-
杜鹃花Rhododendron是世界十大名花之一,是城市重要的绿化植物。杜鹃花根系细而分布浅,水分管理十分重要。城市生态环境的一个主要特点就是土壤水分缺失,即使是南方城市也有季节性干旱问题,因此,对杜鹃花的抗旱性进行研究显得尤其必须。近年来,国内部分学者对云锦杜鹃Rh. fortunei,露珠杜鹃Rh. irroratum,大白花杜鹃Rh. decorum等高山野生杜鹃花在干旱胁迫下的形态表现和生理响应进行了研究,为高山杜鹃花在平原地区的引种驯化提供了理论支持。另有一些学者对部分杜鹃花和毛鹃品种的抗旱性从生理指标方面进行了评价,未涉及到品种的叶片解剖结构对抗旱性的影响,而且未对影响抗旱性的相关指标进行分析[1-4]。国外的专家学者也进行过杜鹃花抗旱性的相关研究,如ANISKO等[5]主要研究了杜鹃花品种在干旱胁迫下的水势变化以及干旱胁迫对杜鹃花抗寒性的影响;MAYR等[6]对高山杜鹃花的木质部解剖结构与其抗旱性的关系进行了研究。本研究通过测定杜鹃花的叶片解剖结构指标及干旱胁迫下其各项生理指标,利用隶属函数法评价其抗旱性,筛选与杜鹃花抗旱性相关的叶片解剖结构与生理指标,以期为杜鹃花抗旱品种的选择以及杜鹃花在园林绿化中的应用提供更好的理论依据。
HTML
-
本试验选取西洋杜鹃‘蓝茵’Rh. ‘Lan Yin’ ‘国旗红’Rh. ‘Guoqihong’ ‘爱丁堡’Rh. ‘Aidingbao’ ‘粉珍珠’Rh. ‘Fenzhenzhu’等4个品种为研究对象。采用长势基本一致的3年生杜鹃花扦插苗作为试验材料。2015年10月将杜鹃花苗移植于普通塑料花盆中,花盆上口径为27.9 cm,下口径20.9 cm,高18.5 cm,盆土为松针泥炭土[V(松针):V(泥炭)=1:1],1株·盆-1,盛土量一致,进行正常管理。田间最大持水量为71.125%。试验于2015年12月在陕西省西北农林科技大学园林植物试验室进行。实验室温度为18~25 ℃,空气湿度为50%~60%,光照良好,平均每日光照为6 h,光照强度为500~700 lx。
-
2015年12月进行水分胁迫,充分灌溉,使土壤含水量一致,停止灌溉后等土壤自然落干进行干旱胁迫。待土壤水分降至试验要求后,每天早上8:00用电子秤(载量30.0 kg,感量1.0 g)称量法将土壤含水量控制在设定范围内,并补充其水分消耗,准确记录加水量,胁迫第8天早上进行采样,选取植株新梢成熟叶片置入冰盒中带回试验室,立即剪碎混合均匀后对样品进行理化指标测试。试验重复3次。
试验设置3个水分处理:① 正常水分处理,田间持水量的75%~80%(对照);② 中度干旱胁迫处理,田间持水量的40%~45%;③ 重度干旱胁迫处理,田间持水量的30%~35%。6盆·处理-1,1株·盆-1。
-
叶片萎蔫情况是判断植物受干旱胁迫程度最为直观的依据[7-11]。干旱胁迫下,杜鹃花的生长会受到一定影响,尤其是对新生叶片的影响较大。主要表现为幼叶反卷,新梢成熟叶片依次出现萎蔫、下垂、皱缩等现象,老叶会出现加速脱落的现象。因此,以叶片的形态表现为依据,划分干旱胁迫对杜鹃花影响的程度,以方便观察。具体区分方法为:Ⅰ级:幼叶反卷,10%以下叶片出现萎蔫、下垂、皱缩现象;Ⅱ级:幼叶反卷,10%~29%以下叶片出现萎蔫、下垂、皱缩现象;Ⅲ级:幼叶反卷,30%~59%以下叶片出现萎蔫、下垂、皱缩现象;Ⅳ级:幼叶反卷,60%以上叶片出现萎蔫、下垂、皱缩现象。隔2 d观察1次。
-
分别取4个杜鹃花品种对照组的叶片,进行叶片解剖结构的观察。取样时间为晴天的9:00-11:30。具体为用刀片取每个叶片的中部各5份,不超过0.7 cm × 0.3 cm,放入小瓶中保存。将已切好的材料尽快地浸入体积分数为4%戊二醇固定液中,材料固定完毕, 保存于加盖的容器内,抽气,贴上标签。一般固定时间不低于24 h。磷酸缓冲液(0.1 mol·L-1,pH 6.8)漂洗4次,用体积分数30%,50%,70%,80%,90%,100%乙醇依次脱水,乙酸异戊酯置换后,用二氧化碳临界点干燥仪(K-850)进行干燥,离子溅射仪(E-1045)喷金,再用JSM-6360LV扫描电镜进行观察。观测指标有:叶片总厚度、角质层厚度、栅栏组织厚度、海绵组织厚度、气孔长宽、气孔密度,并计算栅栏组织厚度与海绵组织厚度的比值、叶片组织结构紧密度和叶片组织疏松度和气孔面积。
-
叶片的水分测量参考明冬风[12]的方法。叶绿素质量分数采用丙酮浸提法测定;质膜透性采用相对电导率法;丙二醛(MDA)质量摩尔浓度采用硫代巴比妥酸法测定;脯氨酸质量分数(Pro)采用磺基水杨酸提取茚三酮显色法测定;可溶性糖质量分数采用蒽酮比色法测定;可溶性蛋白质和过氧化氢(H2O2)质量分数采用南京建成生物有限公司的试剂盒测定;超氧化物歧化酶(SOD)活性采用氮蓝四唑(NBT)光还原法测定; 叶片过氧化物酶(POD)活性采用愈创木酚法测定[13]。吸光度采用日本岛津UV-2450型紫外可见分光光度计测定吸光值。生理指标测定均重复3次。
-
采用隶属函数法对4种杜鹃花的抗旱性进行综合评价,与形态表现进行对比。采用灰色关联分析法计算各指标与品种平均隶属函数值的关联系数,进而求得各指标与抗旱性的关联度,排序并筛选与抗旱性关联度大的指标。隶属函数值的计算方法:如果某一指标与抗旱性成正相关,则采用以下公式进行计算:U(x)=(x-xmin)/(xmax-xmin)。如果某一指标与抗旱性成负相关,则采用以下公式进行计算:U(x)=[1-(x-xmin)/(xmax-xmin)]。然后求出每个品种的平均隶属函数值,以平均隶属函数值排序,数值越大,抗旱性越强。
关联系数计算的方法为:① 建立灰色系统:以各品种的平均隶属函数值组成参考函数序列,记为x0;以各品种各个指标的平均值为比较数列,记为xi(i=1,2,…,21),分别代表叶绿素a,叶绿素b含量,…,气孔密度。x0={x0(k)丨k=1,2,…,4}。xi={x1(k)丨k=1,2,…,4},(i=1,2,…,21)。② 用内插法将比较数列无量纲化。③ 计算关联系数和关联度。关联系数计算公式如下:
其中:ε为分辨系数,0<ε<1,一般取ε = 0. 5。关联度计算公式如下:
采用Excel 2013和SPSS 22.0分析软件对数据进行统计分析。
1.1. 试验材料
1.2. 试验设计
1.3. 形态表现观察方法
1.4. 叶片解剖结构观测
1.5. 生理指标测定方法
1.6. 数据处理
-
由表 1可知:‘爱丁堡’的气孔面积、栅栏组织厚度、角质层厚度远大于其他3个品种。‘国旗红’和‘粉珍珠’的气孔密度远小于‘蓝茵’和‘爱丁堡’的气孔密度。‘国旗红’的栅栏组织厚度和海绵组织厚度的比值以及叶片组织结构紧密度远高于其他3个品种;叶片组织结构松散度远低于其他3个品种,说明其叶片结构较其他3个品种更加紧致。
品种 气孔面积/μm2 气孔密度/(个·mm-2) 叶片厚度/μm 栅栏组织厚度/μm 栅栏组织与海绵组织厚度比值/% 角质层厚度/μm 叶片组织结构紧密度/% 叶片组织结构疏松度/% ‘蓝茵’ 24.847 ± 3.881ab 220.079 ± 7.744 b 143.102 ± 6.508 ab 33.883 ± 1.685 a 40.340 ± 2.054 a 1.148 ± 0.169 a 23.777 ± 0.886 a 59.323 ± 1.096 c ‘国旗红’ 30.966 ± 4.517 a 132.765 ± 11.577 a 133.444 ± 4.085 a 44.551 ± 2.913 ab 66.687± 6.210 c 1.240 ± 0.131 a 33.381 ± 1.932 c 51.425 ± 1.818 a ‘爱丁堡’ 40.131 ± 4.061c 241.130 ± 8.480 b 159.570 ± 3.919 b 45.069 ± 2.794 c 55.780 ± 3.782 b 2.956 ± 0.336 b 28.204 ± 1.437 b 51.076 ± 1.314 b ‘粉珍珠’ 22.425 ± 3.458 a 116.259 ± 7.672 a 136.642 ± 5.998 a 39.892 ± 2.596 b 55.714 ± 3.221 b 1.320± 0.193 a 29.130 ± 1.166 b 52.983 ± 1.800 b 说明:根据Duncan检验,表中字母在同一参数内纵向比较,相同者表示无显著差异(P>0.05)。 Table 1. Characteristics of leaf anatomical structure of four Rhododendron cultivars (mean ± ES)
-
由表 2可知:随着干旱胁迫程度和干旱天数的增加,杜鹃花的受胁迫表现也越来越明显。对照组的杜鹃花生长良好;中度干旱胁迫下,‘蓝茵’达到Ⅱ级的受胁迫程度的处理天数为第3天,‘国旗红’‘爱丁堡’‘粉珍珠’为第7天;重度干旱胁迫下,‘蓝茵’达到Ⅲ级的受胁迫程度的处理天数为第3天,达到Ⅳ级的受胁迫程度的处理天数为第5天,‘国旗红’‘爱丁堡’和‘粉珍珠’第7天分别达到Ⅳ级、Ⅲ级、Ⅱ级。以干旱胁迫下杜鹃花的形态表现综合评价来说,抗旱性可排序为‘蓝茵’<‘国旗红’<‘爱丁堡’<‘粉珍珠’。
品种 处埋 不同处理天数下的形态表现 品种 处理 不同处理天数下的形态表现 1 3 5 7d 1 3 5 7d 对照 Ⅰ Ⅰ Ⅰ Ⅰ 对照 Ⅰ Ⅰ Ⅰ Ⅰ ‘蓝茵’ 中度干旱 3 Ⅱ Ⅱ Ⅱ ‘爱丁堡’ 中度干旱 Ⅰ Ⅰ Ⅰ Ⅱ 重度干旱 4 Ⅲ Ⅲ Ⅳ 重度干旱 Ⅰ Ⅰ Ⅱ Ⅲ 对照 5 Ⅰ Ⅰ Ⅰ 对照 Ⅰ Ⅰ Ⅰ Ⅰ ‘国旗红’ 中度干旱 6 Ⅰ Ⅰ Ⅱ ‘粉珍珠’ 中度干旱 Ⅰ Ⅰ Ⅰ Ⅱ 重度干旱 7 Ⅰ Ⅱ Ⅳ 重度干旱 Ⅰ Ⅱ Ⅱ Ⅱ Table 2. Morphological features of four Rhododendron cultivars under drought stress
-
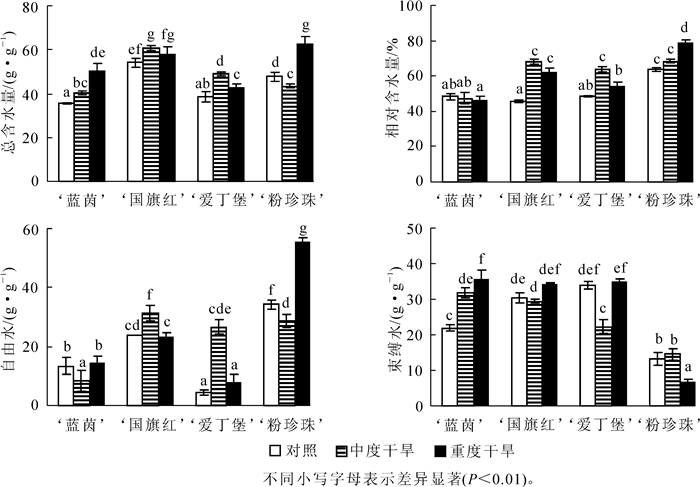
由图 1可得:随着干旱胁迫程度的增加,4个杜鹃花品种的总含水量均有显著的增加,其中‘蓝茵’的增幅最大,为41.8%,‘国旗红’的增幅最小,为6.8%。‘爱丁堡’和‘粉珍珠’的增幅介于两者之间。除‘粉珍珠’自由水含量有显著增加外,其余3个品种在重度干旱胁迫下的自由水含量和对照组相比变化不显著,但是中度干旱胁迫下,‘蓝茵’自由水含量显著下降,而‘国旗红’和‘爱丁堡的’的自由水含量显著升高。束缚水含量的变化趋势为‘粉珍珠’的显著下降,其余3个品种有少量的增加。说明在重度胁迫下,‘粉珍珠’的束缚水向自由水转化,并且自由水的增量比束缚水减量大,其余3个品种总含水量的增量是由束缚水含量增加引起的。就叶片相对含水量而言,‘蓝茵’的叶片相对含水量和对照组相比变化不显著,而其余3个品种都有显著的增加。
-
随着干旱胁迫程度的增加,4个品种杜鹃花的叶绿素和类胡萝卜素均有显著的降低,且中度干旱胁迫的降幅小于重度干旱胁迫。在重度干旱胁迫下,‘蓝茵’的叶绿素a,叶绿素b,总叶绿素和类胡萝卜素的降幅均为最小,分别为30.6%,43.0%,67.2%和61.4%;而‘粉珍珠’的叶绿素a,叶绿素b和类胡萝卜素降幅均为最大,分别为47.5%,56.6%和70.6%,‘爱丁堡’的叶绿素b降幅最大,为76.0%(图 2)。
-
由表 3可得:4个杜鹃花品种随着干旱胁迫程度的增加,可溶性糖质量分数均有一定程度的增加。‘粉珍珠’的可溶性糖质量分数比其他3个品种都高,且升高十分显著,而其他3个品种升高不显著。随着干旱胁迫程度的增加,‘蓝茵’的可溶性蛋白质质量分数变化不显著,其他3个品种的质量分数先显著下降,再略有上升,但上升不显著。说明相对于其他3个品种,可溶性蛋白质质量分数对‘蓝茵’的渗透调节作用不是很大。随干旱胁迫程度的增加,‘粉珍珠’脯氨酸的质量分数先降低再升高,而其他3个品种均先升高再降低。重度干旱胁迫下,‘蓝茵’和‘粉珍珠’的脯氨酸质量分数增量显著,而‘国旗红’和‘爱丁堡’的增量不显著。
品种 处理 可溶性糖/(μg·g-1) 可溶性蛋白质/(mg·g-1) 脯氨酸/(μg·g-1) 对照 17.474 ± 0.797 a 3.524 ± 0.792 a 1.426 ± 0.011a ‘蓝茵’ 中度干旱 22.635 ± 1.489 a 1.061 ± 0.240 a 28.282 ± 0.064 7 重度干旱 22.711 ± 2.280 a 1.500 ± 0.264 a 9.114 ± 0.801b 对照 21.951 ± 1.253 a 11.121 ± 1.993 d 14.060 ± 0.008 d ‘国旗红’ 中度干旱 20.424 ± 0.888 a 2.135 ± 0.307 a 18.199 ± 0.032f 重度干旱 23.596 ± 1.365 a 5.427 ± 0.972 a 14.393 ± 0.085 de 对照 16.591 ± 2.027 a 9.980 ± 0.288 d 15.008 ± 0.062 e ‘爱丁堡’ 中度干旱 18.670 ± 3.485 a 5.650 ± 0.667 bc 17.987 ± 0.321f 重度干旱 19.070 ± 2.706 a 6.645 ± 0.959 c 14.489 ± 0.659 de 对照 100.4, 5 ± 8.967 b 14.820 ± 1.578 f 14.360 ± 0.052 d ‘粉珍珠’ 中度干旱 123.183 ± 10.974 c 11.662 ± 2.570 de 12.797 ± 0.074 c 重度干旱 230.304 ± 6.085 d 12.697 ± 1.184 ef 20.755 ± 0.707 g 说明:根据Duncan检验,表中字母在同一参数内纵向比较,相同者表示无显著差异(P>0.05)。 Table 3. Effects of drought stress on osmotic adjustment substance content in leaf of four Rhododendron cultivars (x ± ES)
-
随着干旱胁迫程度的增加,‘蓝茵’和‘爱丁堡’的细胞膜透性变化不显著,‘国旗红’和‘粉珍珠’的胞膜透性显著变大。‘蓝茵’‘国旗红’‘爱丁堡’‘粉珍珠’增幅分别为30.9%,51.6%,2.9%和47.7%(表 4)。而‘国旗红’的丙二醛质量摩尔浓度有显著增加,其他3个品种的丙二醛质量摩尔浓度变化不显著,增幅分别为5.6%,15.2%,1.7%和10.5%。综合来看,4个品种的细胞膜稳定性顺序由弱到强为:‘国旗红’<‘粉珍珠’<‘蓝茵’<‘爱丁堡’。由表 5可得:‘蓝茵’和‘国旗红’的超氧化物歧化酶活性随干旱胁迫程度的增加,先增加再降低,而‘爱丁堡’和‘粉珍珠’的超氧化物歧化酶活性一直增加。说明前2个品种的超氧化物歧化酶活性随干旱胁迫程度的增加受到了抑制,而后2个品种的活性没有受到抑制。而过氧化物酶活性先显著升高再降低,说明4个品种的过氧化物酶活性随干旱胁迫程度的增加,先增强后受到了抑制。另外,‘蓝茵’和‘爱丁堡’的过氧化氢质量分数随干旱胁迫的增加而下降,‘国旗红’和‘粉珍珠’的过氧化氢质量分数先降低再升高,说明随着干旱胁迫程度的增加前2个品种的过氧化氢酶活性不断增强,而后2个品种的过氧化氢酶活性,先升高后受到了抑制。
品种 处理 细胞膜透性/% 丙二醒/(μmol.g-1) 对照 10.390 ± 1.515abc 0.124 ± 0.010f ‘蓝茵’ 中度干旱 10.919 ± 1.420abc 0.120 ± 0.008 f 重度干旱 13.596 ± 1.414c 0.131 ± 0.008 f 对照 13.130 ± 0.642 bc 0.066 ± 0.005 cd ‘国旗红’ 中度干旱 16.739 ± 1.073 d 0.085 ± 0.005 e 重度干旱 19.905 ± 2.440e 0.076 ± 0.003 de 对照 9.623 ± 0.860 a 0.058 ± 0.001 bc ‘爱丁堡’ 中度干旱 9.451 ± 0.361a 0.046 ± 0.005 a 重度干旱 9.902 ± 0.340ab 0.059 ± 0.006 bc 对照 16.546 ± 3.092 d 0.057 ± 0.009 abc ‘粉珍珠’ 中度干旱 21.849 ± 2.371ef 0.053 ± 0.006 ab 重度干旱 24.438 ± 2.858 f 0.063 ± 0.005 bc 说明:根据Duncan检验,表中字母在同一参数内纵向比较,相同者表示无显著差异(P>0.05)。 Table 4. Effects of drought stress on membrance permeability in leaf of four Rhododendron cultivars (x ± ES)
品种 处理 超氧化物歧化酶/(×16.67 nkat·g-1) 过氧化物酶/(×16.67 nkat·g-1·min-1) 过氧化氢/(mmol·g-1) 对照 247.166 ± 8.086 g 34.630 ± 4.855 c 6.184 ± 0.079 a ‘蓝茵’ 中度干旱 249.410 ± 8.731g 55.467 ± 5.823 d 2.244 ± 0.434 a 重度干旱 145.156 ± 7.869 d 25.794 ± 3.942 b 2.532 ± 0.061 a 对照 78.012 ± 6.332b 10.607 ± 2.940 a 18.232 ± 0.091ef ‘国旗红’ 中度干旱 178.117 ± 4.411e 25.143 ± 3.556 b 2.600 ± 0.825 a 重度干旱 120.120 ± 6.200 c 10.588 ± 2.587 a 12.802 ± 2.247 cd 对照 124.076 ± 3.686 c 6.389 ± 0.194 a 16.580 ± 5.561 de ‘爱丁堡’ 中度干旱 150.305 ± 3.100d 26.408 ± 0.221b 6.013 ± 0.084 ab 重度干旱 226.944 ± 3.597 f 4.289 ± 0.867 a 9.231 ± 0.466 bc 对照 38.522 ± 4.393 a 39.074 ± 6.239 c 25.545 ± 3.433 g ‘粉珍珠’ 中度干旱 79.680 ± 1.028 b 59.477 ± 4.849 d 14.802 ± 2.417 de 重度干旱 117.686 ± 8.840 c 37.366 ± 2.038 c 20.964 ± 3.329 f 说明:根据Duncan检验,表中字母在同一参数内纵向比较,相同者表示无显著差异(P>0.05)。 Table 5. Effect of drought stress on H2O2 and active oxygen scavenging systems in leaf of four Rhododendron cultivars (x ± ES)
-
以每种杜鹃花各项指标的平均隶属函数值作为杜鹃花抗旱能力的综合评价标准,数值越大说明其抗旱能力越强。由表 6可得:4种杜鹃花的抗旱能力从小到大为‘蓝茵’<‘国旗红’<‘粉珍珠’<‘爱丁堡’。
品种 平均隶属函数值 排序 ‘蓝茵’ 0.432 4 ‘国旗红’ 0.491 3 ‘爱丁堡’ 0.593 1 ‘粉珍珠’ 0.505 2 Table 6. Synthetically membership function value of four Rhododendron cultivars
-
由表 7可得:与杜鹃花抗旱性关联度最大的3个叶片解剖结构指标分别为:栅栏组织厚度、栅栏组织厚度与海绵组织厚度比和叶片结构紧密度,对应的相关度为:0.850,0.733,0.711;与杜鹃花抗旱性关联度最大的3个生理指标分别为脯氨酸质量分数、叶片相对含水量和过氧化氢质量分数,对应的相关度为0.893,0.703,0.699,相关度接近0.700。
指标 关联度 关联序 气孔面积 0.691 9 气孔密度 0.544 16 叶片厚度 0.592 14 栅栏组织厚度 0.850 2 栅栏组织与海绵组织厚度比值 0.733 3 角质层厚度 0.700 6 叶片组织结构紧密度 0.711 4 叶片组织结构疏松度 0.379 24 总含水量 0.687 10 自由水含量 0.681 11 束缚水含量 0.589 15 相对含水量 0.703 5 叶绿素a 0.389 22 叶绿素b 0.469 18 叶绿素 0.389 21 类胡萝卜素 0.416 20 可溶性糖 0.609 13 可溶性蛋白 0.697 8 脯氨酸 0.893 1 细胞膜透性 0.637 12 丙二醛 0.388 23 超氧化物歧化酶 0.455 19 过氧化物酶 0.471 17 过氧化氢 0.699 7 Table 7. Correlative modulus, correlative degree and correlative order of the indexes of drought resistance of Rhododendron cultivars
2.1. 杜鹃花的形态表现
2.1.1. 杜鹃花叶片解剖结构观察
2.1.2. 干旱胁迫下杜鹃花的形态表现
2.2. 不同程度干旱胁迫对杜鹃花生理生化指标的影响
2.2.1. 干旱胁迫对4个杜鹃花品种叶片水分含量的影响
2.2.2. 干旱胁迫对4个杜鹃花品种叶片叶绿素和类胡萝卜素质量分数的影响
2.2.3. 干旱胁迫对4个杜鹃花品种叶片渗透调节物质的影响
2.2.4. 干旱胁迫对4个杜鹃花品种叶片质膜稳定性和活性氧代谢的影响
2.3. 抗旱性综合评价
2.4. 杜鹃花各生理指标与抗旱性的关联度
-
水分是植物生命活动的重要组成部分,是保证植物正常生长及发育的重要因子。本研究表明:4个杜鹃花品种的总含水量显著升高,而且除‘蓝茵’的叶片相对含水量无显著变化外,其余3个品种都有显著的增加。这虽然与安玉艳等[14]对杠柳Periploca sepium的研究结果不一致,但是杜鹃花叶片的总含水量和自由水含量均上升,说明干旱胁迫提高了杜鹃花对土壤水分的吸收。
干旱胁迫会抑制叶绿素的合成,并且加快叶绿素的分解,使叶绿素含量快速下降[15-16]。类胡萝卜素在植物光合作用中担负着光吸收辅助色素和保护叶绿素氧化破坏的重要功能,有些类胡萝卜素还是脱落酸(ABA)合成的前体[17]。在本研究中,干旱胁迫下杜鹃花的叶绿素和类胡萝卜素质量分数均显著降低,说明杜鹃花的光合产量有所减少。在哈申格日乐等[18]对樟子松Pinus sylvestris var. mongolica等耐干旱植物以及白志英等[19]对小麦Triticum aestivum的研究中也有相似的结果。
干旱胁迫下,植物会通过提高细胞液中的渗透调节物质的浓度来维持细胞彭压,防止细胞脱水,为正常生命活动创造条件[20-21]。渗透调节物质主要有可溶性糖和可溶性蛋白质。本研究表明:随着干旱胁迫程度的增加,杜鹃花的可溶性糖质量分数升高,可溶性蛋白质质量分数降低,杜鹃花可溶性糖的变化与吴敏等[22]对栓皮栎Quercus variabilis的研究结果一致,说明杜鹃花可以通过积累可溶性糖来调节细胞的渗透势。另外,杜鹃花可溶性蛋白质的变化与吴敏等[22]研究结果不一致。根据魏良民[23]和葛体达[24]研究,在干旱胁迫下,蛋白质的合成受到抑制,并且干旱胁迫会诱导蛋白质降解。因此,杜鹃花叶片的可溶性蛋白质质量分数下降的主要原因是其叶片蛋白质发生了降解。
另外,脯氨酸在植物的渗透调节中也起重要作用。植株体内脯氨酸质量分数在一定程度上反映了植株体内的水分状况,因而可以作为植物缺水情况的参考性生理指标[25]。脯氨酸与植物抗旱性的关联性有很多争论,有的研究认为可以用植物体内脯氨酸质量分数的多少来衡量植物的抗旱性,也有的研究认为其两者的关联性并不显著[26-28]。本研究表明:随着干旱胁迫程度的增加,杜鹃花的脯氨酸质量分数显著增加,说明杜鹃花的抗旱性与其叶片中的脯氨酸积累有一定的关系。范苏鲁等[29]对大丽花Dahlia pinnata和毛永成等[30]对鸡爪槭Acer palmatum的研究也有相似的结果。
细胞膜具有选择透性。干旱胁迫会造成细胞膜过氧化,选择透过性的降低或丧失,细胞内大量离子外渗,从而使组织浸出液的相对电导率升高,并且丙二醛是细胞膜脂过氧化的产物之一,是检测膜损伤程度的公认指标[31]。在本研究中,随着干旱胁迫程度的增加,杜鹃花的细胞渗透率增加,但丙二醛摩尔质量浓度变化不显著,但均有小幅升高,并且杜鹃花品种间丙二醛质量摩尔浓度差异比较大,总的来说杜鹃花的细胞膜稳定性降低。这与范苏鲁等[29]对大丽花和吴敏等[22]对栓皮栎的研究结果一致。
干旱胁迫下,植物体内会产生过量的活性氧,包括超氧自由基、过氧化氢等,但由超氧化物歧化酶、过氧化物酶等抗氧化酶组成的抗氧化防御体系可以清除植物体内产生的活性氧,维持细胞膜稳定[12]。本研究结果表明:随着干旱胁迫程度的增加,超氧化物歧化酶、过氧化物酶活性先增强后会受到一定的抑制,‘蓝茵’和‘爱丁堡’的过氧化氢质量分数随干旱胁迫程度的增加而下降,‘国旗红’和‘粉珍珠’的过氧化氢质量分数先降低再升高,说明随着干旱胁迫程度的增加前2个杜鹃花品种的过氧化氢酶活性不断增强,而后2个品种的过氧化氢酶活性先升高后受到了抑制。这与柯世省等[4]对云锦杜鹃,丁玲等[32]对黄瓜Cucumis sativus幼苗的研究结果一致。
根据平均隶属函数值,4个杜鹃花品种的抗旱能力从小到大为‘蓝茵’<‘国旗红’<‘粉珍珠’<‘爱丁堡’,这与干旱胁迫下杜鹃花的形态表现有一定的差异,主要体现在‘粉珍珠’与‘爱丁堡’的顺序上。结合叶片的形态解剖结构来看,其主要原因是‘粉珍珠’叶片革质,叶片组织结构紧密度相对较大,相对含水量大。因此干旱胁迫下‘粉珍珠’的叶片形态变化不显著。
从叶片解剖结构来说,植物抗旱性主要与叶片厚度、叶片角质层厚度、栅栏组织厚度、栅栏组织与海绵组织厚度比值、叶片组织结构紧密度、气孔密度呈正相关,与叶片气孔面积、叶片组织结构疏松度呈负相关[33-36]。与杜鹃花抗旱性关联度最大的3个叶片解剖结构指标分别为栅栏组织厚度、栅栏组织厚度与海绵组织厚度比和叶片结构紧密度,相关度为均达70%以上。这与邱权等[34]的研究结果基本一致。与杜鹃花抗旱性关联度最大的3个生理指标分别为脯氨酸质量分数、叶片相对含水量和过氧化氢质量分数相关度接近0.700。这与黄承玲等[1]对高山杜鹃花的研究结果有差异,一方面是因为杜鹃花与高山杜鹃花的差异显著,另一方面可能是因为其测量的生理指标覆盖面小。











 DownLoad:
DownLoad:
