-
磷是植物生长和发育的必需营养元素,通过多种途径参与植物的代谢过程,对植物的生长发育起到关键作用[1]。在植物生长发育过程中,植物体所需的养分、水分主要通过植物根部进行吸收和运输供应。根部作为直接接触土壤或基质的器官,在低磷逆境中最先受到胁迫[2]。为了适应低磷环境,植物根在进化过程中形成了多种调节磷的吸收以及平衡策略。例如,植物根系会分泌大量的酸性磷酸酶与有机酸,其通过根系,降低土壤的pH,使植物在低磷的土壤中能够活化、动员有机磷[3],进而提高了有效磷质量分数,促进了植物对土壤中磷的吸收;并且,酸性磷酸酶还可以促进植物体内的磷脂化合物发生水解,并促进植物体内磷的循环,促进有机磷的重复利用[4]。
在正常情况下,植物体细胞内活性氧(ROS)的产生和清除处于一种相对稳定的平衡状态[5],但在低磷胁迫时,ROS原有的状态被打破,其过量产生会使植物细胞发生膜脂过氧化,并且生成有害物质,破坏细胞膜的结构并影响其功能[6]。ROS的增加导致丙二醛(MDA)过量生产,MDA作为植物细胞膜脂过氧化的产物之一,其含量高低可以反映膜脂过氧化的水平以及细胞膜的损伤程度,可视为植物抗逆性的重要指标。为了消除活性氧对植物造成的伤害,植物自身进化出了一系列措施,包括超氧化物歧化酶(SOD)、过氧化物酶(POD)、过氧化氢酶(CAT)等主要的抗氧化酶等协同作用的抗氧化体系,从而维持了植物细胞膜的稳定性,提高了植物低磷胁迫下的生理抗性;同时,植物还会通过提高根系活力等共同参与调节,以应对低磷胁迫所带来的损伤[7]。
马尾松Pinus massoniana是重要的用材树种,主要分布在中国的亚热带和热带地区,占中国森林总面积的3.6%,是中国亚热带地区荒坡造林的主要先锋树种。马尾松适应能力强,具有耐干旱、耐瘠薄、速生丰产等特点,同时在保持水土、涵养水源、维持区域生态平衡等方面发挥巨大作用[8−9]。然而,在中国南方热带和亚热带地区的酸性土壤中,磷易与铁、铝等金属元素及土壤黏粒等通过吸附、固定等方式保持不溶形式,使土壤中有效磷转化为难溶性磷,最终加重土壤磷对植物的限制[10],因此,中国南方黄红壤普遍存在pH低、有效磷缺乏等问题[11]。近年来,大气氮沉降逐渐加剧,土壤环境中的氮素有效性随之增加,造成原有低磷土壤中的有效磷水平相对更低,这不但扰乱了土壤的养分平衡,还使得植物对土壤有限磷素的吸收和利用受到影响[12−14]。
结合全国土壤调查和全国第2次土壤普查养分分级标准得知:土壤磷素在马尾松人工林中存在严重亏缺,马尾松林地土壤有效磷在0~20和20~40 cm土层中分别处于缺和极缺状态[15−16]。对全国马尾松林调查发现:大部分地区马尾松并未表现出缺磷症状并且生长依旧良好,这说明马尾松对低磷胁迫的适应有应对机制[17]。而马尾松幼苗对低磷胁迫研究所设置的磷质量分数多高于马尾松生长的实际磷质量分数,如谢钰容等[18]对不同种源的马尾松盆栽低磷胁迫的研究中设置0~100 mg·kg−1的有效磷添加;唐敏[19]对不同种源马尾松种子及幼苗的低磷胁迫响应中设置磷质量分数为0~20 mg·kg−1;徐向华等[17]对马尾松低磷胁迫下生理生化响应研究中设置磷质量分数为0~31 mg·kg−1。以上研究相较于大量样地调查(马尾松分布区14个省32个研究地点113个样地)和文献资料分析所得的磷质量分数(2.250 0 mg·kg−1)要高很多[16]。鉴于此,本研究选择易控制的马尾松幼苗为对象,通过研究幼苗根系生理变化和根抗氧化酶系统的响应,探究马尾松应对低磷胁迫的生理生化特征,以期为阐明其耐低磷的机制提供支持。
-
研究地点位于湖北省宜昌市秭归县湖北长江三峡库区(秭归)森林生态系统国家定位观测研究站(30°53′N,110°54′E,海拔296 m),该区属亚热带大陆性季风气候,年均气温为19.0 ℃。
2021年5月1日,选择苗高、地径长势基本一致的2年生马尾松幼苗(苗高为61.0 cm,地径为5.4 mm)移栽入直径16.5 cm,高17.0 cm的花盆中,按m(石英砂)∶m(蛭石)∶m(珍珠岩)=7∶2∶1的比例混合作为幼苗培养基质。移栽后的马尾松幼苗放入透明遮雨棚中,1个月内用Hoagland营养液培养,进行缓苗处理,1个月后对苗木进行不同磷处理。根据中国马尾松林土壤肥力特征调查所获得的马尾松林0~20 cm土层土壤有效磷质量分数的中位数(2.250 0 mg·kg−1)作为对照(ck)[16],将磷酸二氢钾(KH2PO4)配制成不同浓度的溶液,用氯化钾(KCl)调整使钾离子(K+)浓度一致,用稀盐酸(HCl)调整氯离子(Cl−)浓度,使营养液pH为5.5,并保持其他营养元素浓度相同。每隔3 d,将40 mL营养液沿基质表层幼苗茎干浇下,共浇灌5次,营养液浇灌完成时间为6月15日。使马尾松幼苗盆栽基质中的有效磷质量分数分别为:0 (P0)、0.562 5 (P1)、1.125 0 (P2)、2.250 0 (ck)、4.500 0 (P3)、9.000 0 mg·kg−1 (P4),每个处理15盆,进行相同光照和水分条件管理。
-
在磷处理完成后,结合马尾松生长季节(3月下旬至10月上旬)[20],分别于处理后1.5个月(8月1日)和3个月(9月16日),从每个处理中随机选取3株马尾松整株幼苗,共计收获36株。采集时,将植株用剪刀从根茎交界处剪取地上部、地下部,取根系用清水冲洗干净并轻轻擦去表面水分,以备后续生理生化指标测定。
-
采用北京索莱宝科技有限公司生产的试剂盒对酸性磷酸酶(ACP)活性、丙二醛(MDA)质量摩尔浓度、过氧化物酶(POD)活性、超氧化物歧化酶(SOD)活性、过氧化氢酶(CAT)活性进行测定。取根鲜样剪碎混匀,用万分之一天平称取0.100 0 g的样品,将样品放入研钵中,加入1 mL提取液在冰上研磨至匀浆,4 ℃下离心10 min,ACP离心转速为10 000 r·min−1,MDA、SOD、POD、CAT离心转速为8 500 r·min−1,取上清液处理后于酶标仪并计算,酶活性单位为16.67 nkat·g−1,MDA质量摩尔浓度单位为nmol·g−1。根系活力采用氯化三苯基四氮唑(TTC)法测定[21],有机酸采用酸碱滴定法测定[22]。
-
数据采用Excel 2016和SPSS 26统计分析软件进行处理。运用双因素方差分析比较不同磷质量分数和不同采样时间及其交互作用对马尾松幼苗根系的ACP酶活性、有机酸质量摩尔浓度、根系活力、MDA质量摩尔浓度、SOD活性、POD活性、CAT活性的影响;用单因素(ANOVA)和多重比较(LSD, α=0.05)分析不同磷质量分数处理间生理生化指标的差异;用t检验比较2个采样时间生理生化指标的差异;用Pearson相关系数分析各生理生化指标间的相关性。所有处理在SPSS 26中进行,用Origin 2022软件作图。
-
由图1A可知:不同磷质量分数处理下马尾松幼苗根ACP活性在不同采样时间差异极显著(P<0.01)。2个采样时间的马尾松幼苗根ACP活性随磷质量分数的增加而降低。在8月,P1、P2、P3处理的ACP活性与ck相比均无显著差异;P0处理的ACP活性显著高于ck (P<0.05),是ck的1.45倍;P4处理的ACP活性显著低于ck (P<0.05),相较ck降低了60.92%。而随着胁迫处理时间的增加,9月P3和P4处理的ACP活性均显著低于ck (P<0.05),而P0、P1、P2处理与ck无显著差异。

图 1 不同磷质量分数下马尾松幼苗根ACP活性和有机酸质量摩尔浓度
Figure 1. ACP activity and organic acid content in root of P. massoniana seedlings in different phosphorus concentrations
由图1B可知:不同磷质量分数下马尾松幼苗根有机酸质量摩尔浓度存在显著差异(P<0.05),而不同采样时间差异不显著,且磷质量分数与采样时间对有机酸质量摩尔浓度无交互影响。其中,P2、P3处理的根有机酸质量摩尔浓度与ck差异不显著;P0、P1处理的有机酸质量摩尔浓度显著高于ck (P<0.05),分别是ck的1.25、1.14倍,P4处理的有机酸质量摩尔浓度显著低于ck (P<0.05),相比于ck降低了12.53%。整体而言,根系有机酸总量随着磷质量分数的增加呈下降趋势。说明在低磷条件下,马尾松幼苗根系可通过自身调节提高ACP活性,增加有机酸质量摩尔浓度以提高对磷的利用效率,从而适应低磷胁迫环境。
-
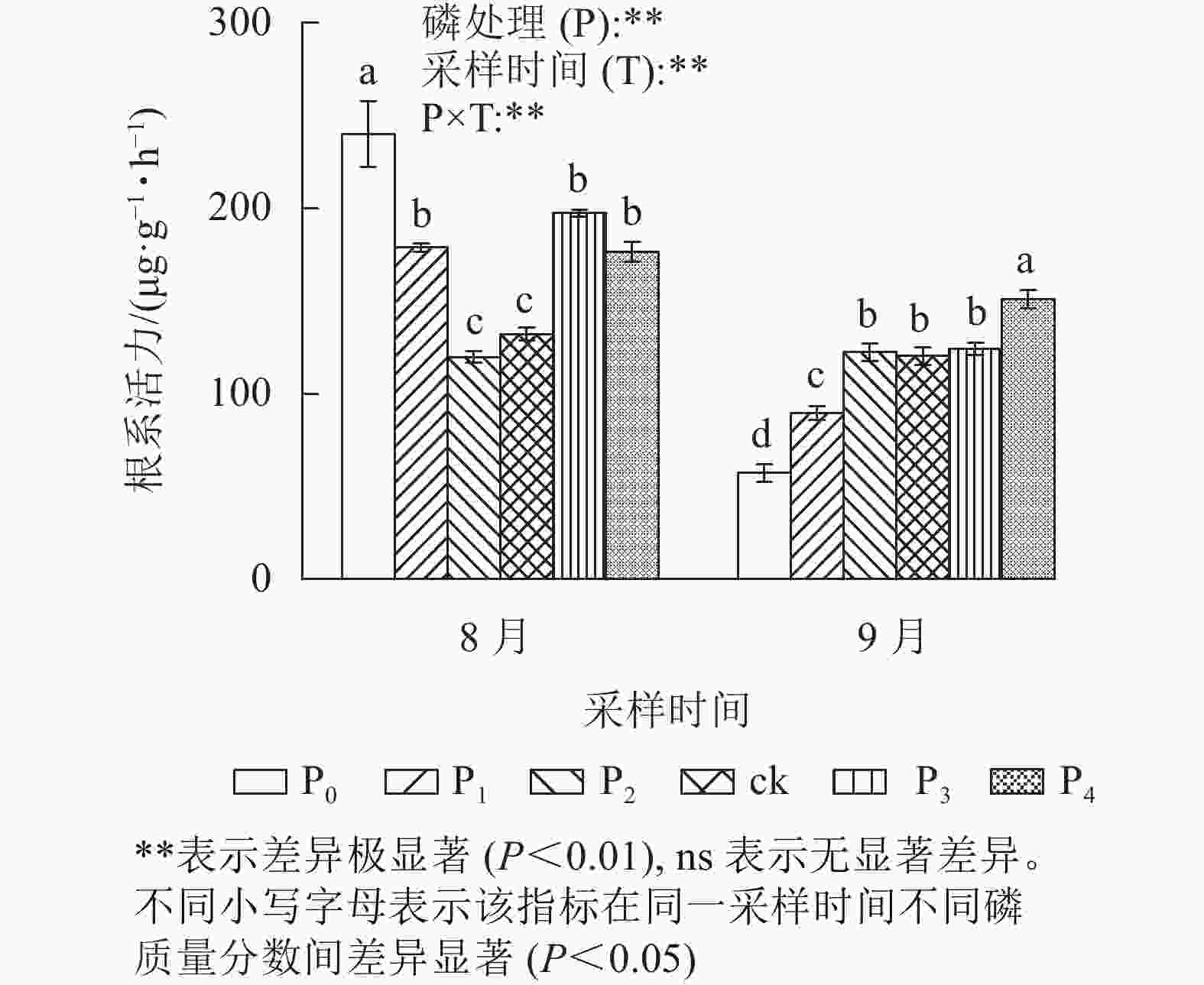
由图2可见:不同磷质量分数处理间的马尾松幼苗根系活力在不同采样时间下存在极显著差异(P<0.01)。其中:在8月,ck处理的马尾松幼苗根系活力与P2处理无显著差异;P0、P1、P3、P4处理的根系活力显著高于ck (P<0.05),分别是ck的1.82、1.35、1.49、1.34倍。而在9月,ck处理与P2、P3处理差异不显著;P0和P1处理的根系活力显著低于ck (P<0.05);仅P4处理根系活力显著高于ck (P<0.05),是ck的1.25倍。在9月,马尾松幼苗根系活力表现出随磷质量分数的升高而增强。综上所述,在低磷胁迫前期,根系活力虽然保持较高水平,但随着胁迫时间的增加,根系活力急剧下降。
-
MDA是植物经受逆境情况下产生的,是一种广泛使用的损伤标志物,其质量摩尔浓度随胁迫程度发生变化[23]。从图3A可以看出:马尾松幼苗根MDA质量摩尔浓度在不同磷质量分数间和不同采样时间下存在极显著差异(P<0.01)。ck处理的MDA质量摩尔浓度与P1、P2、P3处理无显著差异,马尾松幼苗根MDA质量摩尔浓度随着磷质量分数的降低呈不同程度的增加,尤其在P0处理下,MDA质量摩尔浓度显著高于ck (P<0.05),是ck的1.37倍,P4处理则显著低于ck (P<0.05),相比于ck降低了18.98%。同时,随着时间的推移,MDA质量摩尔浓度也明显增加,9月马尾松幼苗根MDA质量摩尔浓度显著高于8月(P<0.05),涨幅为21.34%。由此可见,缺磷导致MDA积累增加,植物损伤程度增强,且随着胁迫时间增加,MDA也不断积累。MDA积累是马尾松幼苗应答低磷环境的生理变化之一。
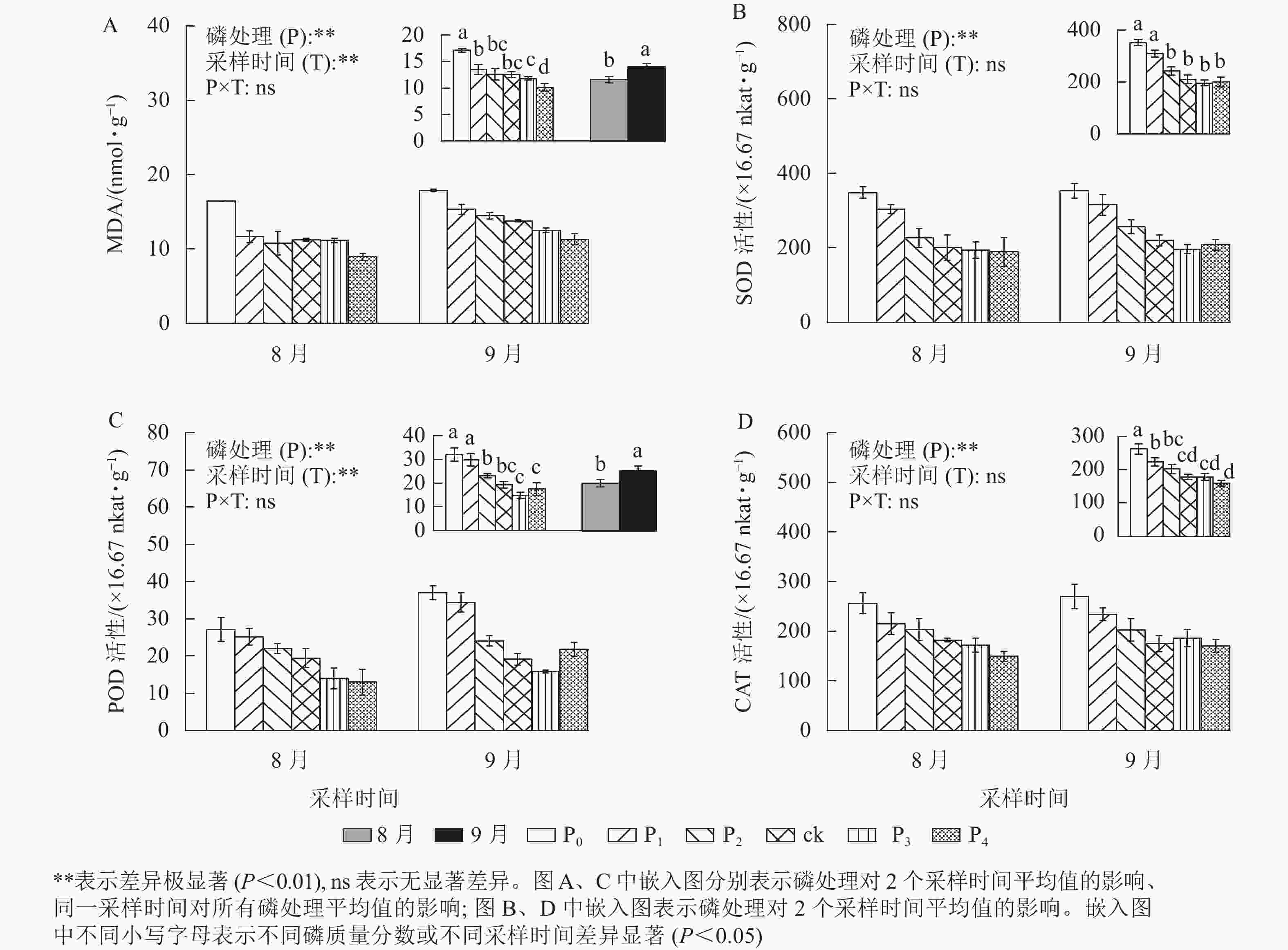
图 3 不同磷质量分数下马尾松幼苗根MDA质量摩尔浓度及SOD、CAT、POD活性差异
Figure 3. MDA content and the SOD、CAT and POD activity in root of P. massoniana seedlings in different phosphorus concentrations
不同磷质量分数处理下的马尾松幼苗根SOD、CAT和POD活性均存在极显著差异(图3,P<0.01),MDA质量摩尔浓度以及SOD、CAT、POD活性随着磷质量分数的增加而降低。P2、P3、P4处理的SOD、POD、CAT活性均与ck无显著差异,P0、P1处理的SOD、POD、CAT活性显著高于ck (P<0.05)。其中:P0、P1处理的SOD活性分别是ck的1.67、1.47倍(图3B);相较于ck处理,P0、P1处理的POD活性分别增加了66.11%和54.43%(图3C);P0、P1处理的CAT活性分别是ck的1.47、1.26倍(图3D)。
同时,随着处理时间的增加,MDA质量摩尔浓度和POD活性显著增加了21.34%、26.15%(P<0.05)。SOD、CAT活性在不同采样时间无差异,而POD活性在9月显著高于8月(P<0.05),是8月的1.26倍。上述结果说明马尾松幼苗可通过提高抗氧化酶的活性来应对低磷胁迫。
-
由表1可知:马尾松幼苗根ACP活性与有机酸质量摩尔浓度相关系数为0.472,呈极显著正相关(P<0.01),即有机酸质量摩尔浓度的增加有利于ACP活性的提高;MDA质量摩尔浓度与SOD、POD、CAT活性相关系数分别为0.695、0.694、0.712,呈极显著正相关(P<0.01),即在低磷胁迫下膜脂过氧化产物MAD质量摩尔浓度增加,使细胞膜受到破坏,SOD、POD、CAT酶活性增强以适应低磷胁迫的损伤;根系活力与ACP活性呈显著正相关(P<0.05),与POD活性呈极显著正相关(P<0.01)。
表 1 不同磷质量分数下马尾松幼苗根生理变化指标的Pearson相关性分析
Table 1. Pearson correlation analysis of the physiological indexes of P. massoniana seedlings under different phosphorus concentrations
指标 有机酸总量 根系活力 MDA SOD活性 POD活性 CAT活性 ACP活性 0.472** 0.392* 0.215 0.466** 0.287 0.415* 有机酸总量 1 −0.190 0.702** 0.658** 0.577** 0.640** 根系活力 1 −0.308 −0.095 −0.432** −0.162 MDA 1 0.695** 0.694** 0.712** SOD活性 1 0.670** 0.671** POD活性 1 0.672** 说明:*表示相关显著(P<0.05);**表示相关极显著(P<0.01) -
在逆境中,植物的根部是最先受到环境胁迫影响的器官,而在植物对低磷环境的响应中,ACP与根的联系最紧密,可以促进根有机磷的重复利用[24]。如ZAHEER等[25]研究认为:植物的ACP活性可衡量植物生理的磷营养情况;程丽莉等[26]对落叶松Larix gmelinii幼苗的研究发现:低磷胁迫下,幼苗根组织内ACP活性增加。本研究也发现:在处理1.5个月后,马尾松幼苗根ACP活性随磷胁迫程度的增加而增强,说明幼苗为满足自身生长对磷元素的需要,通过提高根中ACP活性以增强体内磷的利用效率[27]。但是随着胁迫时间的推移,幼苗生长自身对磷元素的需求有所差异,使得各磷质量分数根ACP活性的差异在2个采样时间有所不同。此外,为维持植物体内有效磷水平,根需要保持旺盛的有机酸分泌活动。俞元春等[28]研究表明:缺磷胁迫下杉木Cunninghamia lanceolata和马尾松苗木根系有机酸分泌量显著增加。在本研究中,马尾松幼苗根有机酸分泌量随着磷质量分数的增加而下降,且有机酸质量摩尔浓度与ACP活性呈极显著正相关,这说明有机酸质量摩尔浓度的增加有利于ACP活性的增强。在磷胁迫下,马尾松幼苗通过提高根ACP活性和增加有机酸分泌量来提高磷的利用效率,进而适应低磷环境。
在逆境条件下,根系活力是表征植物抵御逆境胁迫能力高低的重要生理指标,会直接影响植物的生长状况。研究表明:低磷胁迫下根系活力会降低[29−30]。如崔博文[31]对7个种源马尾松幼苗研究发现:其中4个种源在轻度、中度低磷胁迫时,其根系活力较对照升高,而当低磷胁迫达到重度时,根系活力下降;另外3个种源根系活力随磷胁迫程度增加而降低。然而本研究中,第2次采样时根系活力随磷质量分数的降低而降低,这与上述研究结果一致,说明随磷胁迫程度的增加,根系活力显著下降。但是在处理仅为2个月,即第1次采样时,无磷处理根系活力显著高于ck与其他处理,这可能是在低磷胁迫初期,马尾松幼苗为满足自身生长对磷元素的需求,通过提高对基质中磷的吸收能力,促进根系生长,以此提高根系活力[32-35]。
活性氧的产生和清除在植物正常代谢中处于一个动态平衡的状态,而逆境会导致细胞内活性氧含量上升,并破坏植物膜系统[36]。活性氧的积累会引起膜脂过氧化,MDA作为植物细胞膜脂过氧化的产物之一,在一定程度上能够反映植物体的受害程度[37−38]。MDA的积累会刺激植物保护酶(SOD、POD、CAT)系统活性的提高,通过这些保护酶之间相互协调,加快清除MDA,保持稳定平衡的状态。如于姣妲等[39]对低磷胁迫下杉木的生理响应研究发现:杉木幼苗的根系和叶片会通过改变SOD、POD和CAT 3种保护酶活性来抑制MDA的形成,从而降低MDA对细胞膜系统的破坏。本研究也发现:不同磷质量分数处理下MDA质量摩尔浓度与SOD活性、POD活性和CAT活性均显著正相关,且随着磷质量分数的降低,MDA质量摩尔浓度与SOD活性、POD活性、CAT活性均呈上升趋势,这与乔光等[32]对不同种源马尾松幼苗低磷胁迫的生理响应结果一致。与此同时,随着处理时间的增加,第2次采样时间的根MDA质量摩尔浓度和POD活性均显著高于第1次采样,表明马尾松细胞膜受到的破坏随磷胁迫程度和时间的推移而加重,马尾松幼苗根受到磷胁迫的影响产生更多的自由基,膜脂过氧化程度加快,导致MDA质量摩尔浓度增加,但在受到低磷胁迫后,保护酶系统被激活,其活性增强以提高清除自由基的能力。由此可见,马尾松幼苗中存在着MDA质量摩尔浓度和保护酶活性的保持动态平衡的低磷生理应答机制。
本研究发现:不同磷质量分数下,马尾松幼苗根ACP活性与有机酸质量摩尔浓度随磷胁迫程度的增加而升高,以提高植物体对磷的活化、吸收与利用;在胁迫前期,根系活力在无磷处理下显著高于其他处理,但随着胁迫时间增加,根系活力随磷质量分数的降低而下降,尤其是无磷、低磷处理的根系活力急剧下降;根MDA质量摩尔浓度、SOD活性、POD活性、CAT活性,随磷质量分数的下降而升高,低磷环境下MDA质量摩尔浓度增高,SOD活性、POD活性、CAT活性也随之增强以修复MDA带来的损伤。可见,低磷胁迫下马尾松幼苗会通过调整生理、生化的变化机制来应对低磷环境。
Physiological and biochemical responses of seedling roots of Pinus massoniana to different phosphorus concentrations
-
摘要:
目的 研究不同质量分数度下马尾松Pinus massoniana幼苗根系生理生化特征,以探究马尾松应对磷胁迫的生理生化机制,为低磷区域的马尾松人工林经营提供理论支持。 方法 以2年生马尾松幼苗为材料,采用石英砂盆栽的方法,基于全国马尾松林土壤有效磷质量分数(2.250 0 mg·kg−1),设计0 (P0)、0.562 5 (P1)、1.125 0 (P2)、2.250 0 (ck)、4.500 0 (P3)、9.000 0 (P4) mg·kg−1共6个有效质量分数度,分析了2个采样时间[8月(处理1.5个月)与9月(处理3.0个月后)]马尾松幼苗根系活力及酸性磷酸酶(ACP)活性、有机酸质量摩尔浓度、丙二醛(MDA)质量摩尔浓度以及3种抗氧化保护酶活性[超氧化物歧化酶(SOD)、过氧化物酶(POD)、过氧化氢酶(CAT)]的差异。 结果 ①随着磷质量分数的增加,ACP活性与有机酸质量摩尔浓度均呈下降趋势。其中,P0处理的ACP活性在8和9月均显著高于ck (P<0.05),P4处理的ACP活性在8和9月均显著低于ck (P<0.05);在9月,P3处理的ACP活性也显著低于ck (P<0.05);在2个采样时间段,P0和P1处理的有机酸质量摩尔浓度显著高于ck (P<0.05),P4处理的有机酸质量摩尔浓度显著低于ck (P<0.05)。②8月ck的根系活力显著低于P0、P1、P3、P4处理(P<0.05),而9月根系活力随磷质量分数的增加而增加。③MDA随着磷质量分数的降低而增加,P0处理显著高于ck (P<0.05),P4处理则显著低于ck (P<0.05)。仅P0和P1处理的3种抗氧化保护酶活性处理显著高于ck (P<0.05)。 结论 低磷胁迫会使马尾松幼苗根系MAD增加并影响其根系活力,但是马尾松幼苗通过增加根ACP活性与有机酸质量摩尔浓度以及抗氧化系统酶活性来应对低磷胁迫的影响。图3表1参39 Abstract:Objective This study, with an investigation of the physiological and biochemical responses of roots in Pinus massoniana seedlings to different phosphorus (P) concentrations, is aimed to explore the physiological and biochemical mechanisms of P. massoniana in response to low P stress, so as to provide theoretical support for the managements of P. massoniana plantations in low phosphorus area. Method First, two-year-old seedlings of P. massoniana planted in potted with quartz sand were treated with six available P concentrations, including 0 (P0)、0.562 5 (P1)、1.125 0 (P2)、2.250 0 (ck)、4.500 0 (P3)、9.000 0 (P4) mg·kg−1 before a control check (ck, 2.250 0 mg·kg−1) with the effective phosphorus content of the national P. massoniana forest soil survey. Then, the physiological and biochemical characteristics of roots, including activities of acid phosphatase (ACP), total organic acid content (TOC), root vigor, malondialdehyde (MDA) and activities of three antioxidant protective enzymes including superoxide dismutase (SOD), peroxidase (POD) and catalase (CAT), were determined and analyzed in both 1.5 and 3.0 months after treatments. Result (1) Both ACP activity and organic acid content decreased with the increase of phosphorus concentration with the ACP activity of P0 in August and September being significantly higher than that of ck (P<0.05), and the ACP activity of P4 being significantly lower than ck both in August and September (P<0.05) whereas in September, the ACP activity of P3 was also significantly lower than that of ck (P<0.05) with the organic acid content in P0 and P1, and organic acid content in P4 being significantly lower than in ck (P<0.05) in two sampling times. (2) In August, the root vigor in ck was significantly lower than in P0, P1, P3 and P4 (P<0.05), and in September, the root vigor increased with the increase of phosphorus concentration. (3) The root MDA content increased with the decrease of phosphorus concentration with the MDA content in P0 being significantly higher than that in ck (P<0.05), and the MDA content in P4 being significantly lower than that in ck (P<0.05). The antioxidant enzyme activities showed a tendency of increasing as phosphorus concentration decreased whereas SOD, POD and CAT were significantly higher in P0 and P1 than that in ck (P<0.05) though their activities showed no significant variation among ck, P2, P3 and P4 treatments. Conclusion In response to low P stress, P. massoniana seedlings had their MAD, ACP, TOC and antioxidant system enzyme activities increased in roots with the root vigor regulated. [Ch, 3 fig. 1 tab. 39 ref.] -
Key words:
- Pinus massoniana seedlings /
- phosphorus /
- root vigor /
- antioxidant protective enzymes
-
表 1 不同磷质量分数下马尾松幼苗根生理变化指标的Pearson相关性分析
Table 1. Pearson correlation analysis of the physiological indexes of P. massoniana seedlings under different phosphorus concentrations
指标 有机酸总量 根系活力 MDA SOD活性 POD活性 CAT活性 ACP活性 0.472** 0.392* 0.215 0.466** 0.287 0.415* 有机酸总量 1 −0.190 0.702** 0.658** 0.577** 0.640** 根系活力 1 −0.308 −0.095 −0.432** −0.162 MDA 1 0.695** 0.694** 0.712** SOD活性 1 0.670** 0.671** POD活性 1 0.672** 说明:*表示相关显著(P<0.05);**表示相关极显著(P<0.01) -
[1] ZHANG Yi, CHEN Haoting, LIANG Ying, et al. Comparative transcriptomic and metabolomic analyses reveal the protective effects of silicon against low phosphorus stress in tomato plants [J]. Plant Physiology and Biochemistry, 2021, 166: 78 − 87. [2] KIM Y, CHUNG Y S, LEE E, et al. Root response to drought stress in rice (Oryza sativa L. ) [J/OL]. International Journal of Molecular Sciences, 2020, 21(4): 1513[2022-03-01]. doi:10.3390/ijms21041513. [3] MA Xiaofan, LI Haigang, ZHANG Junling, et al. Spatiotemporal pattern of acid phosphatase activity in soils cultivated with maize sensing to phosphorus-rich patches[J/OL]. Frontiers in Plant Science, 2021, 12: 650436[2022-03-01]. doi: 10.3389/fpls.2021.650436. [4] 黄宇, 张海伟, 徐芳森. 植物酸性磷酸酶的研究进展[J]. 华中农业大学学报, 2008, 27(1): 148 − 154. HUANG Yu, ZHANG Haiwei, XU Fangsen. Research summarization on acid phosphatase in higher plants [J]. Journal of Huazhong Agricultural University, 2008, 27(1): 148 − 154. [5] FAN Jingwei, YANG Xiaowei, WANG Tao, et al. Contrasting growth, photosynthesis, antioxidant responses and water use efficiency in two Medicago sativa L. genotypes under different phosphorus and soil water conditions[J/OL]. Agronomy, 2020, 10(10): 1534[2022-03-01]. doi: 10.3390/agronomy10101534. [6] TIWARI P, SHARMA P K. Decrypting the effects of starvation and excess of nitrogen and phosphorus on Nostoc calcicola [J]. Journal of Stress Physiology &Biochemistry, 2021, 17(2): 5 − 19. [7] 欧阳泽怡, 陈雯彬, 欧阳硕龙, 等. 低磷胁迫对赤皮青冈幼苗叶片生理指标的影响[J]. 中南林业科技大学学报, 2021, 41(1): 69 − 79. OUYANG Zeyi, CHEN Wenbin, OUYANG Shuolong, et al. Effects of low phosphorus stress on physiological characteristics of Cyclobalanopsis gilva seedling leaves [J]. Journal of Central South University of Forestry &Technology, 2021, 41(1): 69 − 79. [8] 国家林业和草原局. 中国森林资源报告(2014—2018)[R]. 北京: 中国林业出版社, 2019. National Forestry and Grassland Administration. China Forest Resources Report(2014−2018)[R]. Beijing: China Forestry Publishing House, 2019. [9] PAN Xiaocheng, HU Haibo. Transcriptome analysis of low phosphate stress response in the roots of masson pine (Pinus massoniana) seedlings[J/OL]. Acta Physiologiae Plantarum, 2020, 42(12): 176[2022-03-01]. doi: 10.21203/rs.2.14498/v1. [10] 曹升, 胡华英, 张虹, 等. 我国南方人工林土壤有效磷匮乏原因及对策分析[J]. 世界林业研究, 2019, 32(3): 78 − 84. CAO Sheng, HU Huaying, ZHANG Hong, et al. Causes and countermeasures of plantation soil available phosphorus deficiency in southern China [J]. World Forestry Research, 2019, 32(3): 78 − 84. [11] 胡支舰, 庾金武, 陈健, 等. 氮沉降对低磷胁迫下毛竹实生幼苗生长及土壤化学特性的影响[J]. 浙江林业科技, 2021, 41(2): 17 − 22. HU Zhijian, YU Jinwu, CHEN Jian, et al. Effect of nitrogen deposition on growth of Phyllostachys edulis seedlings and soil chemical properties under low phosphorus stress [J]. Journal of Zhejiang Forestry Science and Technology, 2021, 41(2): 17 − 22. [12] LIU Juxiu, HUANG Wenjuan, ZHOU Guoyi, et al. Nitrogen to phosphorus ratios of tree species in response to elevated carbon dioxide and nitrogen addition in subtropical forests [J]. Global Change Biology, 2013, 19(1): 208 − 216. [13] DONG Chengcheng, WANG Wei, LIU Hongyan, et al. Temperate grassland shifted from nitrogen to phosphorus limitation induced by degradation and nitrogen deposition [J]. Ecological Indicators, 2019, 101: 453 − 464. [14] FUJITA Y, ROBROEK B J M, RUITER P C D, et al. Increased N affects P uptake of eight grassland species: the role of root surface phosphatase activity [J]. Oikos, 2010, 119(10): 1665 − 1673. [15] 全国土壤普查办公室. 中国土壤普查技术[M]. 北京: 农业出版社, 1992. National Soil Census Office. The Technique of Soil Survey in China[M]. Beijing: Agricultural Press, 1992. [16] 简尊吉, 倪妍妍, 徐瑾, 等. 中国马尾松林土壤肥力特征[J]. 生态学报, 2021, 41(13): 5279 − 5288. JIAN Zunji, NI Yanyan, XU Jin, et al. Soil fertility in the Pinus massoniana forests of China [J]. Acta Ecologica Sinica, 2021, 41(13): 5279 − 5288. [17] 徐向华, 丁贵杰. 马尾松适应低磷胁迫的生理生化响应[J]. 林业科学, 2006, 42(9): 24 − 28. XU Xianghua, DING Guijie. Physiological and biochemical responses of Pinus massoniana to low phosphorus stress [J]. Scientia Silvae Sinicae, 2006, 42(9): 24 − 28. [18] 谢钰容, 周志春, 金国庆, 等. 低P胁迫对马尾松不同种源根系形态和干物质分配的影响[J]. 林业科学研究, 2004, 41(3): 58 − 62. XIE Yurong, ZHOU Zhichun, JIN Guoqing, et al. Difference of induced acid phosphate activity under low phosphorus stress of Pinus massoniana provenances [J]. Forest Research, 2004, 41(3): 58 − 62. [19] 唐敏. 磷对不同种源马尾松种子及幼苗影响的研究[D]. 贵阳: 贵州大学, 2007. TANG Min. Study on Effect of Phosphorus to Germination and Seedling of Pinus massoniana Different Provenances[D]. Guiyang: Guizhou University, 2007. [20] 周政贤. 中国马尾松[M]. 北京: 中国林业出版社, 2001. ZHOU Zhengxian. Masson Pine in China[M]. Bejing: China Forestry Publishing House, 2001. [21] 张志良, 李小方. 植物生理学实验指导[M]. 5版. 北京: 高等教育出版社, 2016. ZHANG Zhiliang, LI Xiaofang. Experimental Guide for Plant Physiology [M]. 5th ed. Beijing: Higher Education Press, 2016. [22] 胡华群. 磷对辣椒根系生长发育及生理代谢的影响[D]. 贵阳: 贵州大学, 2009. HU Huaqun. Effect of Phosphate on Growth Development and Physiological Metabolism of Hot Pepper Roots[D]. Guiyang: Guizhou University, 2009. [23] DAVEY M W, STALS E, PANIS B, et al. High-throughput determination of malondialdehyde in plant tissues [J]. Analytical biochemistry, 2005, 347(2): 201 − 207. [24] WANG Liangsheng, LIU Dong. Functions and regulation of phosphate starvation-induced secreted acid phosphatases in higher plants [J]. Plant Science, 2018, 271: 108 − 116. [25] ZAHEER A, MUHAMMAD A, GILL M A, et al. Intra-Specific variations of phosphorus utilization efficiency [J]. Pakistan Journal of Biological Sciences, 2000, 3(4): 1149 − 1171. [26] 程丽莉, 曹伟, 刘智, 等. 低磷胁迫对落叶松幼苗生长及根系酸性磷酸酶活性的影响[J]. 北京林业大学学报, 2006, 28(6): 46 − 50. CHENG Lili, CAO Wei, LIU Zhi, et al. Effects of phosphate deficiency on the growth and acid phosphatase activity of Larix gmelinii seedlings [J]. Journal of Beijing Forestry University, 2006, 28(6): 46 − 50. [27] 谢钰容, 周志春, 廖国华, 等. 低磷胁迫下马尾松种源酸性磷酸酶活性差异[J]. 林业科学, 2005, 41(3): 58 − 62. XIE Yurong, ZHOU Zhichun, LIAO Guohua, et al. Difference of induced acid phosphate activity under low phosphorus stress of Pinus massoniana provenances [J]. Scientia Silvae Sinicae, 2005, 41(3): 58 − 62. [28] 俞元春, 余健, 房莉, 等. 缺磷胁迫下马尾松和杉木苗根系有机酸的分泌[J]. 南京林业大学学报(自然科学版), 2007, 31(2): 9 − 12. YU Yuanchun, YU Jian, FANG Li, et al. Organic acids exudation from the roots of Cunninghamia lanceolata and Pinus massoniana seedlings under low phosphorus stress [J]. Journal of Nanjing Forestry University (Natural Sciences Edition) , 2007, 31(2): 9 − 12. [29] LIU Ruixian, ZHOU Zhiguo, GUO Wenqi, et al. Effects of N fertilization on root development and activity of water-stressed cotton (Gossypium hirsutum L. ) plants [J]. Agricultural Water Management, 2008, 95(11): 1261 − 1270. [30] 徐惠龙. 磷高效水稻根系响应低磷胁迫的生理特性与相关蛋白研究[D]. 福州: 福建农林大学, 2007. XU Huilong. Study on the Physiological Response and Related Proteins in Root of Rice (Oryza satava L. ) with High P Efficiency to Low Phosphate Stress[D]. Fuzhou: Fujian Agriculture and Forestry University, 2007. [31] 崔博文. 马尾松种质评价及生长性状相关QTL的初步定位[D]. 贵阳: 贵州大学, 2017. CUI Bowen. Evaluation of Germplasm Resources and Growth-trait-related QTL Mapping in Masson Pine (Pinus massoniana Lamb) [D]. Guiyang: Guizhou University, 2017. [32] 乔光, 崔博文, 文晓鹏, 等. 不同种源马尾松幼苗对低磷胁迫的生理响应[J]. 种子, 2017, 36(8): 32 − 36, 41. QIAO Guang, CUI Bowen, WEN Xiaopeng, et al. Physiological and biochemical responses to low phosphorus stress for different Masson pine (Pinus massoniana) provenances [J]. Seed, 2017, 36(8): 32 − 36, 41. [33] 姜仕昆, 周运超, 谭伟, 等. 马尾松林近自然不同经营管理措施下土壤肥力[J]. 浙江农林大学学报, 2020, 37(5): 876 − 882. JIANG Shikun, ZHOU Yunchao, TAN Wei, et al. Soil fertility of Pinus massoniana forests under different near-natural management measures [J]. Journal of Zhejiang A&F University, 2020, 37(5): 876 − 882. [34] 周建菲, 史文辉, 潘凯婷, 等. 低磷胁迫对毛竹幼苗生长和养分生理的影响[J]. 浙江农林大学学报, 2022, 39(5): 1010 − 1017. ZHOU Jianfei, SHI Wenhui, PAN Kaiting, et al. Effect of low phosphorus stress on growth and nutrient physiology of Phyllostachys edulis seedlings [J]. Journal of Zhejiang A&F University, 2022, 39(5): 1010 − 1017. [35] 王艺雄, 张华锋, 李全, 等. 氮添加对毛竹林土壤磷组分的影响[J]. 浙江农林大学学报, 2022, 39(4): 695 − 704. WANG Yixiong, ZHANG Huafeng, LI Quan, et al. Effect of nitrogen addition on soil phosphorus fractions in the Phyllostachys edulis plantation [J]. Journal of Zhejiang A&F University, 2022, 39(4): 695 − 704. [36] LIU Yukun, HE Chengzhong. Regulation of plant reactive oxygen species (ROS) in stress responses: learning from AtRBOHD [J]. Plant Cell Reports, 2016, 35(5): 995 − 1007. [37] HAJLAOUI H, DENDEN M, EI AYEB N, et al. Changes in fatty acids composition, hydrogen peroxide generation and lipid peroxidation of salt-stressed corn (Zea mays L. ) roots [J]. Acta Physiologiae Plantarum, 2009, 31(4): 787 − 796. [38] JANERO D R. Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury [J]. Free Radical Biology &Medicine, 1990, 9(6): 515 − 540. [39] 于姣妲, 夏丽丹, 殷丹阳, 等. 磷素对杉木幼苗耐铝性的影响机制[J]. 林业科学, 2018, 54(5): 36 − 47. YU Jiaoda, XIA Lidan, YIN Danyang, et al. Effects of phosphorus on aluminum tolerance of Chinese fir seedlings [J]. Scientia Silvae Sinicae, 2018, 54(5): 36 − 47. -

-
链接本文:
https://zlxb.zafu.edu.cn/article/doi/10.11833/j.issn.2095-0756.20220226






 下载:
下载: