-
芸香Ruta graveolens又名臭草、香草、百应草、小叶香,是芸香科Rutaceae芸香属Ruta多年生草本植物。植株高达1 m,叶羽状复叶,灰绿或带蓝绿色;花金黄色,花期3-6月;原产地中海沿岸,在中国均有栽培[1]。芸香植株各部分均有浓烈的特殊气味,具有杀虫抑菌效果[2-4];全株均可入药,有清热解毒、凉血散瘀、利尿、消肿、止咳平喘等功效[5-6]。目前,对芸香科的研究主要集中在生理特性[7-8]、分子生物学[9-10]、精油化学成分[3-4, 11-13]及抑菌效果[14-15]等方面,并未见有关芸香花瓣及叶片挥发性有机物成分随时间变化的相关报道。现有的研究探究芸香的有机物成分主要采用精油作为试验材料[16-18],精油的提取常常会受提取温度和方法的干扰,造成某些挥发性物质受到破坏,不能完全反映芸香释放的挥发性有机物的成分。因此,本研究采用顶空固相微萃取技术吸附采集不同时期芸香叶片释放的挥发性有机成分,结合气质联用仪检测并分析不同季节及1 d中不同时间释放的挥发性有机物成分及相对含量,揭示其叶片挥发性有机物的季节变化规律和日变化规律;并用相同的方法探究叶片和花瓣释放的挥发性有机物成分的差异,为科学利用芸香营造生态型、保健型、芳香园林景观提供理论依据。
HTML
-
芸香苗龄为5~6年生,株高70~75 cm,长势繁茂,无病虫害,栽植于深圳职业技术学院西校区芳香植物园内。
-
分别于3月、6月、9月、12月的16日上午9:00-10:00在同一位置的植物上摘取向阳侧上、中、下部位的健康无损伤的叶片10片,分别剪碎混匀,称取0.5 g放置5 mL萃取瓶中密封,静置30 min,环境温度为(22.0±3.0) ℃。将型号为DVB-CAR-PDMS 100 μm的SPME纤维头(美国Supelco公司)通过聚四氟乙烯瓶垫插入到萃取瓶中,置于样品正上方0.5 cm左右,顶空萃取40 min,然后将纤维头插入6890N/5975气相色谱-质谱联用仪(美国Agilent公司)的气相色谱进样口,解吸10 min。每次收集设置3次重复,吸附空萃取瓶中的气体作为空白对照。花瓣挥发性有机物的测定于3月16日进行,9:00-10:00采摘盛开的花5朵;挥发性有机物日动态变化的测定于6月16日进行,8:00-20:00隔2 h采样1次。色谱条件:HP-5MS弹性石英毛细管柱色谱柱,长为30 m,内径为0.25 mm,液膜厚为0.25 μm,载气为高纯氦气,不分流进样,恒流流速为1.0 mL ·min-1,进样口温度为230 ℃,接口温度为280 ℃。季节变化和花瓣挥发物测定初始温度为50 ℃,保持4 min,以6 ℃·min-1升至150 ℃,保持2 min,然后以7 ℃·min-1升至250 ℃,保持8 min。日动态变化规律测定初始温度为50 ℃,保持4 min,以4 ℃·min-1升至150 ℃,保持2 min,然后以8 ℃·min-1升至250 ℃,保持6 min。质谱条件:电子轰击(EI)离子源,电子能量为70 eV,离子阱温度为230 ℃,四级杆温度为150 ℃,原子质量扫描范围为30~500。
-
挥发性有机物成分经气相色谱分离,各色谱峰、质谱峰分别利用气相色谱-质谱仪中MSD Productivity ChemStation和NIST Mass Spectral Database 2008谱库进行分析鉴定。各成分在样品气体中的浓度采用峰面积归一法进行计算。相对含量=(该物质峰面积/样品所有气体峰面积之和)×100%。
1.1. 试验材料
1.2. 试验方法
1.2.1. 植物挥发性有机物的采集和测定
1.2.2. 数据处理
-
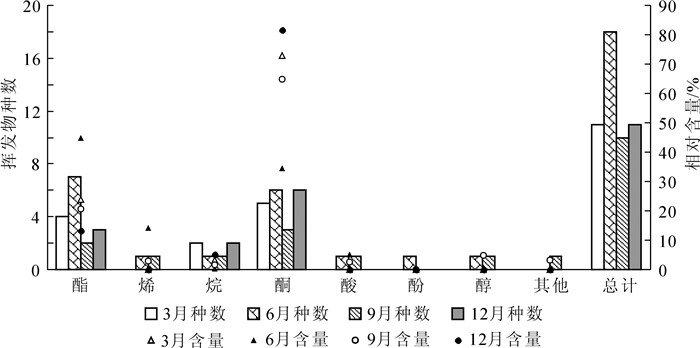
芸香叶片1 a中不同季节释放的挥发性有机物中,共鉴定出29种化合物(图 1,表 1),其中酯类11种,酮类7种,烷类4种,萜烯类,酸类和醇类各2种,以及少量的酚类和其他化合物;主要成分为乙酸壬酯、4-(3, 4-亚甲基二氧基苯基)-2-丁酮、2-壬酮和2-十一(烷)酮等。1 a中检测到叶片释放的挥发性有机物种类数量呈先增多后减少的趋势;6月最多,为18种,9月最少,仅为10种(图 2)。3月挥发性有机物成分检测到酮类、酯类和烷类3类化合物,以酮类最多,其次为酯类;其中2-壬酮的相对含量最高,为48.98%。6月检测到酯类、酮类、萜烯类、酸类、烷类、酚类和醇类7类化合物,以酯类最多,7种。9月检测到的挥发性有机物种类仅为6月的55.56%,酮类和酯类占50.00%,其中2-十一(烷)酮的相对含量最高,为34.13%。12月检测到的挥发物中以酮类为主,占当月总种类的54.55%。1 a中每个季节都可以检测到乙酸壬酯、2-壬酮和2-十一(烷)酮。
挥发性有机物 t/min CAS号 分子式 相对含量/% 3月 6月 9月 12月 2-octanone 仲辛酮 9.37 000111-13-7 C8H16O 0.19 - - - 3-hexen-1-ol, acetate, (Z)- (Z)-乙酸-3-己烯-1-醇酯 10.99 003681-71-8 C8H16O2 5.96 2.41 - 2.15 2-nonanone 2-壬酮 13.86 000821-55-6 C9H18O 48.98 6.22 17.74 34.21 cyclohexene, 3, 4-diethenyl-3-methyl- 3, 4-二乙烯基-3-甲基环己烷 14.99 061141-77-3 C11H16 2.47 0.24 1.53 - cyclohexene 环己烯 15.45 000110-83-8 C6H10 - - 2.91 - cyclotrisiloxane, hexamethyl- 六甲基环三硅氮烷 16.02 000541-05-9 C6H18O3Si3 - - - 4.65 1-nonene 壬烯 16.40 000124-11-8 C9H18 - 14.25 - - butanoic acid, 3-hexenyl ester, (Z)- (Z)-丁酸-3-己烯酯 17.13 016491-36-4 C10H18O2 0.63 0.86 - - cyclopentasiloxane, decamethyl- 十甲基环五硅氧烷 17.54 000541-02-6 C10H30O5Si5 - - - 0.47 chloroacetic acid, octyl ester 氯乙酸辛酯 18.45 005451-98-9 C10H19ClO2 - - 5.08 - acetic acid, sec-octyl ester 乙酸仲辛酯 19.07 054515-77-4 C10H20O3 16.93 - - - 2-decanone 2-癸酮 20.05 000693-54-9 C10H20O 1.14 0.52 - 0.09 2-undecanone 2-十一(烷)酮 20.21 000112-12-9 C11H22O 21.89 20.02 34.13 37.88 tridecyl acetate 十三基醋酸 20.39 1000351-76-9 C15H30O2 - - 2.43 - 2-dodecanone 2-十二烷酮 20.83 006175-49-1 C12H24O 0.68 1.23 - 2.04 acetic acid, nonyl ester 乙酸壬酯 21.38 000143-13-5 C11H22O2 0.41 0.61 15.46 10.20 butylated hydroxytoluene 2, 6-二叔丁基对甲基苯酚 23.38 000128-37-0 C15H24O - 0.42 - - 2-acetoxy tridecane 2-乙酰氧基十三烷 25.26 1000245-61-2 C15H30O 0.72 - - - 2-tridecanone 2-十三烷酮 25.71 000593-08-8 C13H26O - 0.78 - 1.30 2-propenoic acid, 3-phenyl-, methyl ester 肉桂酸甲酯 25.78 000103-26-4 C10H10O2 - - - 0.96 7H-furo[3, 2-g][1]benzopyran-7- one 未命名 30.29 000066-97-7 C11H6O3 - - 4.72 - 4-(3, 4-methylenedioxyphenyl) -2-butanone 4-(3, 4-亚甲基二氧基苯基)-2-丁酮 30.64 055418-52-5 C11H12O3 - 5.64 12.89 6.06 1, 3-benzodioxole, 5-(2, 2-dimethylethyl)- 未命名 33.45 028140-80-9 C11H14O2 - - 3.11 - 2H-furo[2, 3-H]-1-benzopyran-2-one 二氢山芹醇 34.46 000523-50-2 C14H14O4 - 0.59 - - (Z, Z)-9, 13-octadecadienoic acid (Z,Z)-9,13-十八烷二烯酸乙酯 34.78 000060-33-4 C18H32O3 - 16.80 - - (Z, Z)-9, 14-octadecadienoic acid (Z,Z)-9,14-十八烷二烯酸乙酯 34.85 000060-33-5 C18H32O4 - 10.81 - - (Z, Z)-9, 15-octadecadienoic acid (Z, Z)-9, 15-十八烷二烯酸乙酯 35.08 000060-33-6 C18H32O5 - 3.29 - - n-Hexadecanoic acid 十六烷酸 38.06 000057-10-3 C16H32O2 - 5.31 - - (Z, Z)-9, 12-octadecadienoic acid (Z, Z)-9, 12-十八烷二烯酸乙酯 40.92 000060-33-3 C18H32O2 - 10.01 - - 说明:“-”为未检测到或不存在 Table 1. Seasonal variations of components and relative contents of VOCs released from leaves of Ruta graveolens

Figure 2. Component numbers and relative contents of classified VOCs from leaves in different seasons of a year
1 a中不同季节,芸香叶片释放的酯类物质的相对含量呈先上升后下降的趋势,在6月最高,为44.78%,12月最低(图 2)。烷类和酮类化合物均呈先下降后上升的趋势,6月其相对含量最低,12月达最高。萜烯类、酸类和醇类化合物只在6月和9月检测到,且6月检测到的萜烯类和酸类化合物相对含量均高于9月;酚类物质仅在6月检测到。叶片释放的主要成分季节变化趋势如下:2-壬酮和2-十一(烷)酮的相对含量随季节呈高-低-高的趋势,而乙酸壬酯则呈现低—高—低的变化趋势。
-
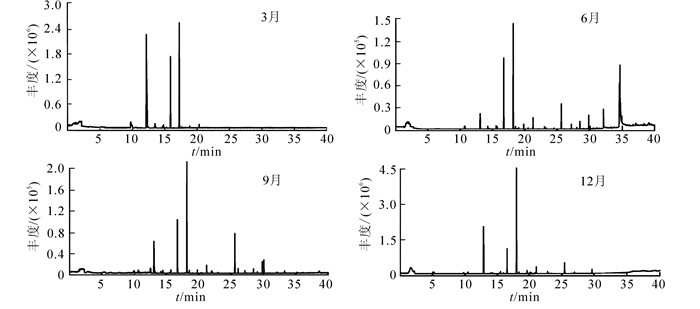
芸香叶片1 d中不同时段释放的挥发性有机物共鉴定出41种(表 2)。其中酯类化合物10种,酮类7种,酸类和醛类各6种,烷类和醇类各4种,以及少量的萜烯类、炔类等化合物;主要成分为乙酸仲辛酯,2-壬酮,2-十一(烷)酮,十六烷酸,油酸等。8:00-20:00叶片释放的挥发性有机物种类呈先增多后减少的趋势(图 3),12:00最多,为22种,20:00最少,为14种。8:00检测到酮类、酯类、酸类和醛类等4类化合物,以酮类最多;但挥发物的相对含量以酸类最高,其中十六烷酸和油酸,分别为39.90%和19.31%。10:00挥发物种类较8:00增加了6种,出现了烷类、醇类和炔类;酯类数量最多,其次为酮类,2类物质的相对含量较8:00大幅上升,酮类占此时挥发物含量的58.11%,其中2-壬酮占35.10%;而酸类化合物的相对含量急剧下降,仅为3.31%。12:00-14:00挥发物种类较10:00变化不大,14:00出现了萜烯类,但相对含量较低。16:00-20:00种类逐渐减少,主要成分和相对含量均以酮类和酯类化合物为主;18:00酸类相对含量在1 d中最低,而此时萜烯类和醛类含量达最高。1 d中,每个时段均可检测到(Z)-乙酸-3-己烯-1-醇酯,乙酸壬酯,2-壬酮,2-癸酮,2-十一(烷)酮,2-十三烷酮和十六烷酸7种物质。
挥发性有机物 t/min 化合物相对含量/% 8:00 10:00 12:00 14:00 16:00 18:00 20:00 3-hexen-1-ol, acetate, (Z)- (Z)-乙酸-3-己烯-1-醇酯 10.99 2.32 0.96 1.35 1.41 1.82 1.32 0.68 2-nonanone 2-壬酮 13.86 3.84 35.10 28.04 31.54 34.76 36.96 36.39 (R)-(-)-5-hexen-2-ol (R)-(-)-5-己烯-2-醇 14.60 - - - - - 0.94 - cyclohexene, 3, 4-diethenyl-3 -methyl- 3, 4-二乙烯基-3-甲基环己烷 14.99 - 1.58 - - 2.02 - 1.92 1, 3 -cycloheptadiene 1, 3-环庚二烯 16.07 - - - - - 1.77 - 3-cyclohexene-1-methanol 3-环己烯-1-甲醇 16.07 - - 1.78 1.83 - - - butanoic acid, 3-hexenyl ester, (Z)- (Z)-丁酸-3-己烯酯 17.13 - 0.38 0.49 0.49 0.44 0.41 0.31 acetic acid, octyl ester 乙酸辛酯 18.69 - 0.42 0.40 0.38 0.40 0.40 - acetic acid, decyl ester 乙酸癸酯 18.69 - - - - - - 0.48 acetic acid, sec-octyl ester 乙酸仲辛酯 19.07 - 21.81 22.65 21.49 23.25 22.88 23.48 2-decanone 2-癸酮 20.05 0.37 1.24 1.11 1.22 1.31 1.56 1.34 2-undecanone 2-十一(烷)酮 20.21 17.24 20.86 22.31 21.46 24.25 23.75 26.73 pentafluoropropionic acid, nonyl ester 未命名 20.63 5.69 - - - - - - 2-dodecanone 2-十二烷酮 20.83 0.36 - - - - - - acetic acid, nonyl ester 乙酸壬酯 21.38 0.52 0.48 0.71 0.42 0.47 0.51 0.63 2-acetoxy tridecane 2-乙酰氧基十三烷 25.26 - 1.01 1.34 1.12 1.22 0.18 1.47 2-tridecanone 2-十三烷酮 25.71 0.90 0.70 0.85 0.73 0.90 1.66 1.03 2-propenoic acid, 3-phenyl-, methyl 肉桂酸甲酯 25.78 0.39 - - - - - - ester 4- (3, 4-methylenedioxyphenyl) -2-bu tanone 4-(3, 4-亚甲基二氧基苯基)-2-丁酮 30.64 3.61 0.21 0.26 0.37 0.30 0.26 - 2H-furo [2, 3-H]-1-benzopyran-2-one 二氢山芹醇 34.46 - 0.11 - 0.12 - - - hexadecanal 棕榈醛 35.58 2.09 - - - - - - tetradecanoic acid 十四烷酸 35.65 0.58 0.32 0.37 0.28 - - - n-hexadecanoic acid 十六烷酸 38.06 39.90 2.46 5.78 2.89 2.69 0.28 1.35 cyclopentadecanone, 2-hydroxy- 2-羟基环十五烷酮 38.45 - - - - 0.33 - - (Z)-ll-hexadecenoic acid 反-11-十六烯酸 38.45 - - - 0.07 - - - 9-tetradecenal, (Z)- (Z)-9-十四烯醛 38.72 1.51 - - - - - - 9-oxabicyclo [6.1.0] nonane 9-双环氧辛烷[6.1.0]壬烷 39.95 - - 0.09 - - - - cis-9-hexadecenoic acid 顺-9-十六碳烯酸 40.29 - - 0.91 - - - - hexadecanoic acid, trimethylsilyl ester 棕榈酸三甲基硅脂 40.81 1.37 - - - - - - (Z, Z)-9, 12-octadecadienoic acid (Z, Z)-9, 12-十八烷二烯酸乙酯 40.92 - 7.23 0.76 1.67 - - - cis, cis-7, 10, -hexadecadienal 顺, 顺-7, 10-十六碳二烯醛 41.27 - - 0.22 - - 5.35 - oleic acid 油酸 41.84 19.31 0.36 7.20 9.23 4.21 - 3.66 (Z) -9, 17-octadecadienal (Z)-9, 17-十八烷烯醛 41.84 - 1.95 0.71 - - - - cis-13-octadecenoic acid 顺-13-十八碳烯酸 42.19 - - 1.08 - - 0.02 - 2-octyl-cyclopropaneoctanal 2-辛基-环丙基辛醛 42.47 - 1.87 1.58 2.35 0.37 - 0.54 cis-9-oxabicyclo [6.1.0] nonane 顺-9-双环氧辛烷[6.1.0]壬烷 42.72 - - - - 0.51 1.00 - Z, Z-10, 12-hexadecadien-1-ol acetate 1-乙酯基-Z, Z-10,12-十六二烯 42.91 - - - 0.24 - - - 1, 2-benzisothiazole, 3-(hexahydro-1H-azepin-1-yl)-, 1, 1-dioxide 未命名 43.02 - 0.23 - - - - - Z, Z-3, 13-octadecadienol-2-methyl 未命名 43.15 - 0.05 - - - - - 7-pentadecyne 7-十五炔 43.29 - 0.69 - - - - - (Z)-9-octadecenal (Z)-9-十八烷烯醛 43.47 - - - 0.71 0.74 0.75 - 说明:“-”为未检测到或不存在 Table 2. Components and relative contents of VOCs released from leaves of Ruta graveolens in one day
1 d不同时段,芸香叶片释放的酯类化合物的相对含量呈先上升后下降的趋势,10:00达最大;酮类呈先上升后略微下降再上升的趋势,20:00达最大,为65.49%;酸类呈先下降后上升再下降的趋势,8:00最大,为59.80%。烷类和醛类相对含量变化不明显;萜烯类、炔类和醇类的相对含量较少,仅在少数几个时间段出现(表 3)。其中,主要成分日变化趋势如下:乙酸仲辛酯和2-十一(烷)酮的相对含量呈现低-高的变化趋势,十六烷酸则呈现高—低的变化趋势,2-壬酮的相对含量呈现低—高—低—高的变化趋势,油酸呈现出高—低—高—低的变化趋势。
时刻 相对含量/% 酯 烯 烷 酮 酸 炔 醛 醇 其他 8:00 10.29 - - 26.31 59.80 - 3.60 - - 10:00 31.28 - 2.59 58.11 3.13 0.69 3.82 0.16 0.23 12:00 26.36 - 1.43 52.57 15.34 - 2.52 1.78 - 14:00 25.85 0.24 1.12 55.33 12.46 - 3.05 1.94 - 16:00 26.38 - 3.75 61.86 6.90 - 1.11 - - 18:00 25.51 1.77 1.18 64.20 0.30 - 6.10 0.94 - 20:00 25.57 - 3.39 65.49 5.01 - 0.54 - - 说明:“-”为未检测到或不存在 Table 3. Classified statistic about components and relative contents of VOCs released from leaves in different time of a day
-
通过对芸香花瓣的SPME/GC/MS总离子流图分析(图 4),扣除本底空气中的杂质,3月花期时,花瓣释放的挥发性有机物中检测出8种挥发性成分,其中酮类化合物最多,有5种,其相对含量占花瓣总挥发物的82.24%;其次为萜烯类化合物,其相对含量为11.20%(表 4)。花瓣中检测到的相对含量最多的挥发性成分是2-十一(烷)酮,为66.10%,其次为2-壬酮和壬烯。
挥发性有机物 t/min CAS号 相对含量/% 2-nonanone 2-壬酮 13.86 000821-55-6 13.18 cyclohexene, 3, 4-diethenyl-3-methyl- 3, 4-乙烯基-3-甲基环己烷 14.99 061141-77-3 1.82 1-nonene 壬烯 16.40 000124-11-8 11.20 2-decanone 2-癸酮 20.05 000693-54-9 2.09 2-undecanone 2-十一(烷)酮 20.21 000112-12-9 66.10 tridecyl acetate 乙酸十三酯 20.39 1000351-76-9 4.74 2-dodecanone 2-十二烷酮 20.83 006175-49-1 0.34 2-tridecanone 2-十三烷酮 25.71 000593-08-8 0.53 说明:“-”为未检测到或不存在 Table 4. Components and relative contents of VOCs released from flowers of Ruta graveolens
2.1. 芸香叶片释放挥发性有机物成分和其相对含量的季节动态变化
2.2. 芸香叶片释放挥发性有机物的日动态变化
2.3. 芸香花瓣释放的挥发性有机成分及其相对含量
-
目前,学者对芸香或芸香科其他植物挥发性有机物的研究多采用水蒸气蒸馏法和溶剂提取法[3-5, 11]。蒸馏法简单易行,适于难溶或不溶于水的成分的提取;但属于非活体提取,长时间高温提取会使某些挥发性物质受到破坏;溶剂提取法是根据相似相溶原理,选择适合的溶剂非常关键,但溶剂中的微量杂质沉淀在提取物中,影响提取物的纯度[19]。通过对比各种提取方法,本研究采用了顶空-固相微萃取采集法,此方法所需样品量少,无需溶剂,操作简化,集采样、萃取、浓集、进样于一体,非常适合芳香植物挥发性成分的研究,已在刺山柑Capparis spinosa,茶Camellia sinensis,黄兰Michelia chmpaka,麦秆菊Helichrysum bracteatum等多种植物挥发物的研究中得到应用[20-23]。
植物释放挥发性有机物的成分和相对含量在同种之间存在差异,与植物的品种、自身结构特点、生理状态和株龄等有密切关系[24-25]。如HADDOUCHI等[4]研究得出阿尔及利亚生长的芸香含有10种挥发性有机物,唐祖年等[15]从芸香挥发油中鉴定出21种成分,徐汉虹等[2]从芸香精油中鉴定出39种成分。本研究在芸香叶片中共鉴定出41种,花瓣中检测到8种,虽然与前人鉴定的结果不尽相同,但通过对比发现,芸香挥发性有机物的主要成分均为2-十一(烷)酮和2-壬酮。主要原因是植物的自身特性决定了不同植物所释放的有机物成分、含量和释放速率不同。
前人[26-31]对侧柏Platycladus orientalis,薄荷Mentha×piperita,红松Pinus koraiensis,桂花Osmanthus fragrans等植物挥发性有机物释放规律的研究表明,挥发物释放的种类和相对含量与采样时温度、湿度、气孔导度、光照以及蒸腾速率等关系密切。一般而言,随着温度、湿度、光照等的提高,植物释放的有机物也会增多。本研究表明,不同季节和1 d中不同时段芸香释放的挥发性有机物的种类均呈先上升后下降的趋势;其中,6月最多,为18种;1 d中12:00最多,为22种。在测试的4个月中,6月光照时间较长,温度较高,达30 ℃左右,植物进入生长旺盛阶段,植物自身旺盛的光合作用为有机物合成提供充足的碳源和还原力,体内有机物合成、代谢加快,强烈的蒸腾作用也为有机物的扩散创造了条件,使挥发性有机物的种类增多[32-34]。说明随着温度、光照等的提高,芸香叶片释放的挥发性有机物的种类也随之增多。另外,研究发现芸香花瓣释放的挥发性有机物种类少于叶片,叶片和花瓣挥发性有机物的主要成分均为酮类化合物,叶片释放的物质中以2-壬酮为主,花瓣中以2-十一(烷)酮为主。芸香释放的挥发性有机物种类多少、相对含量的高低与外界环境以及植物自身条件的密切相关,两者之间关系有待进一步深入研究。
植物释放的挥发性有机物具有一定的药用保健作用。前人[35-36]研究已证实,针叶树释放的萜烯类化合物使人情绪趋于放松状态,使人感觉清新、舒爽和愉悦;柠檬油、雪松醇、龙脑和1, 8-桉叶油能够降低心率和血压;萜烯类和生物碱类物质具有一定的抗菌和消炎作用[3-6, 16]。芸香叶片和花瓣释放的挥发性有机物的主要成分均具有一定的药用价值,可以起到杀虫抑菌效果,在某种程度上对调节人体情绪具有一定的作用。如:2-壬酮具有果香、甜香、皂香及椰子、奶油的气味,2-十一(烷)酮是芸香特有的香气,低浓度时有类似桃子的香气;这些有机物的气味或许可以使人趋于放松状态,从而间接起到调节人体情绪的作用。因此,在植物景观设计中,可以适当栽植芸香,营造保健型的生态植物景观。












 DownLoad:
DownLoad: