-
沙棘Hippophae rhamnoides为胡颓子科Elaeagnaceae沙棘属Hippophae植物,是一种广泛分布于亚洲、欧洲和北美洲的落叶树种。在中国,沙棘天然分布于华北、西北、西南等半干旱地区,后经人工引种至东北地区[1]。沙棘不但适应性好,固氮能力强[2],具有良好的改良土壤和水土保持功能,而且沙棘果实及叶子中富含的多种营养成分和生物活性物质,具有医药及日化等工业前景[3-5],是一种生态效益、社会效益与经济效益均十分显著的优良树种[6]。虽然沙棘在长期的进化中已经具备多种抗旱的结构特性和生理机制,但是水分仍然是沙棘生长和发挥功能的主要限制因子。所以,培育生态价值和经济价值高的沙棘品种依然是林业生态建设的焦点。沙棘品种的抗旱性对于其价值的实现具有重要作用。一些学者不仅对沙棘的抗旱表型进行了研究,还探讨了干旱胁迫下沙棘光合作用和生理生化等特性的变化[7-9],并以这些指标作为抗旱性强弱的评价依据,对沙棘品种间的抗旱性进行了比较[10-14]。但其试验方案多采用人为控制土壤含水量不变的方法,不能保证补充散失水分过程中土壤水分均衡和模拟自然环境中动态的土壤干旱过程,同时缺少干旱胁迫后复水过程。本研究减少人为对水分的干预,模拟自然环境中土壤干旱及复水过程,观察中国沙棘‘丰宁’H. rhamnoides subsp. sinensis‘Fengning’和引进品种‘向阳’H. rhamnoides subsp. mongolia‘Xiangyang’在不同土壤含水量的表型变化,以及对不同干旱程度的植株水分关系和光合生理特性,通过数据分析,比较2个品种对水分变化响应的差异性和共性,从而为沙棘抗旱树种的筛选和育林的补水时间提供科学依据。
HTML
-
本试验使用的材料采自中国林业科学研究院沙漠林业实验中心,为2年生沙棘根蘖苗,分别是乡土品种中国沙棘‘丰宁’和引进蒙古品种大果沙棘‘向阳’。2016年4月将苗木栽入高22.5 cm,内径25.5 cm的花盆中,1株·盆-1,盆中装土5.5 kg[m(沙):m(草炭):m(珍珠岩):m(黏土)=5:3:1:1],土壤最大含水量为62%,体积含水量44%。放置于中国林业科学研究院科研温室大棚,生长条件为自然光照,昼/夜温度为20~30 ℃/10~15 ℃。相对湿度为60%~70%[15],使植株正常生长。3个月后选取健康,生长均一的植株分组进行干旱处理试验。
-
本试验共分为2个组,分别为对照组(C)和试验组(T)。2016年7月30日前所有供试苗木保持正常水分,第2天测定供试苗木正常水分条件下的土壤体积含水量,此后试验组停止浇水进行自然干旱胁迫。对照组为正常浇水组,水分控制在(35.00±5.00)%土壤体积含水量。土壤含水量采用WET土壤三参数速测仪Moisture Meter-HH2(Delta-T Devices Ltd,英国)[16]测定,隔1 d测量1次,及时补充对照组散失的水分。隔5 d进行各项生理指标测定[17]。达到沙棘最低耐受程度后,对试验组复水至对照组水平,第3天和第5天测定生理指标。
-
在试验期间,用LI-6400便携式光合仪(LI-COR,美国)分别测定对照组与试验组2个沙棘品种的净光合速率(Pn,μmol·m-2·s-1),蒸腾速率(Tr,mmol·m-2·s-1),气孔导度(Cond,mol·m-2·s-1)和光响应曲线。测定条件:红蓝固定光源,固定光强为1 000 μmol·m-2·s-1,温度28 ℃,相对湿度为70%,二氧化碳浓度为温室中大气浓度。光响应曲线使用自动测量程序,梯度为2 000,1 800,1 500,1 000,600,400,200,100,50,0(μmol·m-2·s-1),同时用叶绿素含量测定仪(SPAD-502Plus,日本)[18]测定相应位置的叶绿素相对含量,选择15片叶片分成3组,测量记录每组平均值。叶片水势变化用WP4露点水势仪(Decagon,美国)[19]测定。
-
利用Excel 2007统计软件整理数据及制作图表。在SPSS 17.0统计软件中进行one-way ANOVA分析和0.05,0.01水平的最小显著性差异(LSD)法多重分析。
1.1. 试验材料
1.2. 试验方法
1.3. 测定指标及方法
1.4. 统计分析
-
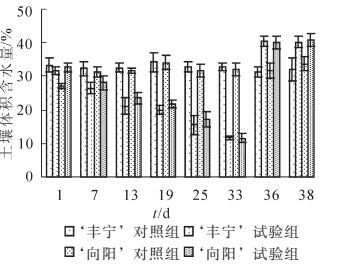
本研究将土壤体积含水量作为土壤干旱的分级标准[20],控水后的土壤体积含水量的变化如图 1所示。‘丰宁’对照组和‘向阳’对照组的体积含水量保持在最大体积含水量75%~80%的正常水分条件,而‘丰宁’试验组和‘向阳’试验组的体积含水量呈现下降趋势并且趋势相同。在自然干旱第7天,2个试验组分别26.37%和28.09%,达到轻度干旱。干旱至第19天,体积含水量分别为19.90%和21.78%,为中度干旱。在第22天观测到‘向阳’有叶片开始变黄,体积含水量约为19.40%。第33天开始对试验组复水至浇透,复水前体积含水量为11.66%和11.63%,为重度干旱,达到‘丰宁’所能耐受的最低含水量。在该水分下植株叶片开始变黄、枯萎。
-
对‘丰宁’和‘向阳’不同控水天数叶片水势的测定(表 1)发现,‘丰宁’试验组与‘向阳’试验组与对照组相比叶片水势降低,其中‘丰宁’试验组下降明显,在第13天达到显著差异水平(P<0.05),第19天达到极显著水平(P<0.01)。‘向阳’试验组下降趋势缓慢,在第25天达到显著差异,第33天达到极显著水平(P<0.01)。在重度干旱情况下,虽然‘丰宁’差值(0.77)高于‘向阳’(0.45),但是‘丰宁’试验组(-2.36)和‘向阳’试验组(-2.31)的大小差异不显著。复水后沙棘叶片水势快速上升,在第3天,‘向阳’恢复正常水平,而‘丰宁’第5天才恢复。
控水
天数/d水势/MPa ‘丰宁'
对照组‘丰宁'
试验组‘向阳'
对照组‘向阳'
试验组1 -1.66±0.23 a -1.80±0.12 a -1.84±0.10 A -2.03±0.04 A 7 -1.65±0.06 a -1.86±0.29 a -1.82±0.29 A -1.95±0.09 A 13 -1.59±0.20 a -1.96±0.07 b -1.82±0.07 A -1.95±0.18 A 19 -1.53±0.09 a -2.03±0.26 b -1.85±0.33 A -2.02±0.17 A 25 -1.72±0.09 a -2.35±0.03 b -1.77±0.08 A -2.14±0.08 B 33 -1.59±0.19 a -2.36±0.40 b -1.86±0.10 A -2.31±0.10 B 36 -1.53±0.19 a -1.64±0.09 a -1.82±0.31 A -1.83±0.12 A 38 -1.56±0.08 a -1.51±0.05 a -1.85±0.23 A -1.8 ±0.05 A 说明:同一列不同小写字母表示‘丰宁'对照组与‘丰宁'试验组差异显著(P<0.05),不同大写字母表示‘向阳'对照组与‘向阳'试验组差异显著(P<0.05)。 Table 1. Changes of leaf water potential of two Hippophae rhamnoides cultivars in different days
-
从图 2可以看出:‘丰宁’对照组和‘向阳’对照组叶绿素相对含量总体呈现稳定趋势,但‘丰宁’对照组在后期有微量上升。两者相比,‘向阳’要高于‘丰宁’。‘丰宁’试验组和‘向阳’试验组叶绿素相对含量分别要高于对照组,在总体趋势上随着干旱程度增强上升,在第33天重度干旱下达到最大增长幅度,分别是10.33%和2.39%,干旱胁迫对‘丰宁’叶绿素相对含量的影响高于‘向阳’。复水后‘向阳’下降至对照组水平,而‘丰宁’仍高于对照组。
绿色植物主要的生命活动之一是光合作用,干旱胁迫对光合作用有重要影响。从表 2可以看出:随着控水时间延长,干旱程度增强,净光合速率(Pn),蒸腾速率(Tr),气孔导度(Cond)总体呈现出下降趋势,复水后恢复或者超过正常水平。‘丰宁’试验组和‘向阳’试验组与对照组相比,在轻度干旱和中度干旱阶段Pn下降趋势不明显,在控水后第33天达到重度干旱,都降到最低值,分别是对照组的32.2%和34.6%,具有显著差异(P<0.05),‘向阳’是‘丰宁’的1.4倍。复水第2天,‘向阳’的Pn稍微上升,复水第3天,超过对照组。而‘丰宁’在复水第3天才出现上升,第5天与对照组没有显著差异;‘丰宁’试验组的Tr呈现下降趋势,与对照组的差异逐渐增加,第25天土壤体积含水量为15.51%时,具有显著差异(P<0.05),在第33天达到最低值,为对照组的16.38%。复水后开始上升,但没有恢复正常水平。‘向阳’试验组变化趋势与‘丰宁’试验组大体相同,但‘向阳’试验组的下降趋势更平缓,在第33天达到显著差异水平(P<0.05),为对照组的48.02%。复水后第3天超过正常水平;‘丰宁’试验组和‘向阳’试验组的Cond变化趋势是随着干旱程度增强而下降,重度干旱程度具有显著差异,分别是对照组的13.75%和36.89%,复水后上升,但是‘向阳’试验组迅速恢复,而‘丰宁’试验组则需要更长时间。Pn与Tr比值为植物水分利用效率(EWU),沙棘在不同的土壤水分条件和生长时期,EWU有很大差异。随着干旱时间的延长,实验组与对照相比先有较大上升,随后出现下降,其中在中度干旱下沙棘的EWU最高,‘丰宁’试验组与‘丰宁’对照组相比增加35%,而‘向阳’试验组与‘向阳’对照组相比增加31%。重度干旱下沙棘生长受抑制,物质积累减少,因此EWU降低。
控水
天数/d净光合速率/(μmol·m-2·s-1) 控水
天数/d蒸腾速率/(mmol·m-2·s-1) ‘丰宁'
对照组‘丰宁'
试验组‘向阳'
对照组‘向阳'
试验组‘丰宁'
对照组‘丰宁'
试验组‘向阳'
对照组‘向阳'
试验组7 5.94±1.53a 6.5±1.95a 6.54±1.11A 6.63±0.86A 7 1.089±0.197a 1.1±0.264a 1.265±0.236A 1.082±0.030A 13 6.01 ±0.79a 6.48±1.53a 6.9±2.88A 6.33±1.11A 13 0.688±0.044a 0.575±0.145a 0.792±0.179A 0.559±0.093A 19 5.81±0.75a 5.18±2.45a 6.64±2.57A 6.61±1.75A 19 0.738±0.131a 0.486±0.134a 0.789±0.33A 0.601±0.118A 25 4.2±0.72a 4.1±0.81a 5.59±0.59A 4.43±1.82A 25 1.165±0.236a 0.539±0.209b 1.126±0.179A 0.884±0.330A 33 4.85±0.49a 1.56±0.74b 6.3±1.65A 2.18±1.5A 33 1.545±0.409a 0.253±0.243b 2.176±0.499A 1.045±0.590B 34 4.85±0.49a 1.23±0.45b 6.3±1.65A 2.76±1.68A 34 1.545±0.409a 0.289±0.079b 2.176±0.499A 0.923±0.413B 36 4.85±0.84a 2.24±1.81b 5.43±2.17A 5.78±1.81A 36 1.582±0.457a 0.845±0.178b 1.879±0.427A 2.027±0.355A 38 5.51±1.61a 4.11±1.20a 3.89±1.49A 5.36±0.31A 38 1.918±0.307a 0.98±0.258b 1.567±0.349A 1.635±0.506A 控水
天数/d气孔导度/(μmol·m-2·s-1) ‘丰宁'对照组 ‘丰宁'试验组 ‘向阳'对照组 ‘向阳'试验组 7 0.036±0.006 a 0.047±0.008 a 0.043±0.009 A 0.036±0.001 A 13 0.019±0.001 a 0.015±0.004 a 0.022±0.005 A 0.015±0.005 A 19 0.020±0.004 a 0.013±0.004 a 0.022±0.010 A 0.014±0.001 A 25 0.034±0.007 a 0.015±0.006 a 0.036±0.005 A 0.024±0.008 A 33 0.080±0.026 a 0.011±0.010 b 0.122±0.016 A 0.045±0.028 B 34 0.078±0.026 a 0.016±0.005 b 0.122±0.016 A 0.074±0.021 B 36 0.097±0.036 a 0.034±0.009 b 0.114±0.034 A 0.120±0.023 A 38 0.119±0.024 a 0.044±0.019 b 0.086±0.022 A 0.096±0.038 A 说明:同一列不同小写字母表示‘丰宁'对照组与‘丰宁'试验组差异显著(P<0.05),不同大写字母表示‘向阳'对照组与‘向阳'试验组差异显著(P<0.05)。 Table 2. Changes of photosynthetic characteristic of two Hippophae rhamnoides cultivars in different days
-
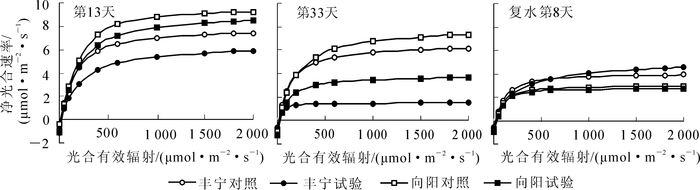
为了全面阐明不同干旱胁迫程度对植物光合作用的影响,除了测定了上述的光合生理特性,还测定了不同控水阶段的光响应曲线。其结果如图 3所示。对光响应曲线拟合后,利用分段式函数[21]计算出净光合最大速度(Pnmax),光饱和点(PLS),表观量子效率(EAQ),光补偿点(PLC),暗呼吸速率(Rd)数值,如表 3所示。由图 3可以看出:所有的光响应曲线呈现二次方程的形式,光合有效辐射为0~400 μmol·m-2·s-1,Pn随着光合有效辐射增加而增加,增幅较大。光照有效辐射进一步增加,Pn增幅变缓,增加到PLS后,Pn不再增加,符合光响应曲线的一般规律。从表 3中看出在第13天轻度干旱阶段,‘丰宁’试验组和‘向阳’试验组与对照组相比,Pnmax,PLS,EAQ都出现下降趋势,而PLC上升。‘丰宁’试验组的Rd出现先下降后恢复趋势,而‘向阳’试验组出现先不变后上升。随着控水时间延长,第33天重度干旱下降趋势更加明显,差异更大。复水第8天后,‘丰宁’试验组的Pnmax和PLS超过对照组,EAQ,PLC和Rd与对照组大体一致。而‘向阳’试验组的Pnmax和PLC没有恢复到正常水平,其他恢复正常。
控水时间/d 分组 最大净光合速率/
(μmol·m-2·s-1)光饱和点/
(μmol·m-2·s-1)表观量子效率 光补偿点/
(μmol·m-2·s-1)暗呼吸速率/
(μmol·m-2·s-1)13 ‘丰宁’对照组 7.26 1 312.5 0.025 5 10.20 -0, 83 ‘丰宁’试验组 5.61 1 125.0 0.017 6 12.84 -0, 46 ‘向阳’对照组 9.57 1 350.0 0.018 7 7.21 -0, 85 ‘向阳’试验组 8.63 1 260.0 0.017 6 23.70 -0, 88 33 ‘丰宁’对照组 6.13 1 433.3 0.021 8 6.24 -0, 66 ‘丰宁’试验组 1.57 465.0 0.008 6 14 19 -0, 62 ‘向阳’对照组 7.32 1 300.0 0.023 4 18.46 -093 ‘向阳’试验组 3.67 1 000.0 0.017 3 38 84 -1.28 8(复水后) ‘丰宁’对照组 3.87 693.8 0.015 5 13.55 -0 58 ‘丰宁’试验组 4.53 1 500.0 0.013 7 14 13 -0 62 ‘向阳’对照组 2.96 700.0 0.015 0 34.13 -1 00 ‘向阳’试验组 2.62 700.0 0.014 9 42 01 -1 01 Table 3. Changes of response characteristics to light of two Hippophae rhamnoides cultivars in different days
2.1. 土壤体积含水量的变化
2.2. 不同程度干旱胁迫对2个沙棘品种植株叶片水势的影响
2.3. 干旱胁迫对光合生理的影响
2.3.1. 不同程度干旱胁迫对2个沙棘品种光合特性的影响
2.3.2. 不同程度干旱胁迫对2个沙棘品种光响应曲线及特征参数的影响
-
在沙棘的生长区,降水不足会导致沙棘生长量和生物量降低[22],水分成为制约沙棘正常生长的主导因素。本研究模拟自然环境中无降水的土壤水分变化,同时对土壤水分进行连续监测。由于温室湿度大导致水分蒸发速率慢,同时沙棘对水分利用率高,对水分吸收较少,所以土壤水分下降缓慢,为植物适应不同强度的土壤干旱提供了时间。在该试验土质和生长条件下,‘向阳’在第22天出现干缩现象,体积含水量约为19.40%,而‘丰宁’在连续干旱33 d后出现同样现象,体积含水量为11.66%,与吴林等[23]在田间观察到的现象一致。
叶片水势代表植物水分运输的能力,是广泛应用于衡量植物抗旱的生理指标,能够反映植物吸收水分的能力和水分状况好坏[24],影响叶水势的因素很多,光照条件、水分供应情况都会对水势大小产生影响,并且同一天不同时间段不同部位的叶片水势也会不同[25]。随着土壤水分降低,植物体内水分产生亏缺,植物为了满足自身生命活动所需水分,降低叶片水势,增加对土壤水分的吸收,抵御外界的干旱条件[26]。‘丰宁’比‘向阳’水势低,差值大,说明2个沙棘品种对水分的吸收能力和利用效率有差异。
植物叶片中叶绿素相对含量高低、叶绿素a和叶绿素b的比值直接影响植物光合作用的强弱[27]。叶绿素的合成和降解与外界环境相关。干旱胁迫下,部分植物叶片中的叶绿素相对含量会增加,表现一定抗旱性[28]。沙棘叶绿素相对含量呈现出稳定或者微量上升的趋势,与韩蕊莲等[29]得到的结论相同。说明在遭受干旱胁迫时,2个沙棘品种为保持较高的生长速率,依旧维持较高的叶绿素含量,‘丰宁’的作用要明显于‘向阳’。
Pn能直接反映光合作用的强度,是衡量光合作用的重要指标,对植物自身来说,Pn大小受Tr和Cond等多因素影响,蒸腾作用可以显示植物体内水分生理特征,Tr反应水分散失情况。Cond一定程度上反映植物内代谢水平,灵敏度可以作为抗旱特征[30]。它们共同保障植物光合作用的顺利进行。Tr和Cond下降,同时Pn出现下降,说明植物水分缺失会影响Tr和Cond,阻碍光合作用的顺利进行[31]。通过EWU的变化,更能直接表现出沙棘的有机物积累变化趋势。EWU在中度干旱下最高,其原因是在中度水分亏缺下,耗水量大幅下降,但新枝生长量下降幅度小。而长时间的重度干旱使沙棘生长受抑制,EWU同样很低。植物对于逆境环境具有抵抗性,在复水的短期内会出现超过正常水平现象,这是植物的防御机制所造成的。
通过测定光响应曲线,发现干旱会对Pnmax,PLS,EAQ,PLC影响显著,PLC上升,EAQ下降说明干旱影响了植物对弱光的利用效率,而PLS下降说明干旱抑制了对光照的利用,在没有达到对照组的光饱和点时水分变成了植物有机质积累的限制因子。干旱对Rd的影响表现出品种差异性,‘丰宁’Rd下降,降低了有机物的消耗,有利于在较低的光合速率下保持生长。复水激发了植物的生理补偿效应,植物表现出很强的恢复力,在很短的时间内恢复或超过正常水平[32],但如果干旱对植物造成不可恢复伤害,即使复水,植物也将恢复不到正常水平。在本试验中,复水后,‘丰宁’和‘向阳’的叶片水势快速恢复正常。‘向阳’叶绿素相对含量降低至对照水平,而‘丰宁’仍高于对照,其他光合特性指标基本恢复正常。由于测定时间较长,跨度较大,出现季节性变化,导致不同时期的对照组指标也出现下降的趋势。
对沙棘抗旱机制的研究不能只停留在生理水平,植物对干旱胁迫的反应主要受基因型和基因表达的影响,而表观遗传修饰在抗逆基因表达调控过程中起到不可忽视的作用[33]。许多研究表明,DNA甲基化程度高,基因表达受到抑制,组蛋白乙酰化水平高促进基因表达[34-35],两者相互协调,从而实现对一些关键抗逆基因的准确调控。通过比较干旱胁迫前后DNA甲基化和组蛋白乙酰化水平的变化[36-37],为阐明DNA甲基化和组蛋白遗传修饰在沙棘抗旱基因表达调控中的功能具有重要的意义。
-
在干旱胁迫下,‘丰宁’和‘向阳’沙棘形态特征、叶片水势和光合生理都表现出明显的变化,真实反映了沙棘在自然干旱环境中的变化。沙棘虽是强抗旱植物,但其抗旱能力有限,在人工林的培育过程中,在极度干旱情况下(土壤体积含水量为15%左右),沙棘叶片出现干缩,要及时灌溉,以免对沙棘生长造成不可恢复的伤害。通过叶片水势和光合生理等比较,‘丰宁’比‘向阳’具有更强的耐旱性,可以将其作为选育良种的亲本。












 DownLoad:
DownLoad: