-
目前,土壤污染问题日益突出,且污染逐渐趋于多元化和复杂化[1],其中重金属和有机污染比较常见。在土壤重金属污染中,锌属于微量元素,但锌矿的采选活动,冶炼废气的沉降和废水的排放以及道路交通活动,均易造成土壤中锌含量的超标。过量锌会降低酶活性甚至使酶失活,易毒害作物甚至直接致死[2-4],且易通过食物链,危及人类健康。此外,农药与化肥的滥用,以及塑料薄膜与橡胶的广泛使用,土壤有机物污染问题也日趋严峻,其中邻苯二甲酸酯(PAEs)的污染问题尤为突出[5]。PAEs为常用的塑化剂,具生殖毒性与致癌性。邻苯二甲酸二乙酯(DEP)为典型的PAEs,可严重影响人体内分泌,具亲脂性和易富集的特点,易与土壤环境中的重金属形成重金属-PAEs复合污染,目前已被检测出普遍存在于土壤、水、大气等环境介质中[6-8]。近年来,生物质炭作为新型土壤污染修复材料受到广泛关注[9-11],对土壤中重金属和有机物污染具有较好的吸附效果[12],在土壤污染修复中具有较好的应用前景和推广价值。然而,目前有关生物质炭修复土壤污染的研究大多集中于单一种类的污染物污染土壤修复的研究上[13-14],关于重金属-有机污染物复合污染土壤,尤其是重金属-PAEs复合污染土壤修复的研究仍鲜见报道。基于此,本研究选取动物源的猪炭和植物源的法国梧桐Platanus orientalis炭为研究材料,以DEP为代表性PAEs,以锌为代表性重金属,采用批量吸附试验,研究其吸附动力学和吸附热力学行为,探究不同因子对施炭土壤吸附重金属-PAEs复合污染溶液中重金属的影响,以期揭示其吸附机理和污染物之间的交互作用,为生物质炭修复土壤重金属和有机物复合污染提供科学依据。
HTML
-
以采自浙江省杭州市临安区竹林村的农业种植土壤作为供试土壤,按照《土壤农业化学分析方法》测定土壤的基本理化性质。土壤pH值为4.89,有机质为7.5 mg·g-1,土壤砂粒、粉粒和黏粒质量分数占比分别为55.00%,26.10%和18.90%。土壤锌全量为48.21 mg·kg-1,远低于国家污染标准,因此,土壤本身所含重金属对试验的影响可忽略。
采用在650 ℃缺氧条件下热解2 h所得的猪炭与法国梧桐炭作为供试生物质炭,利用元素分析仪(Flash EA1112,Thermo Finnigan,意大利)测定生物质炭的碳、氢、氮元素质量分数,运用差减法得到氧元素质量分数,利用比表面仪(TristarⅡ3020,Micromeritica Instument Corporation,美国),采用BET法测定生物质炭的比表面积,用干烧法测定灰分。猪炭和法国梧桐炭的基本理化性质如表 1所示。猪炭相较法国梧桐炭具有较高的灰分含量和比表面积,而法国梧桐炭的碳质量分数远高于猪炭,这些性质差异可能影响其对重金属离子的吸附能力[15]。
生物质炭 PH 电导率/(dS·m-1) 碳/(mg·g-1) 氢/(mg·g-1) 氧/(mg·g-1) 氮/(mg·g-1) 磷/(mg·g-1) 灰分/(mg·g-1) 比表面积/(m2·g-1) 猪炭(PB) 10.04 2.17 37.52 1.74 55.89 4.74 7.85 600 23.10 法国梧桐炭(POB) 9.47 0.22 81.32 2.24 15.74 0.45 0.05 66 2.80 Table 1. Physicochemical properties of biochars used in the experiment
此外,利用发射扫描电镜(SU-8010,日立公司,日本)观察猪炭和法国梧桐炭的孔隙结构和表观形貌特征;利用能谱仪(Aztec X-MaxN,牛津仪器公司,美国)测定表面某点位的元素组成;利用傅里叶变换红外光谱仪(FT-IR)(NICOLET iS10,Thermo Fisher Scientific公司,美国)测定猪炭与法国梧桐炭的表面官能团结构特征[16]。
-
采用批处理振荡平衡法,将猪炭(PB)与土壤按质量分数1%(PB1)、2%(PB2)混合;法国梧桐炭(POB)与土壤按质量分数1%(POB1)、2%(POB2)混合,分别制成含炭量不同的土壤样品,以不施炭处理为对照(ck)。配制0.01 mol·L-1硝酸钠溶液作为背景溶液,添加250 mg·L-1的叠氮化钠作为微生物抑制剂。设置锌离子(Zn2+)溶液初始质量浓度为100 mg·L-1,DEP初始质量浓度分别为0和25 mg·L-1。称取1.00 g土壤样品置于聚四氟乙烯离心管中,加入20 mL含有不同初始质量浓度污染物的溶液,以180 r·min-1的转速在25 ℃条件下振荡24 h,之后采用3 500 r·min-1的转速离心10 min,上清液过滤后用电感耦合等离子体发射光谱(ICP-OES)(Leman Prodigy 7,PE公司,美国)测定溶液中剩余Zn2+质量浓度,计算吸附量。
-
设置吸附试验时间分别为1,2,4,8,16和24 h,获得不同吸附时间的吸附量曲线,利用4种动力学方程进行公式解析,获得吸附动力学曲线和各模型动力学参数。
吸附动力学可描述吸附剂吸附溶质速率的动态变化[17],可推断其吸附机理。本研究采用的吸附动力学方程有准一级线性方程、准二级线性方程、Elovich线性方程、颗粒内扩散线性方程:
其中:
式(1)~(6)中:Qt为t时刻土壤对溶液中重金属的吸附量(mg·g-1);Qe为平衡时土壤对溶液中重金属的吸附量(mg·g-1);t为吸附时间(h);Ct为t时刻溶液中重金属的质量浓度(mg·L-1);C0为溶液中重金属的初始质量浓度(mg·L-1);Ce为溶液中重金属的平衡质量浓度(mg·L-1);V为添加溶液的体积(L);m为加入土壤的质量(g);K1为准一级反应速率常数(h-1);K2为准二级反应速率常数(mg·g-1·h-1);Kti为颗粒内扩散速率常数(mg·g-1·h-0.5);α为初始吸附速率(g·mg·h-1);β是与表面覆盖度和活化能有关的常数(g·mg-1);C为常数。
-
设置吸附实验温度分别为15,25,35,45和55 ℃,恒温振荡24 h,进行吸附热力学实验。利用Gibbs方程获得各吸附热力学参数:平衡吸附系数K,吉布斯自由能变ΔG(kJ·mol-1),焓变ΔH(kJ·mol-1)和熵变ΔS(kJ·mol-1·K-1)。运用Gibbs方程计算热力学参数,研究不同温度对各处理土壤吸附溶液中重金属行为的影响。
由式(8)和式(9)可推导得:
式(7)~(10)中:K为吸附平衡常数;T为绝对温度(K);R为气体常数,R=8.314×10-3(kJ·mol-1·K-1)。
-
数据采用Excel 2013和SPSS 19.0统计处理,不同处理之间差异分析采用单因素方差分析(one-way ANOVA,最小显著差法LSD),利用Origin 8.5作图。
1.1. 供试材料
1.2. 试验方法
1.3. 吸附动力学
1.4. 吸附热力学
1.5. 数据分析
-
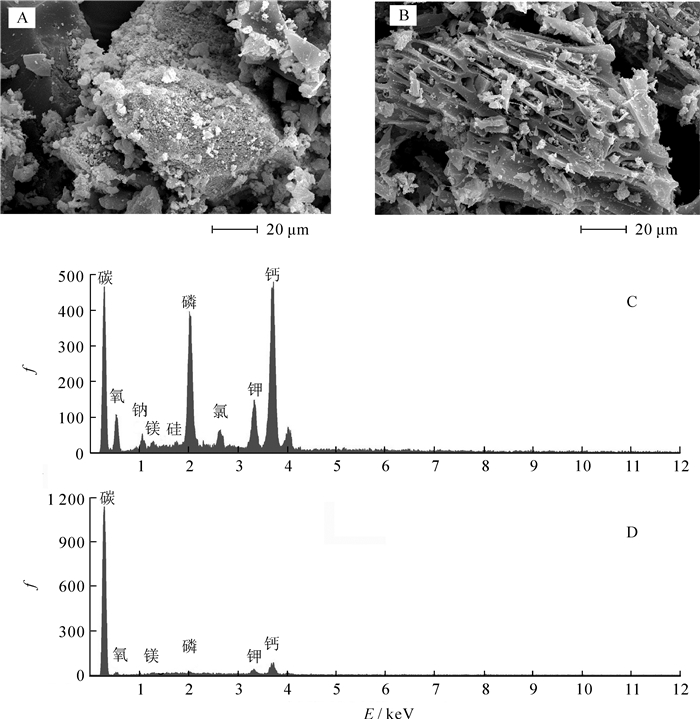
由图 1A和图 1B可知:猪炭表面孔隙致密,而法国梧桐炭表面孔隙较为稀疏,猪炭相较法国梧桐炭具有更加致密的孔隙结构和更大的比表面积(表 1)。由图 1C和图 1D可知:法国梧桐炭的元素组成种类较少,碳元素是构成物质的最主要元素,猪炭表面的矿质元素组成较法国梧桐炭更为复杂,其氧、磷、钾、钙远高于法国梧桐炭。同时,与法国梧桐炭相比,猪炭还有钠、硅、氯等元素检出。此外,猪炭比法国梧桐炭具有较高的矿质元素,这也是造成其较高灰分的原因(表 1)。这些灰分溶于水可使溶液pH值上升,也是猪炭pH值高于法国梧桐炭的主要原因[16]。
-
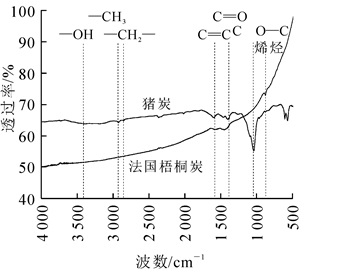
从图 2可见:法国梧桐炭有3个较明显的峰,1 576~1 448 cm-1的峰可能为芳香性中的C=C振动峰[18],650~1 000 cm-1的峰为不饱和烃中C—H的伸缩振动峰[19],1 300 cm-1的峰可能为C=O伸缩振动吸收峰,说明法国梧桐炭表面具有以上3种官能团,而猪炭相较法国梧桐炭吸收峰数量更多,且特征峰振动强度明显大于法国梧桐炭,说明猪炭表面具有更加丰富的官能团。猪炭在3 056~2 924 cm-1的吸收峰表示饱和C—H振动峰,2 850 cm-1附近的吸收峰为C—H2[20],在3 447~3 400 cm-1为—OH基伸缩区,1 271~1 098 cm-1的吸收峰表示C—O—C的伸缩振动[21]。与法国梧桐炭相比,猪炭不仅含有更为丰富的官能团类型,还含有带负电荷的官能团(如羟基和亚甲基等),理论上更有利于吸附重金属。
-
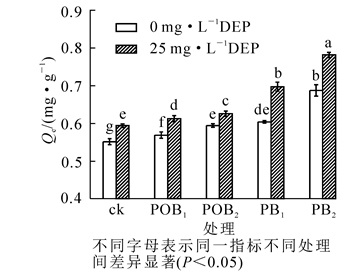
由图 3可知:在DEP添加量为0或25 mg·L-1时,施加猪炭和法国梧桐炭的土壤对Zn2+的饱和吸附量均显著(P<0.05)高于对照,且随施用量的增加效果更明显,说明生物质炭的施加可促进土壤对Zn2+的吸附。这可能是由于2种生物质炭均含有丰富的孔隙结构,其比表面积较大,可为Zn2+提供较多的吸附位点。2种生物质炭pH值均呈碱性(表 1),施用生物质炭可使溶液中氢离子(H+)减少,降低其与Zn2+之间的竞争吸附,且其较高的阳离子交换量,可增加土壤对Zn2+的交换作用,从而达到较好的表面吸附作用[22]。此外,根据2种炭的红外光谱分析(图 2),两者表面均有较丰富的含氧官能团,这些官能团可与重金属离子形成特定的金属配合物[23]。施加生物质炭后土壤对Zn2+较好的吸附作用也可能是炭表面的官能团与Zn2+形成络合物,发生专性吸附。徐楠楠等[24]在其秸秆生物质炭对镉离子(Cd2+)的吸附研究中发现:秸秆炭对Cd2+的吸附其化学机制主要为生物质炭表面羟基和羰基与Cd2+发生络合作用。
本研究发现:施加猪炭的土壤对溶液中Zn2+的吸附能力显著(P<0.05)高于施加等量法国梧桐炭的土壤。相较不加炭的土壤,施加PB1和PB2的土壤对DEP添加量为25 mg·L-1的复合污染溶液中Zn2+的吸附量分别提高了17.43%和31.61%,是施加等量POB的5.51倍和5.90倍。生物质炭对土壤中重金属的作用主要包括以静电吸附为主的物理作用和以离子交换与官能团络合反应为主的分配作用[25-26],而生物质炭理化性质的差异可直接影响其吸附效果[27]。猪炭和法国梧桐炭对土壤吸附溶液中的Zn2+能力存在显著差异,其主要原因可能是猪炭的比表面积远高于法国梧桐炭,可提供更多的吸附点位,且其电导率、阳离子交换量(CEC)以及其钙、镁、钾等矿质元素含量均高于法国梧桐炭,导致其静电吸附和阳离子交换能力均高于法国梧桐炭处理的土壤。此外,猪炭表面含有更丰富的官能团,如法国梧桐炭不具有的—OH,—CH2—,可与Zn2+发生络合作用,且官能团表面带有负电荷,可增加土壤对Zn2+的静电吸附能力。
此外,DEP的添加可显著(P<0.05)提高各处理土壤对溶液中Zn2+的饱和吸附量,与不加DEP对照相比,添加25 mg·L-1DEP分别使ck,POB1,POB2,PB1,PB2的土壤对溶液中Zn2+的饱和吸附量提高了7.75%,7.73%,5.32%,15.18%,13.71%,且猪炭处理下DEP对土壤吸附溶液中Zn2+的影响更明显,施加PB1和PB2处理的土壤添加25 mg·L-1DEP时吸附量分别提高了26.53%和40.52%。结果表明:DEP可促进不同处理土壤对溶液中Zn2+的吸附,其原因可能为DEP与Zn2+发生络合作用,形成带正电荷的金属配合物后,通过离子交换而被吸附,也可能是由于DEP在土壤表面被吸附后,DEP所带的官能团与Zn2+发生络合作用,改变了Zn2+存在形态。周东美等[28]在对土壤中有机污染物-重金属复合污染交互作用的研究中发现:汞、铜、镍和镉等具有比较强的络合能力,其络合点位主要为羧基、羟基以及胺基等。
-
由图 4可知:在吸附反应时间相同时,施加生物质炭均可增加土壤对溶液中Zn2+的吸附量,且施加猪炭比施加等量法国梧桐炭效果更显著,但施加法国梧桐炭处理的土壤达到饱和吸附量的时间更短。DEP为25 mg·L-1的各处理相较未添加DEP的处理在各吸附时间对Zn2+的吸附量均有增加。表明DEP的添加可促进不同吸附反应时间时各处理对Zn2+的吸附。此外,不同处理土壤对溶液中Zn2+的吸附量均随着吸附时间的增加而增加,且主要可分为2个阶段:1~8 h为快速吸附阶段,施炭土壤对溶液中的Zn2+表现出较强的吸附能力,这是因为在吸附初始阶段,不同处理土壤表面均具较多可被利用的吸附位点,Zn2+通过氢键作用被快速吸附到吸附点位上,此过程以物理吸附为主。吸附时间为8 h时,各处理的吸附量均已达到其饱和吸附量的80.00%以上。8~24 h为慢速吸附阶段,吸附速率减慢,逐渐趋于平衡,该过程主要因吸附位点被Zn2+大量占据,剩余可被利用的吸附位点逐渐减少,吸附速率减慢,直至吸附位点均被占据,吸附达到平衡,吸附量不再增加。王璐[29]研究发现:决定吸附速率的主要因素为剩余吸附位点数量。
-
准一级吸附动力学模型多用于描述单一物理吸附影响下的吸附过程,准二级吸附动力学模型则可描述多种吸附作用叠加的吸附过程[30],Elovich方程适合于非均相的扩散过程[31]。本研究用以上3种模型初步探究不同处理土壤对溶液中Zn2+的吸附过程,用颗粒内扩散模型进一步探讨其吸附机理。由图 5和表 2可知:不同处理土壤对溶液中Zn2+的吸附动力学行为可与准二级线性方程较好拟合,其准二级线性方程相关系数R2均达0.99以上,拟合度较高,且远大于其准一级线性方程和Elovich线性方程的相关系数。准二级线性方程拟合所得的饱和吸附量与实际吸附量较接近,且添加DEP的处理均大于不加DEP的处理,进一步验证添加DEP可以提高土壤对溶液中Zn2+的吸附能力。此外,施炭处理的土壤其准二级反应速率常数K2均小于对照,且施加猪炭处理的K2小于施加等量法国梧桐炭的处理,准二级线性方程能较好反映各处理土壤对溶液中Zn2+的吸附过程。为进一步探究具体吸附过程,采用颗粒内扩散模型对数据进行拟合,吸附曲线均为不过原点的直线,说明该吸附过程并非单一颗粒内扩散过程,而是由液膜扩散和颗粒内扩散联合控制[32]。其吸附过程分为3个阶段:1~8 h为快速吸附过程,为表面扩散,Zn2+较快扩散到土壤黏粒矿物及生物质炭表面;8~16 h为慢速吸附过程,为颗粒内扩散的过程,Zn2+逐渐由外部向内部层间扩散,扩散阻力增大,扩散速度减慢;16~24 h为平衡吸附过程,随着吸附位点被逐渐占据,吸附逐渐达到平衡。3个过程中吸附速率常数Kt1>Kt2>Kt3。汲广云[33]在巯基硅烷改性多壁碳纳米管对Cd2+吸附性能的研究,以及王彤彤等[34]关于2种木材生物质炭对铜离子(Cu2+)吸附行为的研究均得到类似结果。
处理 DEP/(mg·L-1) 准一级线性方程 准二级线性方程 Elovich方程 颗粒内扩散方程 K1/h-1 R2 Qe/(mg·g-1) K2/(mg·g-1·h-1) R2 β R2 颗粒内扩散速率常数/(mg·g-1·h-0.5) Kt1 Kt1 Kt1 ck 0 0.479 1 0.819 6 0.570 8 1.846 7 0.998 8 16.345 2 0.983 4 0.066 2 0.042 2 0.011 6 POB1 0 0.486 1 0.791 4 0.589 7 1.774 8 0.998 9 15.515 9 0.972 7 0.072 3 0.039 2 0.011 2 POB2 0 0.599 1 0.909 2 0.613 2 1.583 1 0.998 8 13.798 8 0.991 4 0.088 4 0.028 3 0.010 8 PB1 0 0.489 8 0.833 2 0.624 9 1.738 3 0.999 1 14.819 2 0.980 8 0.077 5 0.037 7 0.013 9 PB2 0 0.667 2 0.890 4 0.716 4 1.193 9 0.998 8 10.924 2 0.991 6 0.106 8 0.049 5 0.024 8 ck 25 0.558 8 0.797 7 0.616 1 1.700 3 0.999 0 13.715 5 0.940 6 0.096 2 0.016 6 0.017 1 POB1 25 0.552 1 0.743 4 0.637 7 1.479 2 0.998 7 13.066 8 0.941 9 0.093 7 0.028 1 0.018 1 POB2 25 0.544 3 0.842 2 0.644 5 1.617 3 0.998 9 13.575 9 0.969 0 0.094 6 0.018 1 0.032 4 PB1 25 0.514 2 0.885 1 0.719 1 1.479 1 0.999 0 12.794 3 0.982 9 0.090 3 0.040 7 0.026 7 PB2 25 0.482 7 0.780 0 0.808 4 1.244 3 0.999 8 11.350 7 0.976 4 0.097 2 0.052 7 0.026 9 Table 2. Parameters of four kinetic models for the adsorption of Zn2+ on different treated soils
-
通过吸附热力学试验,根据吉布斯方程计算所得各热力学参数如表 3所示。自由能变ΔG可衡量各处理土壤对溶液中Zn2+的吸附量。从表 3可见:ΔG均为负值,说明吸附过程为自发过程,随温度的升高,ΔG负值的绝对值增大,说明升温有利于吸附的进行[35]。平衡吸附系数K均随温度的上升而增大,根据热力学原理,说明此过程为吸热反应,且温度升高利于吸附过程的自发进行[36]。此外,DEP初始质量浓度为0和25 mg·L-1时,施炭土壤均比对照ΔG绝对值大,且随着施加量的增加,ΔG绝对值增大,而DEP初始质量浓度为25 mg·L-1时,各处理ΔG绝对值均大于不加DEP的处理,其中猪炭处理下的土壤ΔG相较法国梧桐炭绝对值更大,此结果与上述动力学研究结论一致。
DEP 处理 T K ΔG ΔH ΔS 15 5.809 6 -4.213 0 14.506 4 0.065 3 25 7.608 9 -5.027 8 14.506 4 0.065 3 ck 35 9.311 7 -5.713 6 14.506 4 0.065 3 45 10.278 7 -6.160 4 14.506 4 0.065 3 55 12.545 8 -6.897 6 14.506 4 0.065 3 15 6.606 2 -4.520 7 13.267 8 0.062 2 25 8.669 7 -5.351 1 13.267 8 0.062 2 POB1 35 10.324 2 -5.977 9 13.267 8 0.062 2 45 11.7930 -6.523 7 13.267 8 0.062 2 55 13.1110 -7.017 8 13.267 8 0.062 2 15 7.0794 -4.686 4 14.326 2 0.066 4 25 9.333 6 -5.534 0 14.326 2 0.066 4 0 POB2 35 11.138 2 -6.172 3 14.326 2 0.066 4 45 13.243 3 -6.830 4 14.326 2 0.066 4 55 14.724 0 -7.334 2 14.326 2 0.066 4 15 7.955 1 -4.965 6 13.868 0 0.065 7 25 10.387 2 -5.798 9 13.868 0 0.065 7 PB1 35 12.335 3 -6.433 7 13.868 0 0.065 7 45 14.512 3 -7.072 3 13.868 0 0.065 7 55 16.199 1 -7.594 6 13.868 0 0.065 7 15 9.398 1 -5.364 7 12.453 2 0.062 1 25 11.885 7 -6.132 8 12.453 2 0.062 1 PB2 35 13.774 1 -6.716 2 12.453 2 0.062 1 45 16.046 5 -7.338 0 12.453 2 0.062 1 55 17.809 7 -7.853 0 12.453 2 0.062 1 15 6.415 4 -4.450 5 12.729 7 0.059 8 25 7.937 2 -5.132 4 12.729 7 0.059 8 ck 35 9.570 0 -5.783 7 12.729 7 0.059 8 45 10.602 2 -6.242 3 12.729 7 0.059 8 55 12.452 8 -6.877 3 12.729 7 0.059 8 15 6.865 5 -4.612 9 12.566 5 0.060 1 25 8.981 1 -5.438 6 12.566 5 0.060 1 POB1 35 10.934 0 -6.124 9 12.566 5 0.060 1 45 12.031 2 -6.576 6 12.566 5 0.060 1 55 13.110 5 -7.017 7 12.566 5 0.060 1 15 8.031 3 -4.988 4 11.491 3 0.057 6 25 10.159 9 -5.744 1 11.491 3 0.057 6 25 POB2 35 11.667 8 -6.291 2 11.491 3 0.057 6 45 13.631 4 -6.906 7 11.491 3 0.057 6 55 14.337 3 -7.261 6 11.491 3 0.057 6 15 8.676 4 -5.173 4 11.521 1 0.058 4 25 11.261 7 -5.999 2 11.521 1 0.058 4 PB1 35 12.808 6 -6.530 1 11.521 1 0.058 4 45 14.851 5 -7.133 4 11.521 1 0.058 4 55 15.640 3 -7.498 8 11.521 1 0.058 4 15 9.587 1 -5.412 4 12.588 7 0.062 9 25 12.632 2 -6.283 7 12.588 7 0.062 9 PB2 35 14.602 3 -6.865 7 12.588 7 0.062 9 45 16.832 9 -7.464 5 12.588 7 0.062 9 55 18.409 1 -7.943 3 12.588 7 0.062 9 说明:单位DEP为mg·L-1;T为℃;ΔG为kJ·mol-1;ΔH为kJ·mol-1;ΔS为kJ·mol-1·K-1 Table 3. Thermodynamic parameters for the adsorption of Zn2+ on different treated soils
焓变ΔH值可揭示不同处理土壤对溶液中锌吸附的机理类型。结果中,各处理ΔH为8~40 kJ·mol-1,ΔH的大小可用于描述土壤对重金属离子吸附固定的机理[37],说明该吸附过程以氢键作用力为主[38],ΔH<40 kJ·mol-1,属物理吸附[39]。熵变ΔS均为正值,说明吸附过程离子混乱度增加,其原因为Zn2+与活性位点结合的过程中,可能存在其他种类的离子释放到液相中,从而导致熵增。
2.1. 生物质炭的结构表征
2.1.1. 生物质炭的扫描电镜和X射线能量图谱分析
2.1.2. 生物质炭的红外光谱分析
2.2. 不同处理土壤对Zn2+-DEP复合污染溶液中Zn2+的吸附动力学研究
2.2.1. 生物质炭以及DEP对土壤吸附Zn2+-DEP复合污染溶液中Zn2+的影响
2.2.2. 吸附时间对土壤吸附Zn2+-DEP复合污染溶液中Zn2+的影响
2.2.3. 土壤对Zn2+-DEP复合污染溶液中Zn2+的吸附动力学行为研究
2.3. 土壤吸附Zn2+-DEP复合污染溶液中Zn2+的热力学行为
-
施加猪炭和法国梧桐炭均可提高土壤对溶液中重金属的吸附效果,且随生物质炭施加量的增加吸附效果增强。DEP存在条件下,猪炭和法国梧桐炭对溶液中的重金属的吸附效果增强,且猪炭处理相较法国梧桐炭处理效果更显著。准二级动力模型可较好地描述不同处理土壤对重金属的吸附过程,添加DEP可提高各吸附时间土壤对重金属的吸附量,其吸附机制为液膜扩散和颗粒内扩散的共同作用。不同处理土壤对溶液中Zn2+的吸附为物理吸附,其相互作用力以氢键作用力为主,该吸附过程是自发的吸热反应,且高温条件更利于吸附反应的自发进行。














 DownLoad:
DownLoad: