-
山矾Symplocos sumuntia又名山桂花,是山矾科Symplocaceae山矾属Symplocos常绿灌木或小乔木,分布于长江以南各省区[1]。山矾科仅山矾属1属,该属多种植物有清热解毒、理气止痛、止血生肌、化痰止咳等重要的药用价值[2]。由于山矾枝繁叶茂,四季常绿,开花繁密并具有强烈的芳香,又可作为一种极具潜力的观赏植物进行开发利用[3]。目前,关于山矾花香方面的研究报道较少。罗心毅等[4]通过水蒸汽蒸馏法获得山矾精油,共鉴定出68种化学成分;余爱农等[5]利用60H型硅胶吸收山矾花香,再通过溶剂洗脱后,进行气相-质谱联用技术(GC-MS)分析并鉴定出22个挥发性化学成分。由于水蒸汽加热过程中会使一些香味物质变性损失,而用硅胶吸附香气所需时间过长,容易引起花瓣褐化,并且温度升高和缺乏气体交换会影响其正常生理进程,进而影响挥发物的释放[6],所以以上2种方法可能会造成部分花香成分的损失或变化。植物花香成分及释放量在很大程度上受花朵发育程度的影响[7-8],现已发现玫瑰Rosa rugosa,百合Lilium brownii,金鱼草Antirrhinum majus,枇杷Eriobotrya japonica,文心兰Oncidium‘Sharry Baby’,桂花Osmanthus fragrans等多种植物的花香随着开花进程表现出不同的变化规律,基本呈现为半开期和盛开期花香物质种类和释放量明显增加[9-14]。固相微萃取技术作为一种简便且有效的花香采集方法,集采样、浓缩、萃取为一体,通过萃取头表面涂层吸附挥发性物质,可以在花香采样后立即测定,避免了鲜花离体时间过长导致花香成分变化的问题,现已应用于多种植物花香的采样[15]。本研究采用顶空固相微萃取(HS-SPME)结合GC-MS分析山矾不同开花时期花香成分和相对含量的变化,探索山矾鲜花自然环境中真实的香气组成及释放规律,旨在为山矾野生植物资源及其花香的开发利用提供参考。
HTML
-
试验材料为栽于浙江农林大学植物园内5年生以上的山矾原种,生长状态良好,能正常开花结实。采样时间选择2015年3月开花期晴天上午,采集花枝后立即带回实验室进行香气测定,按不同开花状态分为3个时期(图 1):蕾期(花瓣紧闭,已基本显色)、半开期(花药、花丝露出,花瓣微张)和盛开期(花瓣完全开展)。
SPME进样手柄和100 μm PDMS萃取纤维头由美国Supelco公司制造,GC-MS QP 2010 Plus气相色谱-质谱联用仪为日本岛津公司产品。
-
试验方法及条件参照参考文献[14],并作适当修改,具体如下:取样前先将与手柄组装后的萃取纤维头在气相色谱的进样口处老化,老化时间1 h,老化温度230 ℃;然后随机选取20朵处于相同发育阶段的花置于100 mL的锥形瓶中,并用封口膜密封,平衡10 min再插入老化后的萃取头,室温下萃取40 min;最后将取样后的萃取头插回进样口进行GC-MS分析,重复3次·时期-1。
GC条件:色谱柱为Restek Rtx-Wax气相柱(30 m×0.25 mm);载气为高纯氦气(99.999%),柱前压为49.5 Pa,柱流量1 mL·min-1;取样时间1 min。进样口温度为230 ℃,程序升温为柱起始温度40 ℃,保持5 min,以5 ℃·min-1的速度升至250 ℃,保持5 min。
MS条件:电子轰击源为EI;电子能量70 eV;离子源温度230 ℃,接口温度250 ℃,溶剂切除时间2 min,扫描质量范围为33~650,扫描间隔0.3 s,收集时间3~52 min。
根据离子流峰面积归一化法计算各组分在总挥发物中的相对含量。对采集到的质谱图用NTST08及NTST08s进行分析,并按各峰的质谱裂片图与有关文献[16]进行核对,确定挥发性物质的化学成分。
1.1. 样品与仪器
1.2. 试验方法
-
试验得到山矾不同开花时期GC-MS总离子流色谱图(图 2)。通过对各组分图谱分析及资料核实,分别从蕾期、半开期、盛开期检测出35,39,42种挥发物,主要包括醇类、酮类、酯类、醛类、芳香族类和酸类六大类(表 1)。
序号 t/min 化合物 相对含量/% 蕾期 半开期 盛开期 1 10.605 桉油醇 eucalyptol 3.86±1.46 c 11.9±1.54 b 18.17±1.90 a 2 12.844 乙酸己酯 hexyl acetate 0.80±0.00 - 0.59±0.00 3 14.069 乙酸-4-己烯-1-醇酯 4-Dexen-1-ol, acetate 4.89±0.99 0.49±0.18 1.38±0.85 4 14.600 6-甲基-5-庚烯-2-酮 6-methyl-5-hepten-2-one 0.28±0.00 - - 5 15.121 正己醇 1-hexanol 0.24±0.07 - - 6 15.479 十二甲基环六桂氧烷 cyclohexasiloxane,dodecametDyl- 0.24±0.00 - - 7 15.936 叶醇 leaf alcohol 0.73±0.02 - 0.16±0.00 8 16.406 壬醛 nonanal 0.38±0.07 - - 9 17.66 顺式-氧化芳樟醇 cis-linalool oxide 1.52±0.15 a 0.61±0.05 b 0.80±0.12 b 10 18.195 乙二醇丁醚醋酸酯 2-butoxyethyl acetate 0.20±0.00 - - 11 18.431 反式-氧化芳樟醇 trans-linalool oxide 12.68±0.72 a 5.74±0.36 b 6.55±0.83 b 12 19.415 癸醛 decanal 0.68±0.14 - - 13 19.424 苯甲醛 benzaldehyde - 0.38±0.12 0.17±0.01 14 20.244 丁香醛 J Lilac aldehyde A - 0.48±0.00 1.69±0.16 15 20.595 芳樟醇 linalool 20.19±1.18 a 2.10±0.29 c 5.49±0.99 b 16 20.810 丁香醛 L lilac aldehyde L - 0.54±0.00 2.05±0.17 17 21.397 丁香醛 C lilac aldehyde C - 0.45±0.00 1.54±0.12 18 21.922 (-)-4-萜品醇(-)-terpinen-4-ol - 0.16±0.04 0.18±0.04 19 22.128 脱氢芳樟醇 hotrienol 1.32±0.12 a 0.52±0.29 b 0.46±0.08 c 20 22.497 苯乙醛 benzeneacetaldehyde - 0.12±0.00 0.40±0.05 21 22.962 二甲基桂院双醇 silanediol,dimethyl- 1.10±0.32 0.51±0.13 0.79±0.06 22 24.225 α-松油醇 α-terpineol 0.39±0.05 0.24±0.04 0.36±0.05 23 24.755 紫丁香醇 LlilacalcoholL - 0.17±0.00 2.13±0.16 24 25.77 双花醇 2H-pyran-3-ol, 6-ethenyltetrahydro-2,2,6-trimethyl- 19.45±1.31a 10.05±0.77 b 13.09±0.85 b 25 26.295 紫丁香醇 ClilacalcoholC - 0.24±0.04 0.66±0.06 26 27.155 双丙甘醇 2-propanol,1,1’-oxybis- 0.58±0.11 0.46±0.02 - 27 27.221 紫丁香醇 AlilacalcoholA - - 0.75±0.06 28 27.46 二氢-β-紫罗兰酮2H-β-ionone 0.33±0.06 b 0.72±0.11a 0.32±0.03 b 29 27.81 反式-β-紫罗兰酮(E)-β-ionone 0.12±0.00 2.40±0.31 1.52±0.21 30 28.037 香叶基丙酮(E)-Geranylacetone 0.62±0.11 - - 31 28.053 苄醇 benzyl alcohol - 0.22±0.00 0.23±0.02 32 28.257 2-(2-轻基丙氧基)-1-丙醇1-propanol,2-(2-hydroxypropoxy)- 0.49±0.06 a 0.29±0.03 b 0.16±0.01b 33 28.841 苯乙醇 phenylethyl alcohol 0.32±0.03 b 0.40±0.15 b 1.98±0.18 a 34 29.066 苯甲腊 benzyl nitrile 1.26±0.21a 0.41±0.06 b 0.42±0.02 b 35 29.734 β-紫罗兰酮 β-ionone 0.60±0.06 c 33.38±1.76 a 19.14±3.12 b 36 30.046 二氢-β-紫罗兰酮 2H-β-ionone 0.17±0.02 0.31±0.00 0.18±0.04 37 30.546 1-十三醇 n-tridecan-1-ol 0.27±0.01 0.23±0.05 0.17±0.01 38 30.786 4-(2,2,6-三甲基-7-氧杂二环[4.1.0]-1-庚基)-3-丁烯-2-酮 3-buten-2-one, (2,2,6-trimethyl-7-oxabicyclo [ 4.1.0 ] hept-1 -yl) - 0.28±0.07 - 39 31.301 檀花醚 2H-pyran,3,6-dihydro-4-methyl-2-(2-methyl-1-propenyl)- - - 0.15±0.02 40 31.879 3,4,5-三甲氧基甲苯 3,4,5-trimethoxy toluene 7.79±0.38 b 15.29±0.37 a 3.99±0.64 c 41 32.154 邻甲氧基苯甲酸甲酯 benzoic acid, 2-methoxy-, methyl ester 0.51±0.03 a 0.40±0.09ab 0.22±0.04 b 42 33.712 3-甲基-庚-1,6-二亚乙基三胺-3-醇 3-methyl-hepta-1,6-dien-3-ol - 0.21±0.06 1.1±0.12 43 34.322 1,2,4-三甲氧基苯 1,2,4-Trimethoxybenzene 0.31±0.05 0.43±0.00 2.42±0.21 44 36.066 棕榈酸甲酯 hexadecanoic acid, methyl ester 0.28±0.00 0.32±0.00 0.37±0.17 45 37.52 1,2,3-三甲氧基苯 1,2,3-trimethoxybenzene - - 0.36±0.05 46 37.996 邻苯二甲酸二乙酯 diethyl phthalate 0.16±0.00 0.17±0.00 0.10±0.00 47 39.052 吲哚 indole - 0.17±0.00 0.14±0.02 48 40.149 反油酸甲酯 methyl elaidate 4.37±0.70 3.38±0.87 3.92±0.71 49 40.886 亚油酸乙酯 linoleic acid ethyl ester 0.58±0.14 0.26±0.06 0.33±0.05 50 43.134 反式-4-(反-4-戊基)环己基)-1-环己甲酸4-氟苯酯[1,1’-bicy-clohexyl]-4-carboxylic acid, 4’-pentyl-, 4-fluorophenyl ester - 0.27 % 0.02 1.71±0.00 51 47.357 油酸 oleic acid 0.12±0.00 0.69±0.00 0.41±0.20 说明:a,b,c代表多重比较SNK检验在P≤0.05显著性水平下的差异显著;“-”该成分未检测出。 Table 1. Main volatiles compositions and relative contents at different flowering stages of Symplocos sumuntia
-
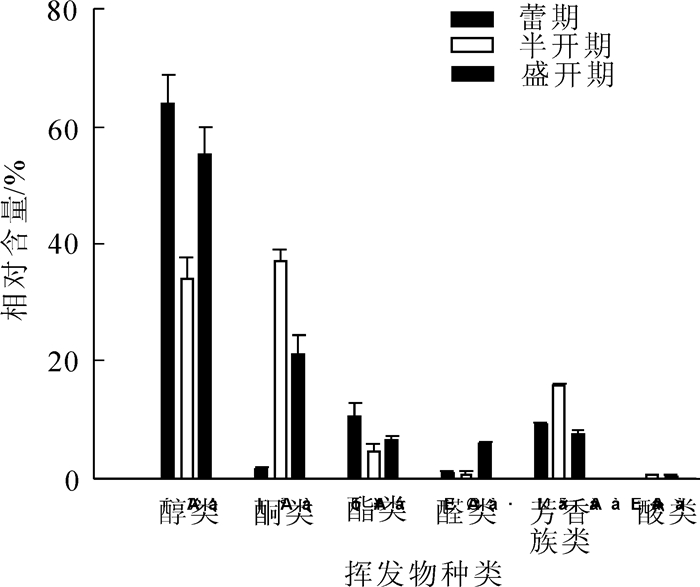
从图 3可以看出:醇类和酮类是各时期相对含量较高的化合物。对各类物质差异性分析可知,醇类在开花过程中呈现高-低-高的变化趋势;酮类和芳香族类在半开期相对含量达到最高,然后逐渐降低;醛类的相对含量在盛花期达到最高;酯类和酸类在各个开花阶段的挥发量并无显著差异。

Figure 3. Comparison of main volatile categories from the flowers of Symplocos sumuntia at different flowering stages
蕾期山矾花的挥发性成分中,醇类的相对含量最高,占63.95%,主要成分包括芳樟醇(20.19%)、双花醇(19.45%),反式-氧化芳樟醇(12.68%),桉油醇(3.86%)等;酯类化合物相对含量占10.68%,主要有乙酸-4-己烯-1-醇酯(4.89%),反油酸甲酯(4.37%)等;芳香类化合物相对含量占9.36%,最高的是3,4,5-三甲氧基甲苯(7.79%)(表 1)。
半开期山矾花的挥发性成分中,以酮类和醇类化合物为主,相对含量分别占36.88%和34.06%,主要包括β-紫罗兰酮(33.38%),反式-β-紫罗兰酮(2.40%),双花醇(10.05%),桉油醇(11.90%),反式-氧化芳樟醇(5.74%),芳樟醇(2.10%)等;芳香族化合物的相对含量占15.90%,其中相对含量最高的是3,4,5-三甲氧基甲苯(15.29%);酯类化合物(4.70%)中相对含量最高的是反油酸甲酯(3.38%)。
盛开期山矾花的挥发性成分中,以醇类化合物为主,相对含量占55.29%,主要成分包括桉油醇(18.17%),双花醇(13.09%),反式-氧化芳樟醇(6.55%),芳樟醇(5.49%),紫丁香醇的不同异构体(3.54%)等;酮类化合物相对含量占21.16%,主要成分是β-紫罗兰酮(19.14%),反式-β-紫罗兰酮(1.52%);芳香族化合物相对含量占7.43%,主要包括3,4,5-三甲氧基甲苯(3.99%),1,2,4-三甲氧基苯(2.42%)等;酯类化合物的相对含量占6.44%,主要包括反油酸甲酯(3.92%)和反式-4-(反-4-戊基-环己基)-1-环己甲酸4-氟苯酯(1.71%)等;醛类化合物的相对含量占5.86%,以丁香醛的不同异构体(5.28%)为主。
-
在所检测的挥发性物质中,相对含量较高的挥发性物质包括β-紫罗兰酮、桉油醇、双花醇、反式-氧化芳樟醇、芳樟醇、3,4,5-三甲氧基甲苯,这些挥发物构成了山矾的主要呈香成分,且各物质在不同开花时期呈动态变化。β-紫罗兰酮的相对含量在蕾期很低(0.60%),到半开期迅速上升,成为相对含量最高的物质(33.38%),在盛开期稍有所降低(19.14%);桉油醇的相对含量在山矾开花进程中呈现不断上升的趋势;双花醇、反式-氧化芳樟醇以及芳樟醇均在蕾期有较高的相对含量,到半开期和盛开期明显降低;3,4,5-三甲氧基甲苯的相对含量从蕾期到盛开期表现为先上升后降低的趋势,半开期分别是蕾期和盛开期的近2倍和近5倍。
2.1. 开花过程中香气物质种类比较
2.2. 开花过程中挥发物成分及其相对含量的变化
2.3. 主要香气成分的动态变化
-
本研究从3个时期的山矾花中共检测到51种挥发物,其中盛开期为42种,而罗心毅等[4]则从山矾花精油中检测到68种化合物,可能是由于精油提取过程中带入了花瓣的内源物质,这种差异性在玫瑰精油与玫瑰鲜花香气中同样存在[17]。余爱农等[5]用硅胶吸附采集花香的方法仅检测出山矾头香成分22种,说明采用该方法有大量花香组分未检测出。本研究还对不同开花时期的花香成分进行测定发现多种挥发物质具有规律性变化,且各组分对呈香起到了一定的作用。
-
山矾醇类化合物中,桉油醇、双花醇、反式-氧化芳樟醇、芳樟醇相对含量较大,其中以萜醇类为主,其具有花香、果香和蜜香香气[18]。桉油醇是桉树Eucalyptus robusta叶和樟树Cinamomum camphora叶挥发油主要组成成分[19-20],在吸引昆虫方面有一定作用[21],其相对含量随着开花进程不断升高,对花香可能有较大的影响。双花醇、芳樟醇均是金银花挥发油的主要成分[22],芳樟醇还是桂花[23]、百合[10]、薰衣草Lavandula angustifolia[24]等多种鲜花的主要芳香物质;反式-氧化芳樟醇是氧化芳樟醇的一种呋喃型立体异构体,具有木香、花香[18],以上3种醇类都在蕾期相对含量最高,到半开期和盛开期降低,说明对蕾期的花香呈现起到重要的作用。
-
酮类化合物在开花进程中呈先升高后降低的变化趋势,其主要成分是β-紫罗兰酮。β-紫罗兰酮作为多种成熟水果中的主要芳香物质[25-27],具有甜香、木香和果香[28],在山矾花的半开期达到非常高的相对含量,说明β-紫罗兰酮对花香的形成发挥了重要的作用。醛类化合物虽然在整个开花过程相对含量都较低,但由于丁香醛及其不同异构体的嗅觉阈值较低[29],且含量呈逐渐上升趋势,所以可能对盛开期花香也有一定的贡献。
-
酯类物质是栀子花Gardenia jasminoides[30]和许多果实[31]香气的主要成分,在山矾不同开花时期的变化并无显著性差异。3,4,5-三甲氧基甲苯是不同开花期的主要芳香族类化合物,还是合成脑功能改善药艾地苯醌和辅酶Q1~Q10等泛醌类化合物的重要中间体[32],如需利用山矾花的药用价值,可以选择在其挥发量达到最高值的半开期进行采摘。
综上所述,不同开花时期山矾花香成分差异显著,挥发物的种类在蕾期最少,盛开期最多。从相对含量上看,β-紫罗兰酮对半开期花香贡献最大,而盛开期的香气是由多种物质共同作用的结果,主要有β-紫罗兰酮、桉油醇、双花醇、反式-氧化芳樟醇、芳樟醇、3,4,5-三甲氧基甲苯和丁香醛等。山矾花香成分的这种变化与花发育的程度密切相关[7-8],可能受多种因素的影响:开花过程中不同酶的激活状态会促进某种香气成分的形成,比如类胡萝卜素裂解酶和芳樟醇合成酶分别与β-紫罗兰酮和芳樟醇的合成有关[33];王洁等[34]对厚朴Magnolia officinalis及徐瑾等[35]对菊花Chrysanthemum×morifloium的研究表明:不同花部器官香气物质有明显的差异,所以花的开展程度、雌雄蕊是否露出可能会影响花香;而激素[36-37]、花朵授粉状态[38-39]也会引起花香成分组成和含量的变化。因此,对于影响山矾在不同开花时期香气变化的机制有待进一步的研究。












 DownLoad:
DownLoad: