L−1重铬酸钾溶液对植硅体进行检验,确保植硅体表面有机物质完全被去除,提取后的植硅体于65 ℃烘箱中烘干48 h,称量[13]。植硅体碳采用碱溶分光光度法测定[14],在样品测定的同时加入植物标准样(GBW07602)对测定的准确性进行检验。每个样品重复3次。样品总硅采用偏硼酸锂熔融-比色法测定[15],样品碳和氮采用碳氮元素分析仪测定。
-
使用SPSS 18.0进行数据统计分析,Duncan新复极差法测验不同处理的差异显著性,Origin 8.5作图。植硅体质量分数(g·kg−1)=植硅体质量(g)/样品干质量(kg),植硅体碳质量分数(g·kg−1) =植硅体碳质量(g)/样品干质量(kg)。
-
由表2可知:在毛竹不同器官及凋落物中,硅、植硅体和植硅体碳质量分数从高到低依次均为凋落物、叶、枝、篼、秆,变化范围分别为2.2~78.4、1.9~151.9和0.78~3.93 g·kg−1。与硅、植硅体和植硅体碳不同,碳质量分数在毛竹不同器官及凋落物中从高到低依次为秆、叶、篼、枝、凋落物,变化范围为372.0~466.0 g·kg−1。不同器官及凋落物氮质量分数则表现为毛竹叶中最高,为18.8 g·kg−1,凋落物最低,为4.4 g·kg−1。差异显著性分析结果表明:凋落物中硅和植硅体质量分数均显著高于其他器官(P<0.05),且植硅体质量分数在枝、秆和篼之间均具有显著差异(P<0.05),而硅质量分数在枝、秆和篼之间不具有显著差异。与植硅体相似,植硅体碳在枝、秆和篼之间具有显著差异(P<0.05),但植硅体碳质量分数在凋落物和叶之间不具有显著差异。碳质量分数除了在叶和篼之间不具有显著差异外,在其他器官及凋落物之间均具有显著差异(P<0.05)。氮质量分数在不同器官之间均具有显著差异(P<0.05),但在凋落物中没有表现出显著低于枝的现象。
样品 硅/(g·kg−1) 植硅体/(g·kg−1) 植硅体碳/(g·kg−1) 碳/(g·kg−1) 氮/(g·kg−1) 叶 26.8±2.8 b 42.3±3.8 b 3.78±0.17 a 452±4 b 18.8±0.9 a 枝 6.7±0.3 c 22.1±1.0 c 2.84±0.19 b 439±1 c 5.8±0.3 d 秆 2.2±0.1 c 1.9±0.1 e 0.78±0.10 d 466±1 a 7.7±0.6 c 篼 5.2±0.4 c 11.7±0.8 d 1.55±0.13 c 448±2 b 11.8±0.3 b 凋落物 78.4±2.8 a 151.9±3.4 a 3.93±0.15 a 372±4 d 4.4±0.2 d 说明:数据为平均值±标准误。不同小写字母表示不同器官间差异显著 (P<0.05)。 Table 2. Content of Si, phytolith, PhytOC, C and N in different organs and litterfall of Ph. edulis
-
由表3可知:毛竹叶、枝、秆、篼硅质量分数均表现为N0Si2处理最高,分别为42.2、8.4、2.9和6.6 g·kg−1,处理N2Si0最低,分别为18.4、5.6、1.8和3.5 g·kg−1。差异显著性分析结果表明: N0Si2和N0Si1处理叶硅质量分数显著高于除N0Si0处理以外的所有处理(P<0.05),而枝、秆和篼硅质量分数在不同处理之间均无显著差异。与叶、枝、秆、篼不同,凋落物硅质量分数表现为N2Si2处理最高,为91.8 g·kg−1,在N0Si0处理中最低,仅为60.9 g·kg−1。差异显著性分析结果表明: N2Si2处理中硅质量分数显著高于N0Si1和N0Si0处理(P<0.05),凋落物硅质量分数在其他处理间均不具有显著差异。
处理 各处理毛竹不同部位硅质量分数/(g·kg−1) 叶 枝 秆 篼 凋落物 N0Si0 33.1±4.7 ab 6.1±3.0 a 2.3±1.0 a 3.7±0.7 a 60.9±5.3 c N0Si1 36.5±2.8 a 6.8±0.4 a 2.3±0.4 a 5.7±1.6 a 74.1±2.2 bc N0Si2 42.2±2.1 a 8.4±0.3 a 2.9±0.2 a 6.6±0.3 a 78.9±7.0 ab N1Si0 20.0±2.5 c 5.9±2.8 a 2.0±0.5 a 3.5±0.8 a 77.0±2.1 ab N1Si1 24.3±2.4 bc 6.2±0.5 a 2.1±0.5 a 5.4±0.2 a 77.2±2.9 ab N1Si2 24.4±1.2 bc 8.0±1.1 a 2.5±0.6 a 6.3±0.1 a 79.2±1.7 ab N2Si0 18.4±3.8 c 5.6±1.5 a 1.8±0.2 a 3.5±0.7 a 80.3±0.3 ab N2Si1 20.3±1.5 c 5.9±0.2 a 1.9±0.4 a 5.6±2.2 a 86.1±1.7 ab N2Si2 21.9±1.9 c 7.5±0.9 a 2.4±0.2 a 6.3±0.8 a 91.8±9.3 a 说明:数据为平均值±标准误。不同小写字母表示不同处理间差异显著(P<0.05)。 Table 3. Contents of Si in different organs and litterfall of Ph. edulis under different treatments
由表4可知:随着硅添加量的增加,大部分处理植硅体质量分数呈增加的趋势。具体来看,不同处理毛竹叶植硅体质量分数为30.3~59.5 g·kg−1,其中N0Si2处理叶植硅体质量分数最高,N2Si0处理最低。N2Si2处理枝植硅体质量分数最高,为26.6 g·kg−1, N1Si0处理最低,仅为18.6 g·kg−1。不同处理秆和篼植硅体质量分数变化规律较为一致,均表现为N2Si2处理最高,分别为2.2和14.9 g·kg−1, N0Si0处理最低,分别为1.6和9.1 g·kg−1。N0Si2处理凋落物植硅体质量分数最高,为169.5 g·kg−1,比N1Si0处理高24.3%。差异显著性分析结果表明:仅叶中植硅体质量分数在N0Si0、N0Si1、N0Si2处理与其他处理间具有显著差异(P<0.05)。
处理 各处理毛竹不同部位植硅体质量分数/(g·kg−1) 叶 枝 秆 篼 凋落物 N0Si0 51.8±7.8 a 19.8±3.3 a 1.6±0.1 a 9.1±2.8 a 156.6±23.0 a N0Si1 59.2±2.3 a 20.6±4.0 a 1.9±0.4 a 9.5±0.2 a 160.8±10.4 a N0Si2 59.5±2.8 a 24.2±4.3 a 2.0±0.2 a 14.0±4.7 a 169.5±3.8 a N1Si0 35.4±1.2 b 18.6±2.4 a 1.8±0.1 a 10.1±2.8 a 136.4±19.5 a N1Si1 35.7±0.9 b 20.8±2.2 a 1.9±0.5 a 12.3±0.8 a 154.6±9.9 a N1Si2 37.8±0.9 b 26.2±3.3 a 2.1±0.5 a 14.7±0.2 a 155.3±6.4 a N2Si0 30.3±0.2 b 20.7±2.5 a 1.9±0.1 a 10.1±2.2 a 144.3±15.6 a N2Si1 34.4±3.0 b 21.1±1.9 a 2.0±0.3 a 11.0±1.7 a 144.7±0.7 a N2Si2 36.3±1.9 b 26.6±5.1 a 2.2±0.2 a 14.9±1.7 a 144.7±28.7 a 说明:数据为平均值±标准误。不同小写字母表示不同处理间差异显著(P<0.05)。 Table 4. Contents of phytolith in different organs and litterfall of Ph. edulis under different treatments
由表5可知:毛竹不同器官植硅体碳质量分数在不同处理之间差异较小。总体来看,随着硅添加量的增加,不同器官及凋落物植硅体碳质量分数均具有上升趋势。不同处理叶、枝、秆、篼及凋落物植硅体碳质量分数分别为3.15~4.68、2.10~3.47、0.30~1.18、1.09~2.15和3.21~4.63 g·kg−1,均表现为N2Si2处理最高, N0Si0处理最低,表明氮和硅的添加能够促进植硅体碳质量分数增加。差异显著性分析结果表明:仅秆植硅体碳质量分数在N0Si1和N0Si0与N2Si2处理间具有显著差异(P<0.05)。
处理 各处理毛竹不同部位植硅体碳质量分数/(g·kg−1) 叶 枝 秆 篼 凋落物 N0Si0 3.15±0.24 a 2.10±0.56 a 0.30±0.04 c 1.09±0.22 a 3.21±0.81 a N0Si1 3.20±1.05 a 2.25±0.66 a 0.39±0.10 bc 1.11±0.07 a 3.83±0.92 a N0Si2 3.82±0.55 a 3.12±0.63 a 0.61±0.40 abc 1.54±0.50 a 4.14±2.25 a N1Si0 3.59±0.67 a 2.28±0.59 a 0.70±0.33 abc 1.13±0.47 a 3.52±0.67 a N1Si1 4.01±0.12 a 3.10±0.54 a 0.91±0.13 abc 1.77±0.51 a 3.73±1.05 a N1Si2 4.31±0.98 a 3.38±0.46 a 1.06±0.14 ab 1.79±0.32 a 4.46±0.20 a N2Si0 3.63±1.17 a 2.45±1.28 a 0.86±0.19 abc 1.58±0.18 a 3.81±0.51 a N2Si1 4.05±0.20 a 3.39±0.28 a 1.00±0.27 abc 1.84±0.62 a 4.01±0.17 a N2Si2 4.68±0.41 a 3.47±0.98 a 1.18±0.05 a 2.15±0.33 a 4.63±1.60 a 说明:数据为平均值±标准误。不同小写字母表示不同处理间差异显著(P<0.05)。 Table 5. Contents of PhytOC in different organs and litterfall of Ph. edulis under different treatments
由表6可知:与植硅体碳质量分数相似,毛竹不同器官碳质量分数在不同处理间差异均较小并具有随着氮添加量增加不断增加,随着硅添加量的增加不断降低的趋势。不同处理叶、枝、秆、篼碳质量分数分别为436~478、436~441、462~471和441~456 g·kg−1,可知叶碳质量分数在不同处理之间差异最大。不同处理凋落物碳质量分数随氮和硅添加量的增加均不断增加,为348~387 g·kg−1。差异显著性分析结果表明: N2Si1处理叶碳质量分数显著高于其他处理,N0Si2处理枝碳质量分数显著低于N1Si0处理, N1Si0处理和N2Si0处理秆碳质量分数显著高于除N2Si1处理以外的其他处理, N2Si2处理凋落物碳质量分数显著高于N0Si0处理(P<0.05)。
处理 各处理毛竹不同部位碳质量分数/(g·kg−1) 叶 枝 秆 篼 凋落物 N0Si0 440±2 b 440±3 ab 463±2 b 443±5 b 348±17 b N0Si1 439±2 b 437±3 ab 463±2 b 442±3 b 372±6 ab N0Si2 436±3 b 435±2 b 462±2 b 441±4 b 376±5 ab N1Si0 453±2 b 442±2 a 471±0 a 452±3 ab 365±11 ab N1Si1 453±2 b 439±1 ab 466±2 b 450±5 ab 368±14 ab N1Si2 453±3 b 438±3 ab 465±1 b 449±4 ab 369±3 ab N2Si0 454±4 b 441±1 ab 471±2 a 456±1 a 381±16 ab N2Si1 478±21 a 441±1 ab 467±0 ab 451±4 ab 384±2 ab N2Si2 459±4 ab 439±2 ab 465±1 b 449±2 ab 387±11 a 说明:数据为平均值±标准误。不同小写字母表示不同处理间差异显著(P<0.05)。 Table 6. Contents of C in different organs and litterfall of Ph. edulis under different treatments
由表7可知:毛竹不同器官氮质量分数在不同处理之间差异均较大,叶、枝、秆、篼和凋落物氮质量分数最低的处理分别为N0Si1、N0Si2、N0Si1、N0Si0和N0Si1处理,其氮质量分数分别为14.5、4.3、5.0、10.0和3.4 g·kg−1;最高的处理分别为N2Si1、N2Si0、N1Si2、N2Si0和N2Si0处理,比最低值分别增加54.5%、79.1%、90.0%、29.0%和50.0%。差异显著性分析结果表明:叶、枝、秆、篼和凋落物氮质量分数在不同处理间变化规律均不明显,氮质量分数在部分处理间存在显著差异(P<0.05)。
处理 各处理毛竹不同部位氮质量分数/(g·kg−1) 叶 枝 秆 篼 凋落物 N0Si0 16.9±0.8 cde 5.4±0.9 bcd 6.5±1.0 abc 10.0±0.2 b 3.4±0.1 c N0Si1 14.5±0.9 e 4.6±0.1 cd 5.0±0.3 c 10.7±0.3 b 3.6±0.1 bc N0Si2 15.7±0.9 de 4.3±0.1 d 5.6±0.2 bc 10.8±0.1 b 3.4±0.1 c N1Si0 19.1±1.0 bc 6.3±0.6 abc 8.7±1.6 ab 12.3±0.2 ab 4.4±0.2 ab N1Si1 20.8±0.8 ab 6.1±0.2 abcd 8.6±0.6 abc 12.4±0.8 ab 4.8±0.3 a N1Si2 18.4±1.0 bcd 5.4±0.3 bcd 9.5±0.1 a 12.1±0.3 ab 4.8±0.3 a N2Si0 20.9±1.1 ab 7.7±0.7 a 9.3±2.2 a 12.9±1.1 a 5.1±0.4 a N2Si1 22.4±0.4 a 6.7±1.0 ab 7.7±0.8 abc 12.3±0.4 ab 5.1±0.6 a N2Si2 20.2±0.6 ab 5.8±0.5 abcd 8.4±1.3 abc 12.6±0.2 a 4.7±0.1 a 说明:数据为平均值±标准误。不同小写字母表示不同处理间差异显著(P<0.05)。 Table 7. Contents of N in different organs and litterfall of Ph. edulis under different treatments
-
相关性分析结果(表8)表明:毛竹硅与植硅体、植硅体与植硅体碳以及硅与植硅体碳质量分数之间均存在极显著正相关关系(P<0.01),硅与氮质量分数之间存在极显著二次相关关系(P<0.01),植硅体碳与碳质量分数之间存在极显著负相关关系(P<0.01)。
x y 拟合方程 决定系数(R2) 显著性水平 植硅体 硅 y = 0.088 + 0.517x 0.958 4 P<0.001 植硅体 植硅体碳 y = 1.839 + 0.016x 0.463 2 P<0.001 硅 植硅体碳 y = 1.837 + 0.031x 0.481 8 P<0.001 硅 氮 y = 7.004 + 0.507x−0.007x2 0.493 3 P<0.001 碳 植硅体碳 y = 472.8−14.5x 0.318 3 P<0.001 Table 8. Correlation between Si and phytolith, phytolith and PhytOC, Si and PhytOC, Si and N, C and PhytOC contents of Ph. edulis
-
交互作用分析结果表明:对于毛竹植硅体、植硅体碳与碳质量分数来说,氮与硅之间均不存在交互作用的显著性(表9)。
指标 自由度 均方 F 显著性水平 $ {\eta }_{p}^{2} $ 植硅体 4 15.858 0.005 1.000 0.000 植硅体碳 4 0.069 0.030 0.998 0.001 碳 4 0.555 0.044 0.996 0.001 说明:$ {\eta }_{p}^{2} $表示植硅体和植硅体碳分别对组间变异的贡献率。 Table 9. Interaction between N and Si for phytolith, PhytOC, and C contents
-
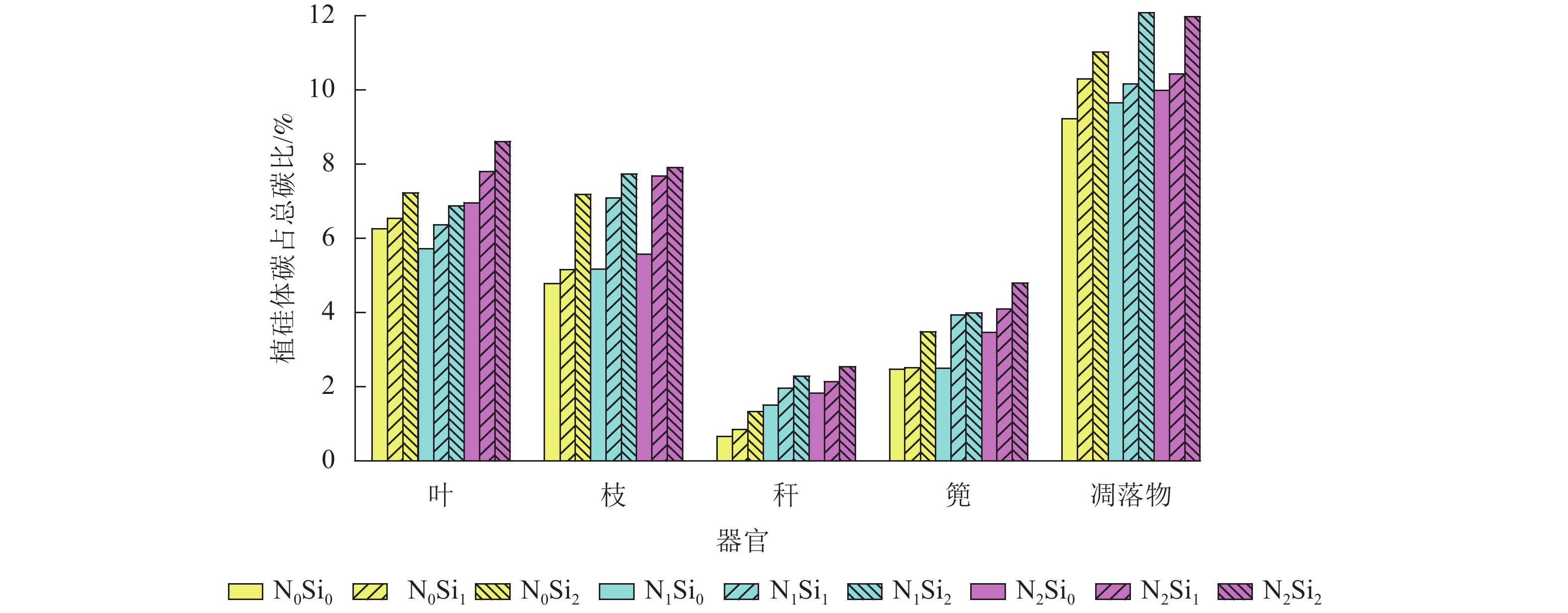
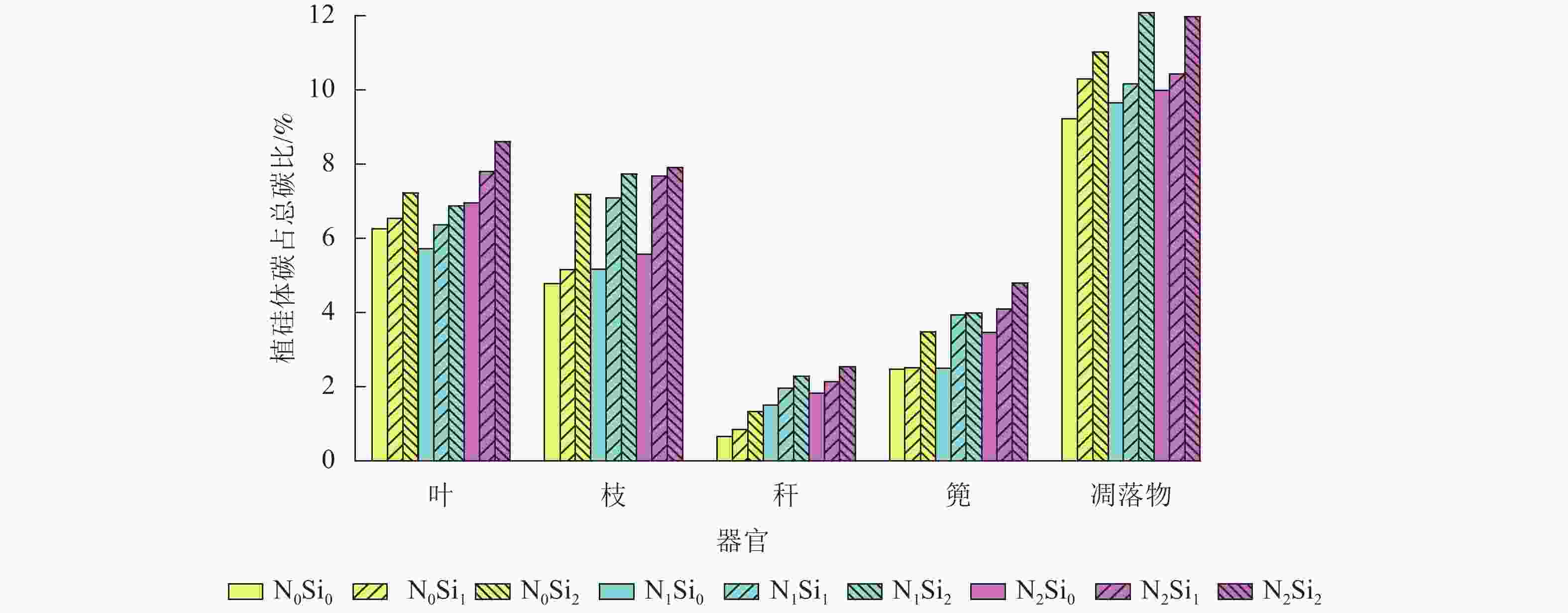
由图1可知:毛竹叶、枝、秆、篼和凋落物中植硅体碳占总碳的比例分别为5.7%~8.6%、4.8%~7.9%、0.7%~2.5%、2.5%~4.8%和9.2%~12.1%。与不同器官相比,凋落物中植硅体碳占总碳的比例平均最高,为10.5%。不同处理凋落物之间,N1Si2处理植硅体碳占总碳的比例最高,N0Si0处理最低。不同器官之间,不同处理植硅体碳占总碳的比例从高到低依次为叶、枝、篼、秆,平均分别为6.9%、6.5%、3.5%和1.7%。同一器官不同处理之间,在同一氮添加水平下随着硅添加量的增加,植硅体碳占总碳的比例不断升高。
-
硅是地壳中第二大元素,其蕴藏量仅次于氧,在地球化学碳循环中起着重要的作用[16]。研究表明:植物对硅的吸收利用分为对单硅酸的跨膜吸收和沿维管束的运输2个过程,其中植物对硅的吸收能力取决于土壤溶液中单硅酸的浓度和植物根系硅转运蛋白的表达量[17]。动力学研究表明:外部单硅酸浓度的升高能够有效促进其以被动扩散或主动运输的形式被跨膜吸收[18−19]。土壤中的有效硅到达植物根部后才能开始随蒸腾流沿着维管束运输的第2个过程,并且具有边运输边形成聚合硅酸的能力[20],最终在植物组织的蒸腾末端沉积,形成稳定的无定型二氧化硅颗粒,表明植物对硅的吸收利用最终依赖于特异性硅转运蛋白和蒸腾作用。
植硅体在植物抗寒、抗逆等方面[4, 21]具有重要的作用。硅被植物吸收后主要以植硅体的形式存在于植物体内,因此植物体内植硅体质量分数取决于植物吸收利用硅的能力。本研究中毛竹不同器官之间硅与植硅体质量分数差异较大,但表现出相同的变化规律和极显著相关关系,表明毛竹不同器官对硅利用的能力不同,也表明了硅与植硅体之间存在的密切联系。这与ZUO等[22]对黍Panicum miliaceum、粟Setaria italica的研究,LI等[23]对湿地芦苇Phragmites australis的研究,PARR等[24]对竹林的研究及SONG等[25]对中国草原植被的研究结果相同,证明了植物体中植硅体质量分数明显受植物对硅富集能力的影响。
植硅体碳形成于植硅体积淀的过程中。LI等[26]在对白洋淀芦苇的研究中指出:植硅体碳质量分数与植物吸收利用CO2速率有直接的关系。SONG等[13]在中国不同森林类型植硅体碳封存估测的研究中提出:植硅体碳质量分数与硅质量分数之间存在密切的联系,并且以硅质量分数的3%作为计算植硅体碳的标准。本研究设计硅外源添加以增加毛竹对硅的吸收利用,同时设计氮外源添加提高毛竹光合作用效率及吸收利用CO2的能力,并对不同处理硅、植硅体和植硅体碳质量分数进行分析,发现硅、植硅体和植硅体碳在毛竹不同器官之间的变化规律呈现高度的一致性,并且随着外源氮和硅添加量的变化,毛竹不同器官植硅体与植硅体碳仍然表现出相似的变化规律;除此之外,植硅体与植硅体碳之间及硅与植硅体碳质量分数之间均存在极显著的正相关关系。这一结果与已有竹类植物植硅体碳相关研究结果一致[8, 13, 27],反映了竹林生态系统中硅与植硅体碳之间的内在联系,表明外源硅的添加是毛竹不同器官植硅体碳质量分数增加的主要原因,证明了硅的添加能够促进毛竹植硅体碳汇能力提升。
-
氮是植物生长最重要的营养元素之一,对于植物光合效率的提高及养分的吸收、利用、积累具有直接的影响[12]。植物对氮的吸收过程复杂且多样,主要包括主动性和被动性吸收2个途径,与光照、温度、pH等环境因素密切相关,与硅的吸收利用相关性较小。本研究中氮-硅交互作用分析结果表明:氮与硅之间均不存在交互作用的显著性,也进一步说明毛竹对氮和硅的吸收是2个相互独立的过程。毛竹叶中碳、氮和植硅体碳质量分数均随着外源氮添加量的增加不断提高,但硅与植硅体质量分数却不断下降 ,主要是由于外源氮的添加极大地促进了叶的生长,导致净生物量在短期内快速积累,而植物对硅的吸收动力主要来源于蒸腾作用[12],在短期内并不会出现大幅变化,因此造成了净生物量积累与植硅体积累不协调的现象,也说明了虽然本研究中硅的添加促进了毛竹对硅的吸收,但氮的添加增加了有机物质的积累,对植硅体形成了稀释效应,因此表现为低氮添加处理植硅体质量分数更高的现象。
随着叶片的老化,大部分氮被转移再利用[28],凋落物氮和碳质量分数均大幅降低,但硅、植硅体和植硅体碳质量分数却并未随之下降,且随着外源氮添加量的增加,凋落物中硅和植硅体碳质量分数有所增加,植硅体质量分数却有所降低,表明尽管外源氮的添加促进了硅的吸收和有机物质的积累,但被吸收的硅并没有形成稳定的植硅体,对植硅体碳的贡献有限,也说明尽管毛竹对氮和硅的吸收利用交互作用不显著,但硅的吸收仍然与氮的添加有关。
进一步对不同处理不同器官及凋落物中植硅体碳占总碳比进行分析,可以更清晰地表明:单纯的氮添加能够促进有机物质的快速积累,但对于提高植硅体碳在总碳中的比例作用有限,而硅的添加对提高植硅体碳在总碳中的比例作用更为明显,这一点也被外源氮添加后碳和植硅体碳呈极显著负相关所证明。
-
植硅体的稳定性主要决定于其形态、颗粒大小、组分和结构[29−32]。有研究表明:外源硅的添加增加了土壤溶液中单硅酸的浓度,外源氮和硅的添加共同促进了毛竹对硅的吸收,进而增加植硅体在植物体内的积累[33−35],但是这种靠人为因素增加的植硅体已被证明主要是轻组植硅体[31],其稳定性、抗腐蚀能力等均远小于重组植硅体[34, 36],且氮的添加促进了毛竹对氮的吸收利用,改变了植硅体组成成分[31],因此尽管高氮处理促进了毛竹凋落物硅质量分数的增加,但植硅体质量分数仍较低。表明虽然氮的添加对毛竹吸收硅的影响不显著,但对毛竹吸收硅后形成的植硅体的稳定性有显著影响。
-
毛竹叶中氮质量分数最高,秆中碳质量分数最高,凋落物中硅、植硅体和植硅体碳质量分数均最高。外源氮添加有助于毛竹对硅的吸收和有机物质的积累,外源硅添加有助于毛竹植硅体和植硅体碳质量分数的增加以及植硅体碳占碳比例的提高。
Effect of N and Si addition on phytolith-occluded organic carbon in Phyllostachys edulis
doi: 10.11833/j.issn.2095-0756.20230460
- Received Date: 2023-09-07
- Accepted Date: 2023-12-22
- Rev Recd Date: 2023-11-29
- Available Online: 2024-03-21
- Publish Date: 2024-04-01
-
Key words:
- Phyllostachys edulis /
- silicon /
- nitrogen /
- PhytOC
Abstract:
| Citation: | YANG Jie. Effect of N and Si addition on phytolith-occluded organic carbon in Phyllostachys edulis[J]. Journal of Zhejiang A&F University, 2024, 41(2): 369-378. DOI: 10.11833/j.issn.2095-0756.20230460 |









 DownLoad:
DownLoad: