-
花香是植物育种的一个重要目标,也是园林植物的重要美学特征之一[1]。随着核磁共振和色谱分析技术的发展,包括月季Rosa chinensis,玫瑰Rosa rugosa,梅花Prunus mume,蜡梅Chimonanthus praecox等数百种园林植物花香的成分已被分析鉴定[2-7]。现在认为,花香由多种挥发性有机化合物(VOCs)组成,主要包括萜烯类、苯丙酸类/苯环型和脂肪酸衍生物等[8]。植物开花过程中,花香VOCs的成分和释放量与植物种类和花朵发育程度密切相关,如,释放苯甲酸甲酯的金鱼草Antirrhinum majus和多花黑鳗藤Stephanotis floribunda具有相反的昼夜节律,多花黑鳗藤VOCs在夜间的释放量高于白天[9-10],水仙花Narcissus tazetta var. chinensis主要成分苯甲醛相对含量在第6天达到最高[11]。鲜花VOCs的释放节律得到许多草本植物的证明[9, 12-14],但是,木本植物(尤其木质藤本植物)的研究较少。日本紫藤Wisteria floribunda是豆科Leguminosae紫藤属Wisteria木质藤本,花期4月下旬至5月中旬,是集观赏、食用、药用、芳香精油于一体的优良园林植物,在园林中广泛用于垂直绿化[15]。Jiang等[16]检测了日本紫藤盛花期的VOCs成分,关于日本紫藤花期VOCs变化尚未见报道。因此,本研究以日本紫藤为试验材料,采用活体植株动态顶空气体循环采集法与热脱附-气相色谱-质谱(TDS-GC-MS)联用技术测定日本紫藤鲜花不同花期VOCs的成分及其相对含量,旨在明确日本紫藤鲜花在花期的特征香气成分,探索其花期动态规律,为进一步研究植物VOCs对环境质量的影响以及园林植物的科学配置提供理论依据。
-
以浙江农林大学东湖校区日本紫藤为材料。采集健康无损伤,15年生左右,地径约12 cm的植株。
-
于2012年4月10日-20日上午10:00-11:00,采用动态顶空气体循环法[17]分别采集日本紫藤花序不同开花阶段即花蕾期、初开期和盛开期的VOCs。采集花序1个·次-1,大约40朵小花,采集3次·花期-1。采气袋容积0.1 m3,采气时间为10 min,气体流量0.1 m3·min-1。
-
VOCs分析采用TDS-GC-MS联用技术,仪器及参数设置条件参考文献[17]。TDS(德国GERSTEL公司生产的TD3型)工作条件:系统载气压力为20 kPa;进样口温度250 ℃;脱附温度250 ℃,10 min;冷阱温度-100 ℃,保持3 min;冷阱进样时温度骤然升温至260 ℃。GC(7890A,Agilent)工作条件:色谱柱为30 m×250 μm×0.25 μm的HP-5MS柱;程序升温;初始温度为40 ℃,保持4 min后以6 ℃·min-1的速率升至250 ℃,保持3 min后以10 ℃·min-1的速率升至270 ℃,保持5 min。MS(5975C,Agilent)工作条件:电离方式为EI,电子能量为70 eV,质量范围28~450,接口温度280 ℃,离子源温度为230 ℃,四级杆温度150 ℃。
-
采用NIST2008谱图库兼顾色谱保留时间,同时结合手工检索确定VOCs成分,利用峰面积和相对含量分析比较日本紫藤不同开花阶段VOCs的动态变化。
-
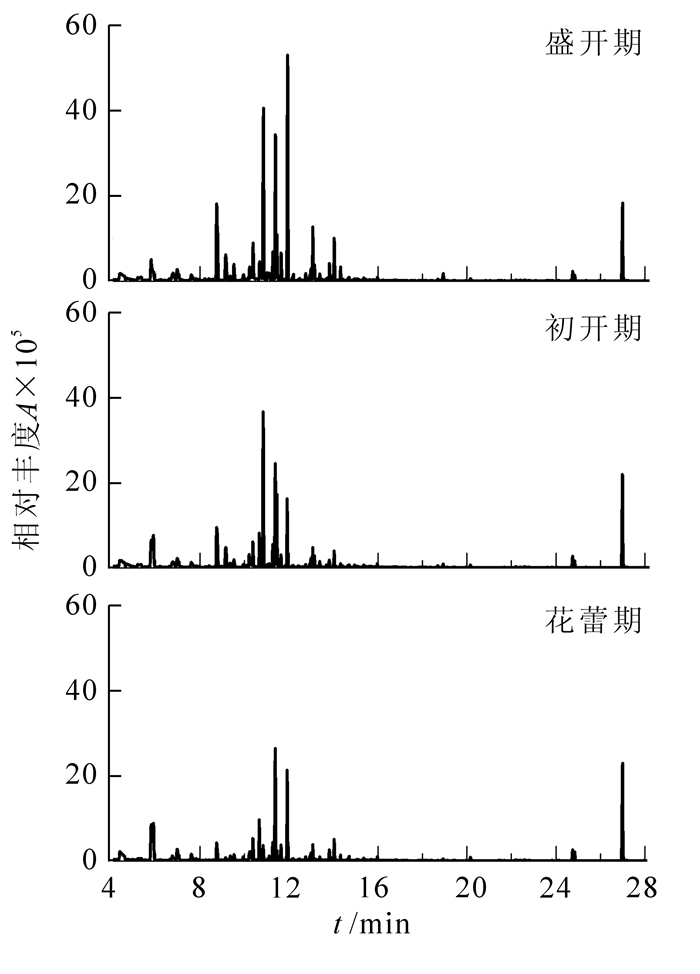
试验得到日本紫藤开花阶段鲜花VOCs总离子流图(图 1)。日本紫藤不同开花阶段鲜花共含34种VOCs,各开花阶段均含萜烯类、酯类、醇类、酮类、醛类、酚类、烷类和芳香烃类(图 1,表 1)。

图 1 曰本紫藤不同开花阶段鲜花挥发性有机化合物总离子流图
Figure 1. Total ion current (TIC) of VOCs from Wisteria floribunda at different flowering stages
表 1 日本紫藤不同开花阶段鲜花挥发性有机化合物组分
Table 1. Constituents of VOCs from Wisteria floribunda at different flowering stages
序号 t/min 挥发性有机化合物 峰面积(A×105) 花蕾期 初开期 盛开期 1 4.697 3-甲基-丁酸甲酯butanoic acid, 3-methyl-, methyl ester - 6.71±0.29 - 2 5.198 已醛hexanal - 3.00±1.17 - 3 6.778 1-乙炔基-1-环己烯1-cyclohexene, 1-ethynyl- - 8.12±0.21 - 4 6.786 叶醇leaf alcohol - - 6.13±0.43 5 8.742 α-薇烯α-pinene 14.47±1.21 38.77±4.89 71.93±8.75 6 9.143 莰烯camphene 2.60±0.24 18.95±2.15 24.29±2.21 7 9.360 3-苯基-2-丁醇3-phenyl-2-butanol 3.65±0.53 3.93±0.75 4.68±0.12 8 9.528 苯甲醛benzaldehyde 4.16±0.31 5.71±1.34 11.30±1.03 9 9.937 β-薇烯β-pinene 2.38±0.67 13.41±1.52 4.41±0.70 10 10.238 苯酚phenol 10.24±0.11 8.81±0.74 8.24±0.31 11 10.297 6-甲基-5-庚烯-2-酮5-hepten-2-one, 6-methyl- - 3.08±0.21 4.04±1.42 12 10.372 月桂烯myrcene 11.87±1.20 - 19.39±2.45 13 10.714 水芹烯phellandrene 3.95±0.79 3.70±1.30 6.80±0.43 14 10.831 乙酸叶醇酯3-hexen-1-ol, acetate, (Z)- 8.19±0.32 80.48±7.10 91.88±8.20 15 11.015 乙酸已酯acetic acid, hexyl ester - - 5.81±1.34 16 11.116 芳構醇linalool 1.92±0.28 - 4.29±0.21 17 11.266 对伞花烃p-cymene 8.80±0.91 11.05±0.62 - 18 11.275 邻位伞花烃o-cymene 5.87±1.64 - 14.37±1.52 19 11.375 柠檬烯limonene 55.27±4.91 54.53±2.76 75.84±6.03 20 11.450 桉叶油醇eucalyptol 5.18±1.54 35.02±2.73 22.73±1.00 21 11.642 反式罗勒烯trans-ocimene 7.07±0.13 5.87±1.01 12.96±1.32 22 11.918 罗勒烯ocimene 42.84±8.02 32.84±1.62 121.05±10.21 23 12.745 2, 6-二甲基-1, 3, 5, 7-辛四烯2, 6-dimethyl-1, 3, 5, 7-octatetraene 2.02±0.08 4.32±0.07 4.04±0.24 24 12.929 5, 6-二亚甲烯基环辛烯cyclooctene, 5, 6-dimethylene- 2.65±0.14 - - 25 12.979 2, 4-二甲基苯乙烯benzene, 1-methyl-4-(1-methylethenyl)- 3.65±0.23 4.90±0.62 10.19±1.13 26 13.063 2-壬酮2-nonanone 7.26±0.77 9.06±0.86 25.72±4.37 27 13.138 苯甲酸甲酯benzoic acid, methyl ester - 5.27±0.77 6.95±0.51 28 13.381 壬醛Sonanal 1.73±0.16 2.68±0.20 - 29 14.032 1, 3, 8对-薄荷三烯1, 3, 8-p-menthatriene 11.48±0.50 8.92±0.32 8.37±0.24 30 14.033 反式-3-曹烯-2-醇trans-3-caren-2-ol - - 22.97±2.19 31 14.308 别罗勒烯allo-ocimene 2.66±0.93 - 6.53±0.52 32 15.971 癸醛decanal 1.96±1.88 - - 33 18.930 甲基正壬酮2-undecanone 1.81±0.21 3.14±0.34 5.92±0.43 34 24.747 十五烷pentadecane 6.00±0.82 5.89±0.31 4.74±0.51 说明:“-”代表未检测到VOCs。 -
花蕾期检测出VOCs成分26种,萜烯类化合物释放量最高,峰面积为159.26,其在花蕾期相对含量达到了总量的69.0%,是主要香气成分;芳香烃类化合物次之,峰面积为18.32,相对含量占9.0%。释放量最多的2个成分是柠檬烯(55.27)和罗勒烯(42.84),分别占VOCs总量的24.0%和19.0%。另外,α-蒎烯(14.47),月桂烯(11.87),1, 3, 8-对薄荷三烯(11.48),苯酚(10.24),对伞花烃(8.80),乙酸叶醇酯(8.19),2-壬酮(7.26),反式罗勒烯(7.07),十五烷(6.00)及邻位伞花烃(5.87)的相对含量均为3%~6%。5, 6-二亚甲烯基环辛烯和癸醛在其他各阶段均未检测到,是花蕾期的特有成分。
-
初开期含VOCs成分25种,峰面积总量为378.16,是花蕾期的1.65倍,其中萜烯类、酯类和醇类化合物是主要香气成分,相对含量各占50.0%,24.0%和10.0%。主要成分包括乙酸叶醇酯(80.84),柠檬烯(54.53),α-蒎烯(38.77),桉叶油醇(35.02),罗勒烯(32.84)等化合物,分别占芳香物质总量的21.0%,14.0%,10.0%,9.0%和9.0%。另外,莰烯、反式罗勒烯、对伞花烃相对含量也较高,为3.0%~5.0%。其中1-乙炔基-1-环己烯、3-甲基-丁酸甲酯和己醛在其他各阶段均未检测到。
-
盛开期含27种VOCs成分,峰面积总量最高,达到605.57,是花蕾期的2.64倍。盛开期主要香气成分也是萜烯类、酯类和醇类化合物,分别各占芳香物质总量的59.0%,17.0%和11.0%。主要成分是罗勒烯(121.05),乙酸叶醇酯(91.88),柠檬烯(75.84)和α-蒎烯(71.93)等,其相对含量分别为总量的20.0%,15.0%,13.0%和12.0%,2-壬酮、莰烯、反式-3-蒈烯-2-醇、桉叶油醇、月桂烯的相对含量均为3.0%~4.0%。叶醇、乙酸己酯和反式-3-蒈烯-2-醇只在盛开期才检测到。
-
从表 1,图 2和图 3可以看出:萜烯类是日本紫藤鲜花的主要芳香成分。在日本紫藤鲜花释放的VOCs中,萜烯类化合物的种类数在花蕾期、初开期和盛开期分别是12,10和11种,在各开花阶段均超过1/3。萜烯类化合物的峰面积在不同开花阶段则占到50.0%~69.0%,远高于其他种类。随着开花进程萜烯类化合物峰面积逐渐增加,在盛开期是初开期的1.88倍,是花蕾期的2.23倍。β-蒎烯在初开期释放量最大,盛开期开始减少,其在初开期(13.41)释放量是花蕾期(2.38)的5.63倍,在盛开期(4.41)则仅是花蕾期的1.85倍;而水芹烯、柠檬烯、反式罗勒烯和罗勒烯在初开期释放量略有减少,盛开期又迅速增加,其中罗勒烯在初开期释放量比在花蕾期减少了近1/4,在盛开期释放量则比花蕾期增加了1.83倍;α-蒎烯和莰烯均随着花朵开放程度的增大释放量逐渐增多,至盛开期达到最高,分别是71.93和24.29,在盛开期释放量分别是花蕾期的4.97和9.34倍;月桂烯和别罗勒烯仅出现在花蕾期和盛开期,均呈增加趋势(表 1)。
-
酯类化合物是日本紫藤的另一类主要芳香成分。从图 2可以看出:酯类化合物在开花阶段的相对含量从4.0%增长至24.0%,伴随花的不断发育,酯类化合物峰面积迅速增加,在花蕾期只有8.19,初开期即迅速增加到92.46,酯类化合物初开期是花蕾期的11.29倍,盛开期为104.64,是花蕾期的12.78倍。乙酸叶醇酯的峰面积在不同开花阶段中占酯类总量的87.0%~100.0%,是酯类中峰面积最高的成分,在初开期(80.84)即迅速增加,是花蕾期(8.19)的9.83倍,盛开期(91.88)是花蕾期的11.22倍;苯甲酸甲酯初开期才开始检测到,其释放量呈平缓增长趋势。
-
日本紫藤鲜花发育过程中醇类化合物呈增加趋势,花蕾期醇类化合物释放量仅为10.75,初开期释放量迅速增加至38.95,是花蕾期的3.62倍,盛开期醇类化合物释放量最高,峰面积为60.80,是花蕾期的5.66倍。桉叶油醇在初开期迅速增加并达到最高,初开期释放量增加至35.02,是花蕾期(5.18)的6.76倍,盛开期即开始减少;3-苯基-2-丁醇的释放量随着开放程度的增加而平缓增加,初开期释放量(3.93)是花蕾期(3.65)的1.08倍,盛开期(4.68)是花蕾期的1.28倍;芳樟醇只出现在花蕾期和盛开期,且释放量迅速增加,盛开期是花蕾期的2.23倍。
-
芳香烃类化合物在初开期(15.95)和花蕾期(18.32)释放量无显著差异,盛开期(24.56)比初开期增长了54.0%。其中2, 4-二甲基苯乙烯呈增长趋势,盛开期(10.19)比初开期(4.90)增长了108.0%;对伞花烃只在花蕾期和初开期检测出,邻位伞花烃仅出现在花蕾期和盛开期,且均呈增长趋势。
-
除萜烯类、酯类、醇类和芳香烃类化合物外,本研究还检测出了酮类、醛类、酚类和烷烃类化合物。酮类化合物释放量伴随花的发育增加而增加,在盛开期释放量(35.68)是花蕾期(9.07)的近4倍,2-壬酮和甲基正壬酮盛开期释放量分别是花蕾期的3.54和3.27倍,6-甲基-5-庚烯-2-酮在初开期才开始出现;醛类化合物释放量呈先升高后略降低的趋势,在初开期相对含量比花蕾期增加了45.1%,盛开期比花蕾期增加了44.0%,其中苯甲醛释放量随花的发育逐渐升高,初开期(5.71)释放量是花蕾期(4.16)的1.37倍,盛开期(11.30)释放量是花蕾期的2.72倍,而壬醛只在花蕾期和初开期检测到,且初开期(2.68)是花蕾期(1.73)的1.55倍。酚类和烷烃类各检测出1种物质,分别是苯酚和十五烷,均随开花阶段逐渐减少。
-
本研究中,日本紫藤开花进程中鲜花VOCs成分包括萜烯类、酯类、醇类、醛类、酮类、酚类、芳香烃类和烷烃类等8大类(表 1),化合物释放量从花蕾期到盛开期逐渐增大,其中萜烯类化合物的数量最多和释放量最高,萜烯类、酯类、醇类、酮类表现出与花香总释放量相同的变化规律(图 2,图 3),与菊花Chrysanthemum morifolium,玫瑰,紫藤Wisteria sinensis,黄兰Michelia champaca,蜡梅,文心兰Oncidium spp.等研究类似[4, 6, 18-21],日本紫藤开花进程中萜烯类、醇类、酯类等化合物呈现上升趋势可能与吸引传粉者有关[22]。Jiang等[16]在田纳西大学校园内采用动态顶空法和GC-MS技术鉴定了日本紫藤挥发物盛开期的成分,发现萜烯类物质释放量最大,芳樟醇和罗勒烯释放量最高,共含有8种成分与本研究相同,依次为芳樟醇、罗勒烯、柠檬烯、α-蒎烯、乙酸叶醇酯、甲基正壬酮、壬醛和癸醛。而本研究中相对含量高的化合物分别是罗勒烯、乙酸叶醇酯、柠檬烯、α-蒎烯,这说明同一种植物挥发物成分有很高相似度,但种类和相对含量受地理条件影响显著。
李祖光等[19]利用固相微萃取法和GC-MS法采集鉴定紫藤不同花期香气成分,鉴定出芳樟醇、2-壬酮和反式罗勒烯等47种化合物,其中芳樟醇各阶段相对含量均大于60%;Jiang等[16]采用动态顶空气体循环采集法和TDS-GC-MS联用技术从紫藤鲜花VOCs中鉴定出芳樟醇、罗勒烯和苯甲酸甲酯等28种化合物,从日本紫藤VOCs鉴定出芳樟醇、罗勒烯和苯甲醇等22种化合物。本研究在日本紫藤鲜花盛开期鉴定出罗勒烯、乙酸叶醇酯、柠檬烯和α-蒎烯等27种化合物(表 1),其中芳樟醇、罗勒烯、苯甲酸甲酯、甲基正壬酮、桉叶油醇、壬醛、癸醛和苯甲醛等8种化合物与紫藤相同。单萜在日本紫藤和紫藤具有明显的差异,日本紫藤盛开期含有15种单萜物质,紫藤只含6种单萜[16],表明日本紫藤和紫藤鲜花主成分相似,但萜类成分上存在显著差异,可能与植物中单萜合成酶有关[23-24]。
研究表明:花香VOCs的成分和释放量变化主要取决于不同发育阶段花香成分相关生物合成酶的活性及基因表达的不同[9, 23-25]。苯甲酸甲酯的释放节律与3种甲基转移酶基因(AmBAMT,SfSAMT和NsBSMT)mRNA的水平波动一致[9, 23-25],初开时NsBSMT的转录水平最高[25],开花后第4天AmBAMT的转录水平达到最高,为苯甲酸甲酯释放的最高期[24]。一般随着开花进程花香VOCs的种类和释放量逐渐升高,授粉时达到高峰,此后逐渐下降直至消失,有的花香VOCs的成分也会发生变化[26]。日本紫藤鲜花VOCs成分和释放量在开花进程中具有明显的变化规律,这种变化是如何受基因表达调控的还有待进一步研究。
Changes in constituents and contents of volatile organic compounds in Wisteria floribunda at three flowering stages
-
摘要: 为探讨植物花期香气成分及其相对含量变化, 采用动态顶空气体循环采集法和热脱附-气相色谱-质谱(TDS-GC-MS)联用技术对日本紫藤Wisteria floribunda不同开花时期释放挥发性有机化合物组分及其相对含量进行测定。结果表明:日本紫藤鲜花释放的挥发性有机化合物包括萜烯类、酯类、醇类、醛类、酮类、酚类、芳香烃类和烷烃类等34种化合物; 其释放量在花蕾期较少(229.68峰面积单位), 初开期逐渐增加(378.16), 盛开期最高(605.57), 其中萜烯类数量最多(10~12种), 其相对含量花蕾期为69.0%, 初开期50.0%, 盛开期59.0%, 在各阶段的总峰面积依次为159.26, 189.43, 355.61。罗勒烯、乙酸叶醇酯、柠檬烯、α-蒎烯等是日本紫藤鲜花的主要香气成分。Abstract: To determine the variation of aromatic constituents and their relative content in different flowering stages of Wisteria floribunda, constituents and their relative content of volatile organic compounds (VOCs) were analyzed using the dynamic headspace air-circulation method and the thermal desorption system/gas chromatography/mass spectrum (TDS-GC-MS) technique at three flowering stages:bud stage, early opening stage, and full opening stage. Results showed that 34 VOCs including terpenes, esters, alcohols, aldehydes, ketones, phenols, aromatic and naphthenic compounds were detected in flowers of W. floribunda. The relative content of VOCs was low at the bud stage (229.68 peak area units), gradually increased at the early opening stage (378.16 peak area units), and peaked at the full opening stage (605.57 peak area units). Among these components, terpenes were the most plentiful (10-12 compounds) and had the highest relative content at each stage (in relative peak area):bud stage——69.0%, early opening stage——50.0%, and full opening stage——59.0%. The major aroma components of W. floribunda were as follows:ocimene, (Z)-3-Hexen-1-ol, acetate, limonene, and α-pinene.
-
Key words:
- botany /
- Wisteria floribunda /
- flowering stage /
- volatile organic compounds /
- constituents /
- relative content
-
表 1 日本紫藤不同开花阶段鲜花挥发性有机化合物组分
Table 1. Constituents of VOCs from Wisteria floribunda at different flowering stages
序号 t/min 挥发性有机化合物 峰面积(A×105) 花蕾期 初开期 盛开期 1 4.697 3-甲基-丁酸甲酯butanoic acid, 3-methyl-, methyl ester - 6.71±0.29 - 2 5.198 已醛hexanal - 3.00±1.17 - 3 6.778 1-乙炔基-1-环己烯1-cyclohexene, 1-ethynyl- - 8.12±0.21 - 4 6.786 叶醇leaf alcohol - - 6.13±0.43 5 8.742 α-薇烯α-pinene 14.47±1.21 38.77±4.89 71.93±8.75 6 9.143 莰烯camphene 2.60±0.24 18.95±2.15 24.29±2.21 7 9.360 3-苯基-2-丁醇3-phenyl-2-butanol 3.65±0.53 3.93±0.75 4.68±0.12 8 9.528 苯甲醛benzaldehyde 4.16±0.31 5.71±1.34 11.30±1.03 9 9.937 β-薇烯β-pinene 2.38±0.67 13.41±1.52 4.41±0.70 10 10.238 苯酚phenol 10.24±0.11 8.81±0.74 8.24±0.31 11 10.297 6-甲基-5-庚烯-2-酮5-hepten-2-one, 6-methyl- - 3.08±0.21 4.04±1.42 12 10.372 月桂烯myrcene 11.87±1.20 - 19.39±2.45 13 10.714 水芹烯phellandrene 3.95±0.79 3.70±1.30 6.80±0.43 14 10.831 乙酸叶醇酯3-hexen-1-ol, acetate, (Z)- 8.19±0.32 80.48±7.10 91.88±8.20 15 11.015 乙酸已酯acetic acid, hexyl ester - - 5.81±1.34 16 11.116 芳構醇linalool 1.92±0.28 - 4.29±0.21 17 11.266 对伞花烃p-cymene 8.80±0.91 11.05±0.62 - 18 11.275 邻位伞花烃o-cymene 5.87±1.64 - 14.37±1.52 19 11.375 柠檬烯limonene 55.27±4.91 54.53±2.76 75.84±6.03 20 11.450 桉叶油醇eucalyptol 5.18±1.54 35.02±2.73 22.73±1.00 21 11.642 反式罗勒烯trans-ocimene 7.07±0.13 5.87±1.01 12.96±1.32 22 11.918 罗勒烯ocimene 42.84±8.02 32.84±1.62 121.05±10.21 23 12.745 2, 6-二甲基-1, 3, 5, 7-辛四烯2, 6-dimethyl-1, 3, 5, 7-octatetraene 2.02±0.08 4.32±0.07 4.04±0.24 24 12.929 5, 6-二亚甲烯基环辛烯cyclooctene, 5, 6-dimethylene- 2.65±0.14 - - 25 12.979 2, 4-二甲基苯乙烯benzene, 1-methyl-4-(1-methylethenyl)- 3.65±0.23 4.90±0.62 10.19±1.13 26 13.063 2-壬酮2-nonanone 7.26±0.77 9.06±0.86 25.72±4.37 27 13.138 苯甲酸甲酯benzoic acid, methyl ester - 5.27±0.77 6.95±0.51 28 13.381 壬醛Sonanal 1.73±0.16 2.68±0.20 - 29 14.032 1, 3, 8对-薄荷三烯1, 3, 8-p-menthatriene 11.48±0.50 8.92±0.32 8.37±0.24 30 14.033 反式-3-曹烯-2-醇trans-3-caren-2-ol - - 22.97±2.19 31 14.308 别罗勒烯allo-ocimene 2.66±0.93 - 6.53±0.52 32 15.971 癸醛decanal 1.96±1.88 - - 33 18.930 甲基正壬酮2-undecanone 1.81±0.21 3.14±0.34 5.92±0.43 34 24.747 十五烷pentadecane 6.00±0.82 5.89±0.31 4.74±0.51 说明:“-”代表未检测到VOCs。 -
[1] CHANDLER S, TANAKA Y. Genetic modification in floriculture[J]. Crit Rev Plant Sci, 2007, 26(4):169-197. [2] WU Shuiqin, WANTANABE N, MITA S, et al. The key role of phloroglucinol o-methyltransferase in the biosynthesis of Rosa chinensis volatile 1, 3, 5-trimethoxybenzene[J]. Plant Physiol, 2004, 135(1):95-102. [3] 王海萍, 晏慧君, 张颢, 等.月季丁香酚合成酶基因RcEGS1的克隆及其表达分析[J].园艺学报, 2012, 39(7):1387-1394. WANG Haiping, YAN Huijun, ZHANG Hao, et al. Cloning and expression analysis of eugenol synthase gene RcEGS1 in Rosa chinensis 'Pallida'[J]. Acta Hortic Sin, 2012, 39(7):1387-1394. [4] 冯立国, 生利霞, 赵兰勇, 等.玫瑰花发育过程中芳香成分及含量的变化[J].中国农业科学, 2008, 41(12):4341-4351. FENG Liguo, SHENG Lixia, ZHAO Lanyong, et al. Changes of the aroma constituents and contents in the course of Rosa rugosa Thunb. flower development[J]. Sci Agric Sin, 2008, 41(12):4341-4351. [5] 赵印泉, 潘会堂, 张启翔, 等.梅花花朵香气成分时空动态变化的研究[J].北京林业大学学报, 2010, 32(4):201-206. ZHAO Yinquan, PAN Huitang, ZHANG Qixiang, et al. Dynamics of fragrant compounds from Prunus mume flowers[J]. J Beijing For Univ, 2010, 32(4):201-206. [6] 周继荣, 倪德江.蜡梅不同品种和花期香气变化及其花茶适制性[J].园艺学报, 2010, 37(10):1621-1628. ZHOU Jirong, NI Dejiang. Changes in flower aroma compounds of cultivars of Chimonanthus praecox (L.) link and at different stages relative to chimonanthus tea quality[J]. Acta Hortic Sin, 2010, 37(10):1621-1628. [7] 周帅, 马楠, 林富平, 等.樟树花挥发性有机化合物日动态变化分析[J].浙江农林大学学报, 2011, 28(6):986-991. ZHOU Shuai, MA Nan, LIN Fuping, et al. Diurnal variation of volatile organic compounds emitted from Cinnamomum camphora flowers[J]. J Zhejiang A & F Univ, 2011, 28(6):986-991. [8] DUDAREVA N, PICHERSKY E, GERSHENZON J. Biochemistry of plant volatiles[J]. Plant Physiol, 2004, 135(4):1893-1902. [9] KOLOSOVA N, GORENSTEIN N, KISH C M, et al. Regulation of circadian methyl benzoate emission in diurnally and nocturnally emitting plants[J]. Plant Cell, 2001, 13(10):2333-2347. [10] POTT M B, PICHERSKY E, PIECHULLA B. Evening specific oscillations of scent emission, SAMT enzyme activity, and SAMT mRNA in flowers of Stephanotis floribunda[J]. J Plant Physiol, 2002, 159(8):925-934. [11] 黄巧巧, 冯建跃.水仙花开放期间香气组分变化的研究[J].分析测试学报, 2004, 23(5):110-113. HUANG Qiaoqiao, FENG Jianyue. Study on the variation in Nacissus armo composition during blossoming[J]. J Instrum Anal, 2004, 23(5):110-113. [12] PICHERSKY E, RAGUSO R A, LEWINSOHN E, et al. Floral scent production in Clarkia (Onagraceae) (I) localization and developmental modulation of monoterpene emission and linalool synthase activity[J]. Plant Physiol, 1994, 106(4):1533-1540. [13] ALTENBURGER R, MATILE P. Circadian rhythmicity of fragrance emission in flowers of Hoya carnosa R.Br.[J]. Planta, 1988, 174(2):248-252. [14] LEE J, SUGAWARA E, YOKOI S, et al. Genotypic variation of volatile compounds from flowers of gentians[J]. Breed Sci, 2010, 60(1):9-17. [15] 陈德昭, 方云忆, 张若蕙.中国植物志:第40卷:被子植物门双子叶植物纲[M].北京:科学出版社, 1999:183-188. [16] JIANG Yifan, CHEN Xinlu, LIN Hong, et al. Floral scent in Wisteria:chemical composition, emission pattern, and regulation[J]. J Amer Soc Hort Sci, 2011, 136(5):307-314. [17] GAO Yan, JIN Youju, LI Haidong, et al. Volatile organic compounds and their roles in bacteriostasis in five conifer species[J]. J Integr Plant Biol, 2005, 47(4):499-507. [18] 徐瑾, 李莹莹, 郑成淑, 等.菊花不同花期及花序不同部位香气成分和挥发研究[J].西北植物学报, 2012, 32(4):0722-0730. XU Jin, LI Yingying, ZHENG Chengshu, et al. Studies of aroma compounds in Chrysanthemum in different florescence and inflorescence parts and aroma releasing[J]. Acta Bot Boreal-Occident Sin, 2012, 32(4):0722-0730. [19] 李祖光, 李建亮, 曹慧, 等.紫藤鲜花在不同花期的头香成分[J].浙江林学院学报, 2009, 26(3):308-313. LI Zuguang, LI Jianliang, CAO Hui, et al. Chemical constituents of fragrances released from fresh flowers of Wisteria sinensis during different florescence periods[J]. J Zhejiang For Coll, 2009, 26(3):308-313. [20] 蒋冬月, 李永红, 何昉, 等.黄兰开花过程中挥发性有机成分及变化规律[J].中国农业科学, 2012, 45(6):1215-1225. JIANG Dongyue, LI Yonghong, HE Fang, et al. The components and changes of VOCs of Michelia champaca L. flower at different developmental stages[J]. Sci Agric Sin, 2012, 45(6):1215-1225. [21] 张莹, 李辛雷, 王雁, 等.文心兰不同花期及花朵不同部位香气成分的变化[J].中国农业科学, 2011, 44(1):110-117. ZHANG Ying, LI Xinlei, WANG Yan, et al. Changes of aroma components in Oncidium sharry baby in different florescence and flower parts[J]. Sci Agric Sin, 2011, 44(1):110-117. [22] NEGRE F, KISH C M, BOATRIGHT J, et al. Regulation of methylbenzoate emission after pollination in snapdragon and petunia flowers[J]. Plant Cell, 2003, 15(12):2992-3006. [23] EFFMERT U, SASCHENBRECHER S, ROSS J, et al. Floral benzenoid carboxyl methyltransferases:From in vitro to in planta function[J]. Phytochemistry, 2005, 66(11):1211-1230. [24] DUDAREVA N, MURFITT L M, MANN C J, et al. Developmental regulation of methyl benzoate biosynthesis and emission in snapdragon flowers[J]. Plant Cell, 2000, 12(6):949-961. [25] POTT M B, HIPPAUF F, SASCHENBRECHER S, et al. Biochemical and structural characterization of benzenoid carboxyl methyltransferases involved in floral scent production in Stephanotis floribunda and Nicotiana suaveolens[J]. Plant Physiol, 2004, 135(4):1946-1955. [26] SCHIESTL F P, AYASSE M. Post-pollination emission of a repellent compound in a sexually deceptive orchid:a new mechanism for maximizing reproductive success?[J]. Oecologia, 2001, 126(4):531-534. -

-
链接本文:
https://zlxb.zafu.edu.cn/article/doi/10.11833/j.issn.2095-0756.2014.04.023






 下载:
下载: