-
竹材具有生长周期短、产量高和强度大等优点,是一种非常有潜力的可再生生物质原料[1]。通过热解技术将竹材等生物质原料制成高品质生物燃料和化学品,已成为生物质能源利用的研究热点[2-3]。综纤维素主要由纤维素和半纤维素组成,是生物质经过脱木质素和抽提物后得到的产物,其热分解机制研究有助于深入了解生物质原料的热解特性。热重红外联用技术(TG-FTIR)不仅可以掌握生物质组分的热解失重规律,还可以对挥发分气体的成分实现在线识别。Liu等[4-5]、Ren等[6]利用TG-FTIR分别对纤维素、综纤维素和木质素的热解特性进行研究,利用挥发分气体成分的特定官能团的红外特征吸收峰进行鉴定,发现其热解挥发分主要成分为一部分小分子物质如CO,CO2,CH4和H2O等,以及一些醛类、酮类、酚类和芳香族有机化合物。木材综纤维素已经得到了研究,而目前还没有竹材综纤维素热解研究报道。竹综纤维素热解过程涉及到复杂的链式反应,通过热解动力学分析,可以深入了解其热解反应机制[7]。因此,本研究以竹综纤维素为原料,采用不同的升温速率,利用TG-FTIR首先分析了竹综纤维素的热解失重规律以及挥发分气体析出特性;然后采用2种无模式函数积分法(model-free method)[8-9],即Flynn-Wall-Ozawa(FWO)法和kissinger-Akahira-Sunose(KAS)法,对其热解动力学进行研究,分析综纤维素热解活化能随转化率的变化规律。
-
竹综纤维素是通过毛竹Phyllostachys edulis脱木质素和抽提物自制获得。精确称取2.0 g毛竹粉末,用定性滤纸包好,按GB/T 2677.6-1994《造纸原料有机溶剂抽出物含量的测定》,先将原料通过苯醇抽提,抽出原料中抽提物。然后将试样包风干后,把里面的试样移入250.0 mL锥形瓶中,加入65.0 mL去离子水、0.5 mL冰醋酸和0.6 g亚氯酸钠,摇匀,扣上25.0 mL锥形瓶,置于75 ℃恒温水浴中加热1 h。之后重复上一步骤,直至试样变白,过滤、洗涤、干燥后得竹综纤维素。
-
采用元素分析仪(德国Elementar Vario EL Ⅲ)测定原料的碳、氢、氮和硫含量,氧元素采用差减法计算;采用GB/T 28731-2012《固体生物质燃料工业分析方法》对原料进行工业分析;采用微机量热仪(中国河南鹤壁仪器有限公司,ZDHW-8A)测定原料热值。采用高效液相色谱(美国安捷伦科技有限公司Agilent-1100)分析原料化学组成,测试标准为NREL 2007 Determination of Structural Carbohydrates and Lignin in Biomass。结果见表 1。
表 1 竹材综纤维素的元素分析、工业分析和组分分析
Table 1. Ultimate, proximate and chemical component analysis of bamboo holo-cellulose
元素分析 工业分析 化学组分分析 元素 百分比/% 成分 百分比/% 成分 百分比/% C 42.12 挥发分 93.56 葡聚糖 62.60 H 6.12 固定碳 6.38 木聚糖 27.90 0# 51.07 灰分 0.06 果胶糖 5.40 N 0.47 S 0.13 低位热值/(MJ.kg-1) 11.43 说明:#氧元素采用差减法得到。 热重红外联用(TG-FTIR)分析仪由热重分析仪TGA Q500(TA Instruments,美国)和傅里叶红外光谱仪Nicolet 6700(Thermo Fisher Scientific,美国)组成。实验用料约为15.0 mg·次-1,氮气流量为70.0 mL·min-1,红外光谱仪的扫描范围为4 000~400 cm-1,分辨率为4 cm-1,扫描时间约为8 s·次-1;升温速率分别为5.0,10.0,15.0,20.0和30.0 ℃·min-1,从室温升至850.0 ℃。
-
固体物质的非等温热反应速率方程(Arrhenius方程)可表示为:
$$ \frac{{{\rm{d}}\alpha }}{{{\rm{d}}\tau }}{\rm{ = }}A\exp \left( { - \frac{E}{{RT}}} \right)f\left( \alpha \right)。 $$ (1) 式(1)中:α为热分解转化率,而α可根据公式(2)计算得到:
$$ \alpha = \frac{{{m_0} - {m_\tau }}}{{{m_0} - {m_\infty }}}。 $$ (2) 式(2)中:τ为热分解时间;m0为生物质初始质量;mτ为τ时刻对应的生物质质量;m∞为热分解终温时残余物质量;A为频率因子,min-1;E为生物质反应活化能,kJ·mol-1;R为摩尔气体常数,8.314×10-3 kJ·K-1·mol-1;T为热力学温度,K;f(α)为反应机制函数。而升温速率β定义为β=dT/dτ,代入式(1)可得热分解反应动力学方程式:
$$ \frac{{{\rm{d}}\alpha }}{{{\rm{d}}T}}{\rm{ = }}\frac{A}{\beta }\exp \left( { - \frac{E}{{RT}}} \right)f\left( \alpha \right)。 $$ (3) 对(3)式求积分整理得:
$$ G\left( \alpha \right){\rm{ = }}\int_0^\alpha {\frac{{{\rm{d}}\alpha }}{{f\left( \alpha \right)}} = \frac{A}{\beta }\int_0^T {\exp \left( { - \frac{E}{{RT}}} \right)} } {\rm{d}}T。 $$ (4) 式(4)中:G(α)为机制函数f(α)的积分形式。
-
根据公式(1)~式(4),可以采用不同的动力学模型对生物质的热解动力学进行研究,本研究采用FWO和KAS积分法对综纤维素的热解机制进行研究。活化能(E)是转化率(α)的函数。FWO和KAS积分法分别用式(5)和式(6)表示。
$$ lg{\beta _i} = \lg \frac{{AE}}{{RG\left( {{\alpha _{ij}}} \right)}} - 2.315 - 1.4567\frac{E}{{R{T_{ij}}}}。 $$ (5) $$ \ln \left( {\frac{{{\beta _i}}}{{T_{ij}^2}}} \right) = \ln \frac{{AR}}{{EG\left( {{\alpha _{ij}}} \right)}} - \frac{E}{{R{T_{ij}}}}。 $$ (6) $$ \left. {\begin{array}{*{20}{c}} {{\beta _1}:{T_{11}},{T_{12}}, \cdots ,{T_{1{k_1}}}}\\ {{\alpha _{11}},{\alpha _{12}}, \cdots ,{\alpha _{1{k_1}}}}\\ {{\beta _2}:{T_{21}},{T_{22}}, \cdots ,{T_{2{k_2}}}}\\ {{\alpha _{21}},{\alpha _{22}}, \cdots ,{\alpha _{2{k_2}}}}\\ {{\beta _L}:{T_{L1}},{T_{L2}}, \cdots ,{T_{Lk{i_L}}}}\\ { \cdots \cdots }\\ {{\alpha _{L1}},{\alpha _{L2}}, \cdots ,{\alpha _{L{k_L}}}} \end{array}} \right\}。 $$ (7) 式(7)中:Tij为αij(i=1,2,…,L;j=1,2,…,ki)时相应的温度,ki为升温速率为βj时的取值数量。在不同的升温速率βj时,取相同的转化率αij为常数,因此G(αij)也是常数。所以,lg(βj)和1/Tij以及ln(βj /T2)和1/Tij为线性关系。经过线性拟合后,活化能E可以根据式(5)和式(6)的斜率-0.456 7E/R和E/R求解得到。本研究采用5个升温速率(5.0,10.0,15.0,2.0和30.0 ℃·min-1)来求解活化能,以期获得更加稳定可靠的活化能值。
-
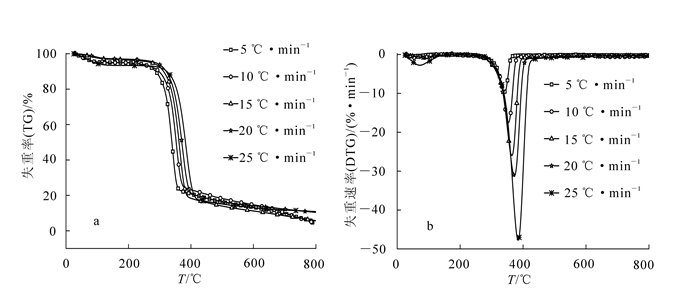
图 1为竹材综纤维素在不同升温速率条件下(5.0,10.0,15.0,20.0和30.0 ℃·min-1)的失重率TG(a)和失重速率DTG(b)曲线。从图 1可以看出:竹材综纤维素热解过程包括3个阶段。第1阶段为干燥阶段,温度范围为室温~150.0 ℃,失重率为2.8%~5.9%,这阶段主要是原料中自由水和结合水的蒸发[10]。第2阶段为快速裂解阶段,温度范围为150.0~452.0 ℃,主要是半纤维素和纤维素的降解,失重率为68.7%~76.2%,不同升温速率此阶段温度范围有所不同。第3阶段为慢速裂解阶段,温度范围为453.0~800.0 ℃,剩余的纤维素和第2阶段热解产生的炭发生进一步降解,失重率为5.5%~19.3%。

图 1 不同升温速率下竹材综纤维素(TG-DTG曲线
Figure 1. TG (a) and DTG (b) cuvers of bamboo holo-cellulose with different heating rates
从图 1可以看出:随着升温速率增加,TG-DTG曲线往高温区域移动。原因是竹材原料试样的导热系数低,热重分析仪记录的坩埚的温度与试样本身的温度有偏差。另外,受试样传质传热的影响,试样颗粒会形成温度梯度,形成热滞后现象,升温速率越高,热滞后越明显[11]。
-
图 2为竹材综纤维素在升温速率10.0 ℃·min-1时的三维红外光谱图。从图 2可以看出:综纤维素热解过程中挥发分主要吸收峰出现在29~43 min时间范围内,与其TG/DTG曲线失重规律吻合,为快速热解阶段。

图 2 竹材综纤维素热解的三维FTIR图
Figure 2. Typical 3D-FTIR spectra of gas products from bamboo holo-cellulose pyrolysis
图 3a为3个典型温度点(热解起始点297.5 ℃,热解峰325.1 ℃和热解接近结束点337.5 ℃)时挥发分的成分分析,图 3b为一些典型热解产物随温度的变化趋势。从图 3可以看出:温度对热解挥发分有明显的影响。在初始热解温度297.5 °C时,所有挥发产物特征峰都已经开始出现,并且在325.1 °C时浓度达到最大值,之后逐渐下降。

图 3 竹材综纤维素热解挥发产物的鉴定(a)以及单种挥发分浓度随温度升高的变化规律(b)
Figure 3. FTIR spectra for validation of the volatile components and the evolution of the concentration in each component in pyrolysis process of bamboo holo-cellulose
以热解失重率最大处时(325.1 ℃)为例,对其挥发分成分进行分析。一些小分子的物质如H2O,CH4,CO2和CO,因其特征吸收峰特别明显,很容易进行判别。4 000~3 400 cm-1为羟基O—H伸缩振动,代表的物质是过氧化氢(H2O)[12]。3 000~2 700 cm-1为C—H键伸缩振动,主要是甲基(—CH3)和亚甲基团(—CH2—),代表的成分是CH4 [5]。2 400~2 250 cm-1区间为CO伸缩振动,代表的是二氧化碳(CO2)。紧靠2 400~2 250 cm-1右侧的波数为2 250~2 000 cm-1处的是一氧化碳(CO)的不显著的特征吸收峰[6]。
另一个显著的特征吸收峰在1 900~1 650 cm-1处,为羰基CO伸缩振动吸收峰,可能的化学物质为醛、酮、酸等。1 690~1 460 cm-1处主要为CC伸缩振动及苯环的骨架振动区。由于在1 600 cm-1,1 580 cm-1和1 500 cm-1处都有吸收峰,因此可判断此区域存在单核的芳环类化合物。1 475~1 000 cm-1为指纹区的一部分,包括C—H键面内弯曲和C—O键伸缩振动等,特征吸收峰相互重叠,很难分辨出单种物质具体成分。然而通过官能团对应的特征吸收峰,可以对其进行类别的分析,如1 460~1 365 cm-1主要是甲基(—CH3)和亚甲基团(—CH2—)的特征吸收峰,可能为烷烃类物质;其他部分,1 200~1 000 cm-1为醇类,1 300~1 200 cm-1为酚类物质,1 275~1 060 cm-1为醚类物质,1 300~1 050 cm-1为脂类物质[13]。
经过以上分析,对其挥发分成分进行分析后,单种挥发分成分随温度的变化趋势如图 3b所示。根据Lambert-Beer定律,处于特定波数物质吸光度数值的大小代表了该物质浓度的高低,因此,吸光度的变化反映出整个热解过程中挥发分气体的浓度变化趋势[12]。气体的产生主要在250.0~450.0 ℃范围内,297.0~325.0 ℃各类挥发分产物浓度随着温度升高而增加,325.0~337.0 ℃各类挥发分产物浓度随着温度升高而减少,之后逐渐降低至0。
-
在1.3.2中说明了,竹综纤维素的活化能值的计算方法,即根据不同升温速率时,同一转化率(α)下对应的温度(T)与升温速率(β)的拟合曲线的斜率来计算。活化能代表的是化学反应发生所需的最低能量,因此活化能越高证明反应发生越困难或者反应速率越低。图 4为竹综纤维素转化率在0.15~0.80范围(间隔0.05)的线性拟曲线,可以看出线性拟合相关系数高于0.98。由于转化率在0.15~0.80范围以外的拟合曲线的线性相关系数太低,故本研究没有采用。

图 4 转化率为0.15~0.80的FWO法(a)和KAS法(b)Arrhenius曲线
Figure 4. Arrhenius plots of FW0 (a) and KAS (b) methods for the conversion rate of 0.15-0.80
活化能随转化率的变化规律如图 5所示。从整体上看活化能值随着转化率的增加而上下浮动,说明竹综纤维素在热解过程中发生了复杂的化学反应,包括重叠、平行和连续反应等。因此,必须对其进行分段讨论,进一步解释其热解反应机制。另外,FWO法和KAS法计算得到的活化能变化趋势一样,两者偏差在5.0%以内。较小的偏差来自于2种方法采用了不同的近似解,FWO法采用的是Doyle近似解lg(P(u)≈-2.315+0.457u,而KAS法采用的近似解为P(u)≈u-2e-u。2种无函数模式法获得的活化能基本一致,说明获得的活化能可靠性。

图 5 FWO和KAS法活化能随转化率增加变化规律
Figure 5. Activation energy distribution dependence on the conversion rate using FWO and KAS methods
根据表 1可知:竹综纤维素主要由纤维素(葡聚糖为代表)和半纤维素(木聚糖和果胶糖为代表)组成,以纤维素为主,占62.5%,半纤维素含量为33.3%。由图 5可以看出:热解第1阶段,0.15<α<0.25,活化能逐渐升高,FWO法活化能为126~133 kJ·mol-1,KAS法活化能为123~130 kJ·mol-1。这一阶段主要是半纤维素的降解,键的断裂首先发生在键能较弱的支链部位,所需的能量较低,因此活化能值也较低;然后半纤维素主链开始发生自由断裂,使得活化能值增加[14]。热解第2阶段,0.25<α<0.80,活化能先出现轻微降低,最后突然快速增加,FWO法活化能为134~151 kJ·mol-1,KAS法活化能为129~148 kJ·mol-1。这一阶段主要是纤维素的降解,初始阶段活化能比中间段高,根据Broido-Shafizadeh提出的纤维素热解模型,纤维素热解首先产生中间产物活性纤维素,聚合度降低,分子链长度减少[15-16]。之后,随着温度升高,活性纤维素开始降解,但此时随着聚合度降低,热降解更易发生,因此活化能跟初始阶段比有所降低。临近纤维素热解结束时,活化能突然快速增加,可能是纤维素表面热解固体产物焦炭含量逐渐增加引起的[17]。一方面炭的反应活性较低,另一方面此时热降解反应主要取决于扩散反应区扩散速度,而随着可降解物质(挥发分)的减少,反应速率急剧降低,造成活化能快速增加[18]。
-
竹材综纤维素热分解可分为干燥、快速裂解和慢速裂解等3个阶段;随着升温速率增加,TG/DTG曲线往高温一侧移动;热解挥发分主要由小分子CO,H2O,CH4和CO2,以及一些醛类、酮类、酸类、烷烃、醇类和酚类等有机物组成。竹综纤维素热解过程发生复杂的化学反应,包括多重、平行和连续反应,使得FWO和KAS积分法计算得到的活化能随着转化率升高而变化。当0.15<α<0.25,活化能逐渐升高,主要是半纤维素的降解,热裂解首先发生在键能较弱的支链部位,然后半纤维素主链开始发生自由断裂;当0.25<α<0.80,此阶段主要是纤维素的降解,纤维素热解首先产生中间产物活性纤维素,活化能轻微降低,随着反应的进行,纤维素表面热解固体产物焦炭含量逐渐增加致使活化能迅速增加。
Pyrolysis characteristics and kinetics study of bamboo holo-celluloce using TG-FTIR
-
摘要: 利用热重红外联用技术(TG-FTIR)研究了竹材综纤维素在不同升温速率下(5.0, 10.0, 15.0, 20.0和30.0℃·min-1)的热解特性和热解动力学。热重分析/热重一次微分曲线(TG/DTG)表明:竹材综纤维素热解可分为干燥、快速裂解和慢速裂解等3个阶段; 随着升温速率增加, TG/DTG曲线往高温一侧移动; 竹综纤维素热解过程发生复杂的化学反应, 包括多重、平行和连续反应; 热解挥发分主要由小分子CO, H2O, CH4和CO2, 以及一些醛类、酮类、酸类、烷烃、醇类和酚类等有机物组成。利用无模式函数积分法, 即Flynn-Wall-Ozawa(FWO)和Kissinger-Akahira-Sunose(KAS)积分法, 对其热解动力学进行研究。结果表明:竹材综纤维素的活化能随着转化率的升高先增大后减小再增大, 活化能数值的变化与纤维素/半纤维素不同的热解特性有紧密联系。Abstract: The pyrolysis characteristic and kinetics of bamboo holo-celluloce with different heating rate (5.0, 10.0, 15.0, 20.0 and 30.0℃·min-1) were investigated using TG-FTIR. TG/DTG curves showed that the pyrolysis process of bamboo holo-celluloce was divided into three stages named drying, fast degradation and slow degradation. With the increase of heating rate, the TG/DTG curves moved toward the side of higher temperature region. And the main components of volatiles were small moleculars, CO, H2O, CH4 and CO2, also along with kinds of organics, such as aldehydes, ketones, organic acids, alkanes, alcohols, phenols. The fluctuation of activation energy can be interpreted as a result of multiple, parallel and simultaneous reaction occoured in the pyrolysis process. The Model-free methods, namely Flynn-Wall-Ozawa (FWO) integral method and Kissinger-Akahira-Sunose(KAS) integral method, were used to research the pyrolysis kinetics. The results showed that with increasing conversion rate, the activation energy of bamboo holocellulose first increased, next decreased and then increased. These changes of activation energy were determined by the pyrolysis characteristics of cellulose and hemicellulose.
-
Key words:
- wood science and technology /
- bamboo /
- holo-cellulose /
- pyrolysis /
- kinetics /
- TG-FTIR
-
表 1 竹材综纤维素的元素分析、工业分析和组分分析
Table 1. Ultimate, proximate and chemical component analysis of bamboo holo-cellulose
元素分析 工业分析 化学组分分析 元素 百分比/% 成分 百分比/% 成分 百分比/% C 42.12 挥发分 93.56 葡聚糖 62.60 H 6.12 固定碳 6.38 木聚糖 27.90 0# 51.07 灰分 0.06 果胶糖 5.40 N 0.47 S 0.13 低位热值/(MJ.kg-1) 11.43 说明:#氧元素采用差减法得到。 -
[1] JIANG Zhehui, LIU Zhijia, FEI Benhua, et al. The pyrolysis characteristics of moso bamboo[J]. J Anal Appl Pyrolysis, 2012, 94:48-52. [2] MA Longlong, WANG Tiejun, LIU Qiying, et al. A review of thermal-chemical conversion of lignocellulosic biomass in China[J]. Biotechnol Adv, 2012, 30(4):859-873. [3] MA Zhongqing, ZHANG Yimeng, ZHANG Qisheng, et al. Design and experimental investigation of a 190 kW(e) biomass fixed bed gasification and polygeneration pilot plant using a double air stage downdraft approach[J]. Energy, 2012, 46(1):140-147. [4] LIU Qian, ZHONG Zhaoping, WANG Shurong, et al. Interactions of biomass components during pyrolysis:a TG-FTIR study[J]. J Anal Appl Pyrolysis, 2011, 90:213-218. [5] LIU Qian, WANG Shurong, ZENG Yun, et al. Mechanism study of wood lignin pyrolysis by using TG-FTIR analysis[J]. J Anal Appl Pyrolysis, 2008, 82:170-177. [6] 任学勇, 王文亮, 白甜甜, 等.热重红外光谱法考察木质生物质综纤维素热转化特性[J].光谱学与光谱分析, 2013, 33(9):2392-2397. REN Xueyong, WANG Wenliang, BAI Tiantian, et al. TG-FTIR study of the thermal-conversion properties of holo-cellulose derived from woody biomass[J]. Spectrosc Spect Anal, 2013, 33(9):2392-2397. [7] VYAZOVKIN S, BURNHAM A K, CRIADO J M, et al. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data[J]. Thermochim Acta, 2011, 520(1/2):1-19. [8] VYAZOVKIN S. Modification of the integral isoconversional method to account for variation in the activation energy[J]. J Comput Chem, 2001, 22(2):178-183. [9] ZHAO Hui, YAN Huaxiao, DONG Shuangshuang, et al. Thermogravimetry study of the pyrolytic characteristics and kinetics of macro-algae Macrocystis pyrifera residue[J]. J Therm Anal Calorim, 2013, 111(3):1685-1690. [10] 陈登宇, 朱锡锋.生物质热反应机理与活化能确定方法(Ⅰ)干燥段研究[J].燃料化学学报, 2011, 39(8):580-584. CHEN Dengyu, ZHU Xifeng. Thermal reaction mechanism of biomass and determination of activation energy(Ⅰ) drying section[J]. J Fuel Chem Technol, 2011, 39(8):580-584. [11] AGRAWAL A, SAIKAT C. A kinetic study of pyrolysis and combustion of microalgae Chlorella vulgaris using thermo-gravimetric analysis[J]. Bioresour Technol, 2013, 128:72-80. [12] GRANADA E, EGUIA P, VILAN J A, et al. FTIR quantitative analysis technique for gases:application in a biomass thermochemical process[J]. Renewable Energy, 2012, 41:416-421. [13] YANG Haiping, YAN Rong, CHIN Terence, et al. Thermogravimetric analysis-Fourier transform infrared analysis of palm oil waste pyrolysis[J]. Energy Fuels, 2004, 18(6):1814-1821. [14] LOPEZ-VELAZQUEZ M A, SANTES V, BALMASEDA J, et al. Pyrolysis of orange waste:a thermo-kinetic study[J]. J Anal Appl Pyrol, 2013, 99:170-177. [15] BROIDO A, NELSON M A. Char yield on pyrolysis of cellulose[J]. Combust Flame, 1975, 24:263-268. [16] BRADURY A G W, SAKAI Y, SHAFIZADEH F. A kinetic model for pyrolysis of cellulose[J]. J Appl Polym Sci, 1979, 23(11):3271-3280. [17] 陈登宇, 朱锡锋.生物质热反应机理与活化能确定方法(Ⅱ)热解段研究[J].燃料化学学报, 2011, 39(9):670-674. CHEN Dengyu, ZHU Xifeng. Thermal reaction mechanism of biomass and determination of activation energy(Ⅱ) drying section[J]. J Fuel Chem Technol, 2011, 39(9):670-674. [18] CHEN Dengyu, ZHENG Yan, ZHU Xifeng. In-depth investigation on the pyrolysis kinetics of raw biomass part (Ⅰ) kinetic analysis for the drying and devolatilization stages[J]. Bioresour Technol, 2013, 131:40-46. -

-
链接本文:
https://zlxb.zafu.edu.cn/article/doi/10.11833/j.issn.2095-0756.2014.04.001






 下载:
下载: