-
随着工农业生产的发展,工业废水和生活污水大量排入水系,造成水污染。重金属污染是水污染中比较突出的问题[1]。在所有的重金属污染物中,镉(Cd)又以其移动性强、毒性高、污染面积最大被列为“五毒之首”[2],在《重金属污染综合防治“十二五”规划》中镉被列为重点防控的重金属污染物之一。镉在自然环境中常以化合物的形式存在,相对于其他重金属来说,更容易被植物吸收,对植物造成的危害更大[3]。镉通过水流和食物链在水中迁移,形成循环危害,对水生植物的危害更大[4];且能同其他重金属发生协同效应,加剧毒害作用[5-6]。有关重金属镉对植物的毒害和植物对镉的抗性机理等方面的研究主要集中在陆生植物[7-12]。植物受到镉毒害后,植株脱水萎蔫[13],株高和根数都显著减少[3, 14],生物量下降[3, 15-16],干物质减轻[17],叶片褪绿变黄并出现坏死斑[13],植株的生长受到强烈抑制[17-19]。镉导致植物膜系统损伤[20-21],膜透性增加[13],线粒体和叶绿体的结构和功能受到破坏[22-23],叶绿素降低[18, 24],进而影响植物的呼吸作用和光合作用[17]。植物遭受镉胁迫后体内会不断积累大量活性氧(ROS),过量的ROS会对植物产生氧化损伤。植物主要通过体内的酶促和非酶促两大类保护系统清除过量的ROS,以维持正常代谢和减轻受到的损伤[25]。酶促清除系统主要包括超氧化物歧化酶(SOD)、过氧化氢酶(CAT)和过氧化物酶(POD)等抗氧化酶。各抗氧化酶活性变化与镉质量浓度相关。郝怀庆等[26]对水鳖Hydrocharis dubia的研究发现:SOD、CAT和POD活性随镉质量浓度的增加呈先升高后降低的趋势;杨海燕等[27]研究发现:竹叶眼子菜Potamogeton wrightii叶片的SOD活性随镉质量浓度的增加持续下降,POD和CAT活性则表现出先升高后降低的趋势。水禾Hygroryza aristata属禾本科Poaceae多年生水生草本,以独特的叶柄气囊漂浮水面,是一种珍稀濒危植物,世界自然保护联盟(IUCN)把它的濒危等级列为易危(VU)。水禾株形清秀,叶色青翠,浙江和广东等地多将它用于水体美化。目前关于重金属对水禾的毒害及水禾的抗性机理在国内外未见报道。本研究以水禾为研究对象,通过镉胁迫对水禾的生长、光合生理和抗氧化酶活性等的影响,探究水禾对镉胁迫的生理响应机制,以期为水禾的进一步保护利用提供理论依据。
HTML
-
供试材料水禾于2017年7月采自浙江省杭州天景水生植物园有限公司苗圃,栽植于浙江农林大学林业与生物技术学院楼旁池塘内。当年9月,选择株高约25 cm,生长良好、长势一致且无病虫害的水禾植株,清水冲洗干净后,在塑料盆中用Hoagland营养液培养,15株·盆−1,室温(25±3) ℃,自然光照培养1周,再转入光照培养箱内,14 h光照/10 h黑暗,光合光子强度为400 µmol·m−2·s−1,温度光照时25 ℃,黑暗时18 ℃,相对湿度50%~60%,适应3 d后用于实验。培养期间,每2 d更换1次营养液。
-
将氯化镉(CdCl2)加入到Hoagland营养液中,设置2(T1)、4(T2)和6(T3) mg·L−1 共3组镉质量浓度处理,每处理3盆。处理0、4、8和12 d时,测定各生长指标和光合参数;采集植株中上部功能叶片测定各生理生化指标;以未进行镉处理为对照(ck)。处理期间每2 d更换1次培养液。
-
测定水禾植株茎的基部至顶芽的长度表示株高。各处理选择5株植株,利用WinRHZO根系分析系统测定水禾根系总长度和根尖数。叶绿素测定采用体积分数为95%乙醇浸提法[28]。利用Li-6800便携式光合仪测定水禾的净光合速率(Pn)、蒸腾速率(Tr)、气孔导度(Gs)和胞间二氧化碳摩尔分数(Ci),测定条件:光合光子强度400 µmol·m−2·s−1,叶室温度25 ℃,相对湿度60%。过氧化氢(H2O2)采用过氧化氢试剂盒测定;脯氨酸(Pro)采用酸性茚三酮法测定[29];丙二醛(MDA)采用硫代巴比妥酸比色法测定[30];过氧化物酶(POD)、过氧化氢酶(CAT)和超氧化物歧化酶(SOD)活性分别采用过氧化物酶试剂盒、过氧化氢酶试剂盒和超氧化物歧化酶试剂盒测定;以上试剂盒均购于南京建成生物工程研究所。
-
利用Excel和Origin进行数据整理和图形制作,结果用平均值±标准误表示;利用SPSS 20.0进行单因素方差分析,并进行Turkey多重比较。
1.1. 材料培养
1.2. 实验处理
1.3. 测定指标与方法
1.4. 数据分析
-
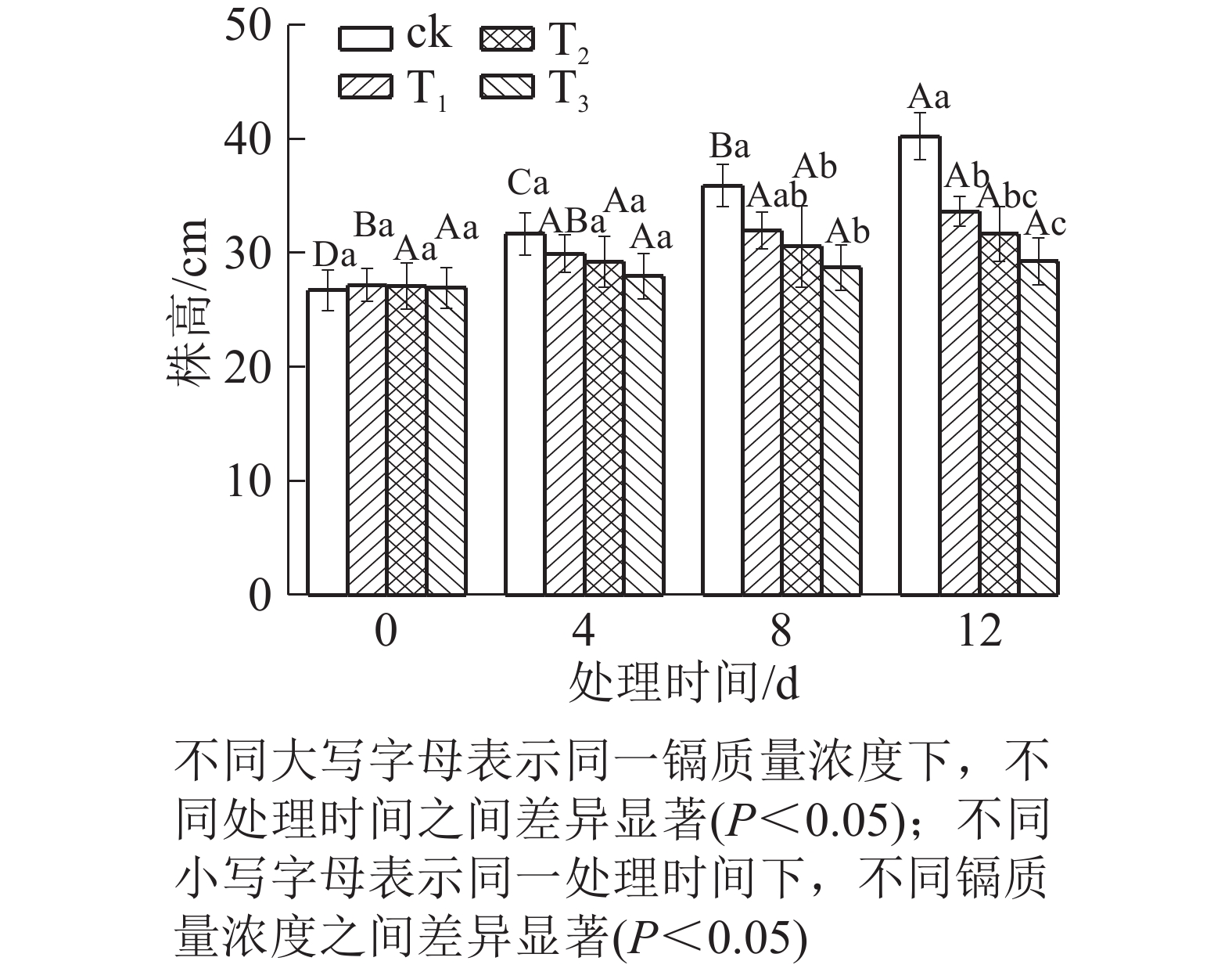
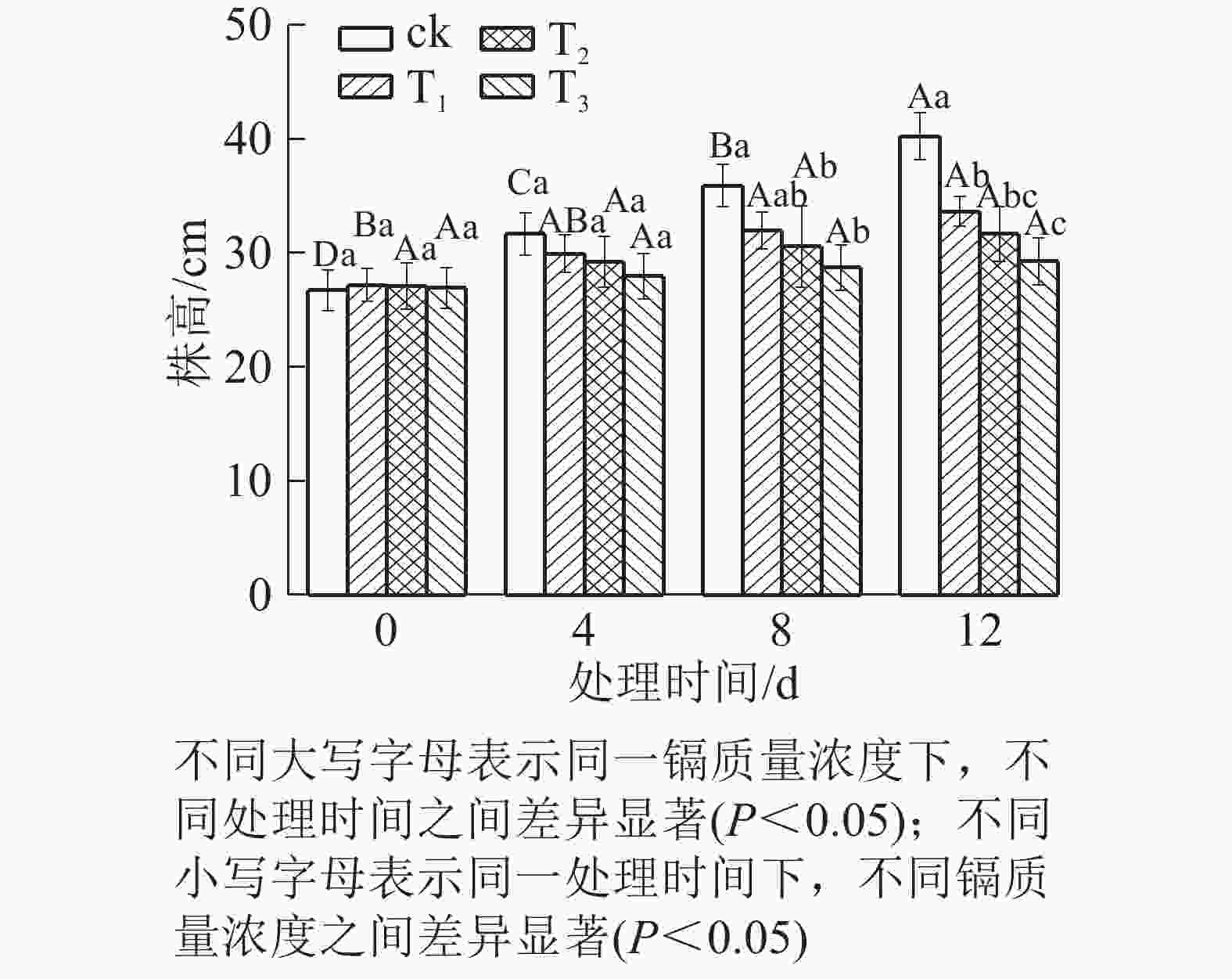
由图1可知:随着镉质量浓度的增加,水禾株高均呈逐渐降低的趋势,到处理12 d时,T1、T2和T3的株高分别比对照降低了16.35%、21.27%和27.29%。说明镉抑制了水禾株高的增长,镉质量浓度越高,抑制作用越强。随着处理时间的延长,各处理的株高增长量均呈下降趋势。说明镉胁迫时间越长,抑制作用越强。
-
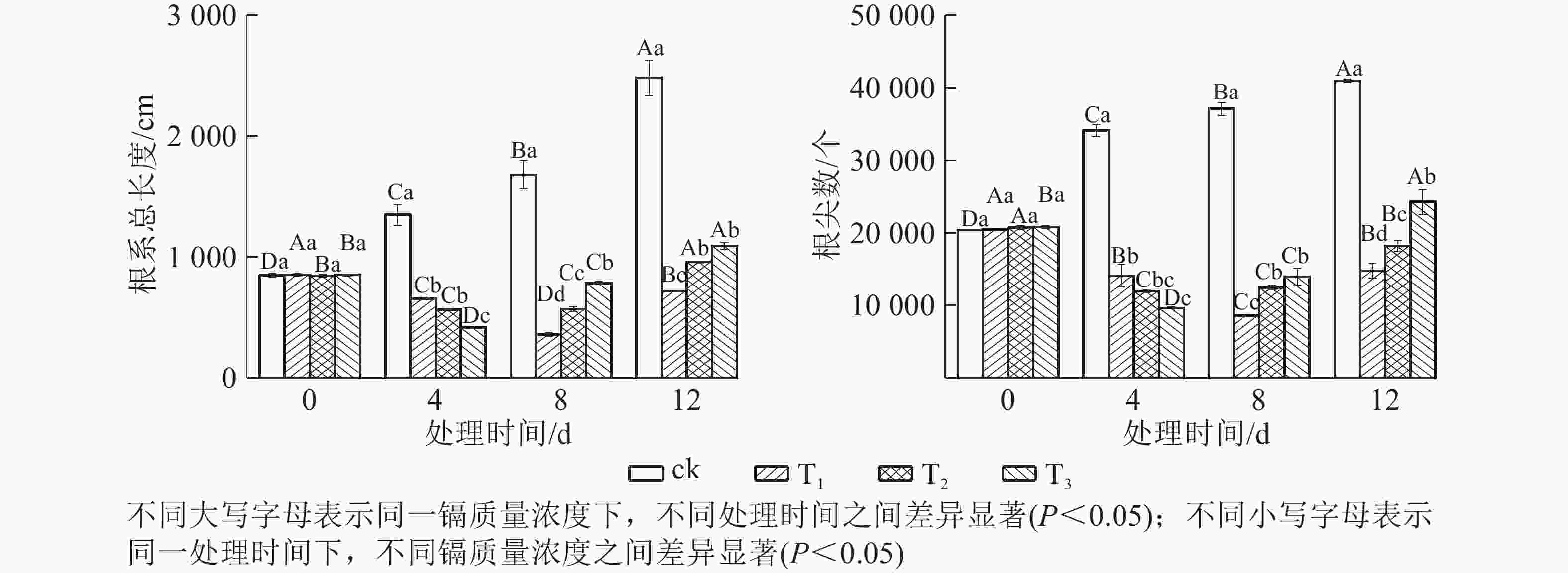
由图2可知:所有镉处理的根系总长度和根尖数均显著小于对照(P<0.05),说明镉胁迫对水禾根系生长产生了明显的抑制作用。随着处理时间的延长,各处理根系总长度和根尖数均呈先减后增的趋势。T1的根系总长度和根尖数最小值均出现在处理8 d时,根系总长度比对照短78.60%,根尖数比对照少76.79%;T2、T3的根系总长度和根尖数最小值均出现在处理4 d时,T2和T3的根系总长度分别比对照短58.22%和69.27%,根尖数分别比对照少64.98%和71.79%。表明随着处理时间的延长,抑制作用得到缓解,且处理质量浓度越高,抑制作用得到缓解也越早。
-
由表1可知:除T1的叶绿素a/b在处理4 d时与对照差异不显著外,其他镉处理的总叶绿素、叶绿素a、叶绿素b和叶绿素a/b均显著低于对照(P<0.05)。说明镉对水禾叶绿素产生了明显的抑制,而叶绿素的变化趋势与镉质量浓度和处理时间相关。处理4 d时,T1、T2和T3的总叶绿素分别比对照降低了20.19%、22.54%和33.33%;到处理12 d时,T1、T2和T3的总叶绿素分别比对照降低了75.65%、78.14%和85.14%。说明镉质量浓度越高,处理时间越长,总叶绿素降幅越大;叶绿素a和叶绿素a/b也表现出同样的趋势。由此推断,随着镉质量浓度的增加和处理时间的延长,镉对叶绿素的抑制作用增强。而T1、T2和T3之间的叶绿素b在处理8 d后就无显著差异。说明叶绿素a比叶绿素b对镉更敏感。
处理天数/d 镉处理 叶绿素质量分数/(mg·g−1) 叶绿素a/b 总叶绿素 叶绿素a 叶绿素b 0 ck 2.02±0.13 1.61±0.10 0.41±0.06 3.89±0.12 4 ck 2.13±0.09 a 1.71±0.08 a 0.42±0.02 a 4.06±0.02 a T1 1.70±0.01 b 1.36±0.01 b 0.34±0.01 b 3.96±0.03 a T2 1.65±0.01 b 1.30±0.01 b 0.35±0.01 b 3.70±0.02 b T3 1.42±0.01 c 1.12±0.01 c 0.31±0.02 c 3.63±0.02 b 8 ck 2.03±0.15 a 1.62±0.12 a 0.41±0.03 a 3.93±0.01 a T1 1.61±0.01 b 1.27±0.01 b 0.34±0.01 b 3.68±0.02 b T2 1.56±0.02 b 1.22±0.02 b 0.34±0.01 b 3.61±0.01 b T3 1.29±0.02 c 0.92±0.01 c 0.36±0.01 b 2.58±0.03 c 12 ck 2.15±0.02 a 1.72±0.01 a 0.43±0.01 a 3.98±0.02 a T1 0.52±0.02 b 0.41±0.01 b 0.11±0.01 b 3.65±0.02 b T2 0.47±0.01 c 0.35±0.01 c 0.12±0.01 b 3.06±0.02 c T3 0.32±0.01 d 0.21±0.01 d 0.11±0.01 b 2.03±0.01 d 说明:不同小写字母表示同一处理时间下不同镉质量浓度间差异显著(P<0.05) Table 1. Changes of chlorophyll contents in leaves of H. aristata under cadmium stress
-
由表2可知:随着镉质量浓度的增加和处理时间的延长,Pn呈逐渐降低的趋势。到处理12 d时,T1、T2和T3的Pn分别比对照降低了55.44%、58.77%和96.47%。说明镉对水禾的光合作用产生了明显的抑制,且处理质量浓度越高,处理时间越长,抑制作用越强。处理4 d时,T1、T2的Tr、Gs和Ci均比对照显著升高(P<0.05),而T3的Tr和Gs比对照显著降低(P<0.05),Ci则显著升高(P<0.05)。处理8 d后,所有镉处理的Tr和Gs均比对照显著降低(P<0.05),Ci则显著升高(P<0.05)。由此推断,较低质量浓度镉短期胁迫对水禾光合作用的抑制是由气孔限制和非气孔限制共同作用的结果,随着处理质量浓度增加,或处理时间延长,抑制作用则主要由非气孔限制引起。
处理天数/d 镉处理 Pn/(µmol·m−2·s−1) Tr/(mmol·m−2·s−1) Gs/(mmol·m−2·s−1) Ci/(µmol·mol−1) 0 ck 10.32±0.55 1.80±0.03 230.76±9.51 334.36±5.92 4 ck 9.98±0.27 a 1.83±0.01 c 234.41±4.15 b 337.72±2.76 c T1 8.35±0.04 b 2.14±0.01 a 264.41±4.06 a 394.94±0.51 a T2 6.35±0.10 c 2.06±0.01 b 259.90±2.63 a 386.37±1.87 ab T3 4.80±0.06 d 1.30±0.01 d 140.55±1.94 c 377.38±3.63 b 8 ck 9.46±0.02 a 2.07±0.01 a 246.28±4.76 a 339.38±0.38 c T1 5.35±0.02 b 0.89±0.01 c 213.64±1.52 b 373.28±5.60 b T2 4.91±0.02 c 1.21±0.02 b 115.23±3.77 c 374.48±3.58 b T3 0.57±0.02 d 0.80±0.01 d 79.83±0.69 d 404.33±1.37 a 12 ck 9.14±0.03 a 2.17±0.07 a 253.39±4.27 a 341.14±0.67 c T1 4.07±0.04 b 1.52±0.01 b 194.70±1.02 b 374.57±7.36 b T2 3.77±0.03 c 1.30±0.01 c 134.09±4.39 c 377.42±1.51 b T3 0.32±0.01 d 0.55±0.01 d 50.56±1.39 d 405.83±0.47 a 说明:不同小写字母表示同一处理时间下不同镉质量浓度间差异显著(P<0.05) Table 2. Changes of photosynthetic parameters in leaves of H. aristata under cadmium stress
-
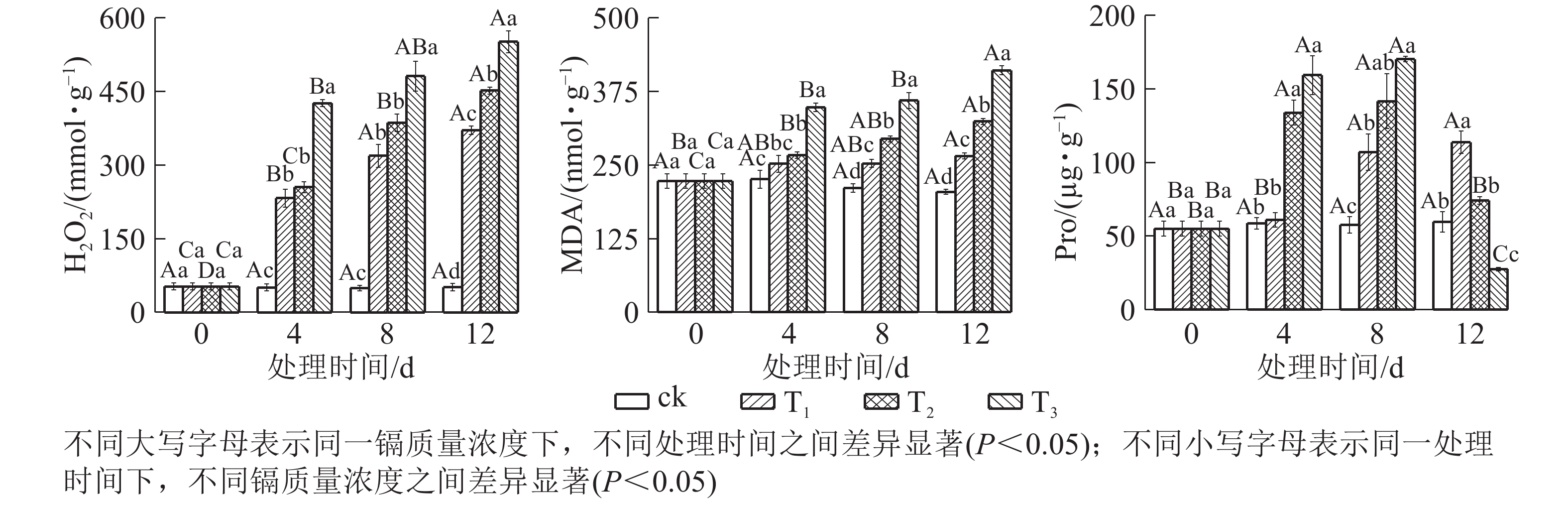
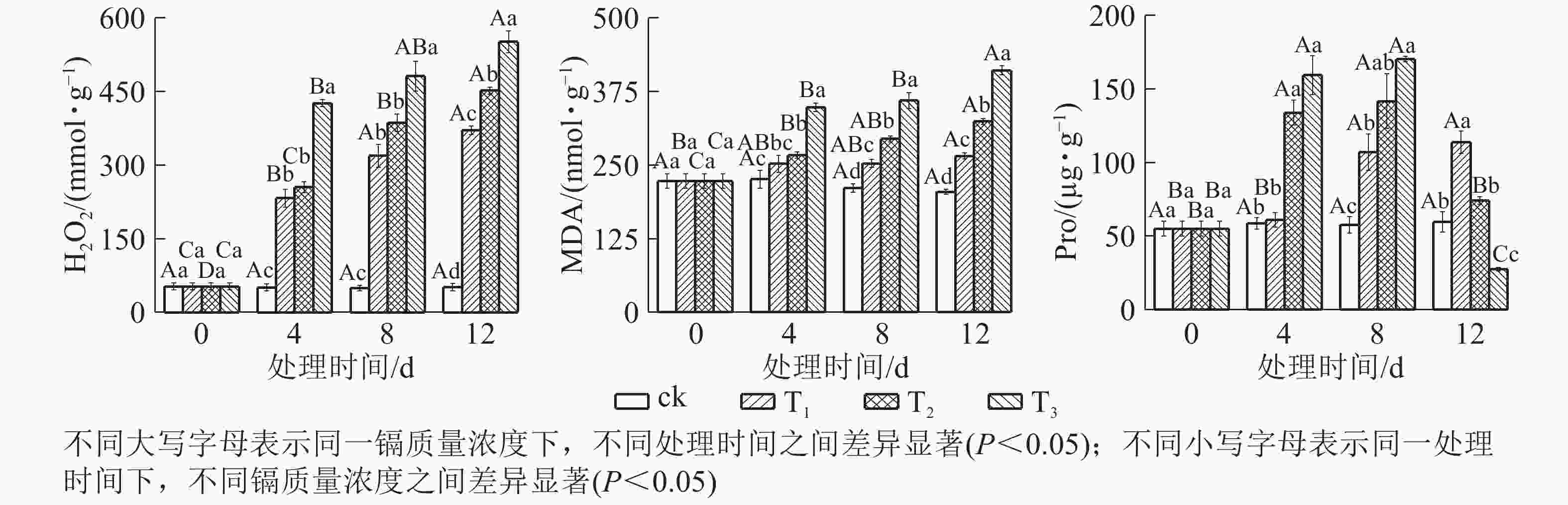
由图3可知:镉胁迫对水禾H2O2、MDA和Pro的影响与处理质量浓度和时间相关。随着镉处理质量浓度的增加,H2O2逐渐升高;处理时间越长,升幅越大。到处理12 d时,T1、T2和T3的H2O2质量摩尔浓度分别是对照的7.21倍、8.79倍和10.73倍。MDA变化趋势同H2O2,到处理12 d时,T1、T2和T3的H2O2分别比对照升高了29.78%、58.34和100.06%。说明镉对水禾产生严重的毒害作用,随着处理质量浓度的增加和处理时间的延长,毒害作用增强。另一方面,随着处理时间的延长,各处理的H2O2和MDA质量摩尔浓度升高速率均呈下降趋势,最大升高速率均出现在0~4 d。说明随着处理时间的延长,H2O2和MDA的产生速率受到一定扼制。随着处理时间的延长,T1的Pro逐渐升高,在处理12 d时达最大值,显著高于对照(P <0.05);T2和T3呈先升高后降低的趋势,在处理8 d时达最大值,显著高于对照(P<0.05),到处理12 d时,T2与对照差异不显著,T3比对照降低了53.85%。说明镉胁迫对Pro的促进作用有一定限度,而高质量浓度镉长期处理使Pro受到抑制。
-
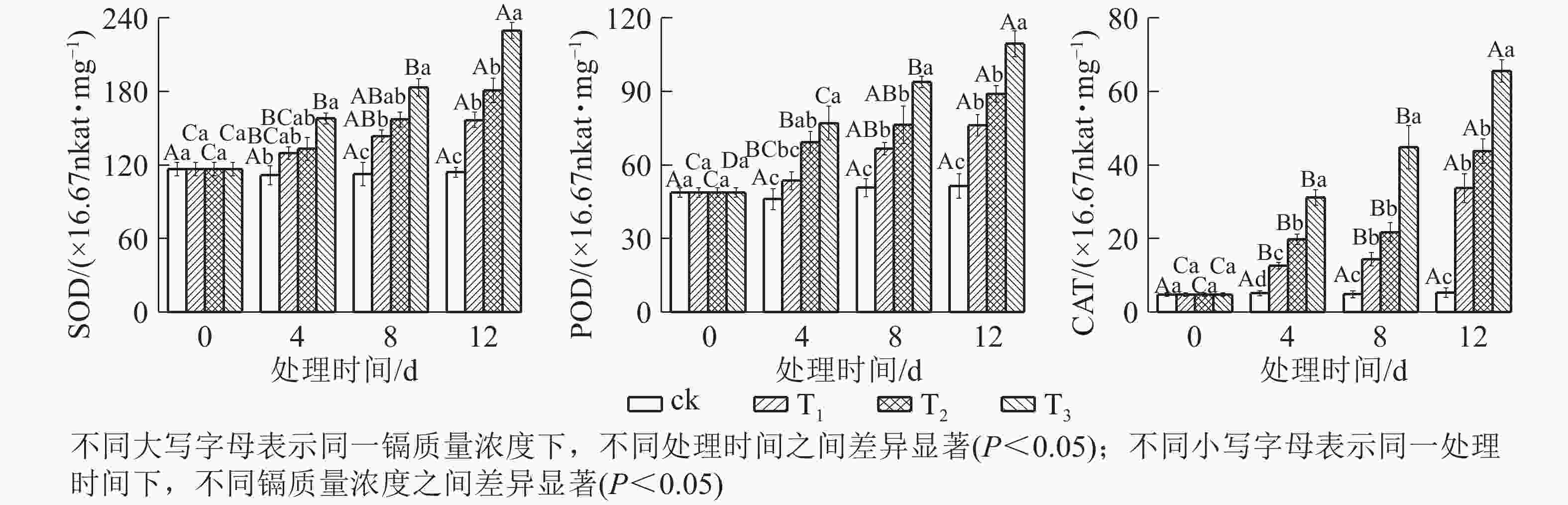
由图4可知:随着镉质量浓度的增加,SOD、POD和CAT活性均逐渐升高。处理4 d时,T1的SOD和POD活性变化不明显,CAT活性显著升高(P<0.05);T2的SOD活性变化不明显,POD和CAT活性显著升高(P<0.05);T3的SOD、POD和CAT活性显著升高(P<0.05);处理8 和12 d时,各处理的SOD、POD和CAT活性均显著升高(P<0.05);到处理12 d时,T1、T2和T3的SOD活性分别比对照升高了37.40%、58.63%和101.45%,POD活性分别比对照升高了48.10%、73.22%和112.83%,CAT活性分别比对照升高了5.32倍、7.23倍和11.32倍。这表明镉诱导了抗氧化酶活性升高,处理质量浓度越高,处理时间越长,抗氧化酶活性升幅越大。
2.1. 镉胁迫对水禾株高的影响
2.2. 镉胁迫对水禾根系的影响
2.3. 镉胁迫对水禾叶绿素质量分数的影响
2.4. 镉胁迫对水禾光合作用的影响
2.5. 镉胁迫对水禾H2O2、MDA和Pro的影响
2.6. 镉胁迫对水禾抗氧化酶活性的影响
-
重金属积累会影响植物的正常生理过程,抑制植株的生长发育,降低植株的生物量。镉对植物生长的影响与其质量浓度有关,一般认为,低质量浓度镉能刺激种子萌发及幼苗生长,而较高质量浓度镉则对其产生抑制作用[31]。本研究发现:各质量浓度镉处理到一定时间后均抑制了水禾株高的生长,随着镉质量浓度的增加,抑制作用增强;镉对水禾株高的抑制作用还与处理时间有关,即随着镉处理时间的延长抑制作用增强,可能是长时间的胁迫导致镉在植株体内不断积累,增强了对植株生长的抑制作用。这与冯君等[32]对大豆Glycine max幼苗的研究结论一致。同时,各质量浓度镉处理均抑制了水禾根系的生长;随着镉处理时间的延长,水禾的根系总长度和根尖数均呈先减后增的趋势。闫志强等[31]认为根部最先接触镉,交换吸附的量比较多,从而导致前期根系的生长受到明显抑制;随着镉被转运至植株上部,抑制作用得到减缓,甚至一定程度上促进了根系生长。由此推断,水禾的根系对镉有一定的耐受性。T2和T3处理下根系总长度和根尖数最小值的出现早于T1处理,说明高质量浓度镉处理对根系的抑制作用缓解得更早。这可能是因为高质量浓度胁迫促使根系活力迅速降低,根对镉的吸收能力减弱,随着镉从根部转运到植株体上部,导致镉在根部的积累量下降,故而其抑制作用缓解更早。
镉胁迫可严重影响植物的叶绿素质量分数[33],并导致黄化这一植物叶片常见的镉毒害症状发生[34]。本研究表明:镉对水禾叶绿素产生了明显的抑制作用,随着镉质量浓度的增加和时间的延长,抑制作用增强。T1、T2和T3处理之间的叶绿素b在处理8 d后就无显著差异,说明叶绿素a比叶绿素b对镉更敏感,受到镉的影响更大。有研究认为:镉可通过影响叶绿素酸酯还原酶的活性而抑制叶绿素的合成[35];直接破坏叶绿体微结构,或抑制叶绿素前体的合成,促进叶绿素分解[36]。叶绿素降低会导致叶片减少对光能的捕捉和转化,光合效率减弱。
本研究表明:镉能抑制植物叶片光合作用,随着镉质量浓度的增加和时间的延长,抑制作用增强。这首先归因于叶绿素的持续降低。再者,较低质量浓度镉短期胁迫对水禾光合作用的抑制是由气孔限制和非气孔限制共同作用的结果;随着镉质量浓度增加,或处理时间延长,抑制作用则主要由非气孔限制引起,即光合作用的限制位点在叶肉细胞内,不是由于气孔导度的下降使二氧化碳供应不足所致,而是由于破坏了光合机构或抑制了暗反应的酶降低了光合作用[37],且随着镉质量浓度的升高和毒害作用的加剧而越来越明显。这与李贺等[11]对大蒜Allium sativum和冯建鹏等[34]对黄瓜Cucumis sativus的研究结论一致。另一方面,Tr的显著降低,可能是镉胁迫下水禾通过降低蒸腾速率以调节水分的损失,保证体内水分的有效利用[38],这也是光合速率降低的重要原因之一。
镉胁迫会产生ROS,如超氧阴离子(O2−·)、H2O2、氢氧根和羟自由基(·OH)等[39]。ROS破坏脂质类物质、蛋白质以及核酸,减缓植物体的生长发育[40]。MDA作为膜脂过氧化作用的终极产物,其变化可解释逆境胁迫对植物细胞膜的破坏程度及植物对逆境的响应[41]。本研究发现:随着镉质量浓度的增加和处理时间的延长,水禾叶片的H2O2和MDA逐渐升高。可能是因为镉胁迫导致水禾叶片产生和积累大量ROS而无法及时清除,引发膜脂过氧化作用增强,这与刘俊等[42]对大豆和何俊瑜等[43]对水稻Oryza sativa的研究结论一致。另一方面,随着处理时间的延长,各质量浓度镉处理的H2O2和MDA产生速率均受到一定扼制,这说明水禾体内对ROS的清除的速率小于ROS的产生速率,ROS的动态平衡被打破[44]。
Pro具有较强的水合能力,在受到逆境胁迫时,Pro可作为溶质来调节细胞水分环境的变化,从而减轻胁迫对植物体内水分亏缺的影响,使细胞维持相对稳定的水环境[45]。张建新等[12]研究表明:随着镉质量浓度的增加,朱砂根Ardisia crenata和虎舌红A. mamillata的Pro都受到抑制。本研究发现:低质量浓度镉长期处理或高质量浓度镉短期处理对Pro有促进作用,而高质量浓度镉长期处理使Pro受到一定程度的抑制,与张建新等[12]的研究结论基本一致。这可能是因为高质量浓度镉长期处理导致水禾植株“失水”严重,破坏了水禾的生理代谢活动。说明水禾的抗胁迫能力有一定限度,与镉质量浓度水平和处理时间有关,高质量浓度镉长期处理下水禾的抗胁迫能力显著降低。
抗氧化酶是ROS的清除系统的重要组成部分。SOD是抗氧化系统中第一道屏障,是防御细胞膜脂过氧化的主要酶,有利于O2−·的清除,减少膜系统的伤害[46],与植物的抗逆性、抗衰老密切相关[39]。POD是植物体内普遍存在、活性较高的一种酶,它与呼吸作用、光合作用以及生长素的氧化等密切相关。POD和CAT使体内某些氧化酶的毒性产物H2O2分解,有效阻止O2−·和H2O2的积累,从而限制这些自由基对膜脂过氧化的启动[47]。当植物体内镉达一定浓度时,膜脂过氧化作用增强,使得POD活性升高;同时,CAT浓度增加,也会导致POD酶活性增加[41]。本研究发现:随着镉质量浓度的增加和处理时间的延长,水禾叶片产生过多ROS,抗氧化酶活性逐渐升高。说明O2−·的积累诱导叶片中SOD活性升高,催化O2−·歧化产生基态分子氧和H2O2;水禾叶片中的H2O2和MDA逐渐升高,由于POD和CAT的作用,其升高速率减缓。总之,在镉胁迫下,水禾的抗氧化酶防御系统能有效清除因胁迫导致过多积累的ROS,使H2O2和MDA的升高速率减缓,表明一方面在胁迫条件下过多ROS的积累能够对水禾叶片产生伤害,另一方面ROS的积累会诱导抗氧化酶活性的升高,有效清除ROS,以维持水禾叶片中ROS的动态平衡,防止氧化损伤的加剧。结合本研究对H2O2和MDA的分析结果推断可知:镉对水禾有严重的毒害作用,长期镉胁迫下,水禾的抗氧化酶在清除ROS、防御氧化伤害上发挥的作用有限,水禾的抗胁迫能力较弱。
综上所述,镉对水禾植株的生长产生了明显的抑制作用,随着镉质量浓度的增加和处理时间的延长,抑制作用增强;水禾根系对镉有一定的耐受性,随着处理时间的延长,镉对水禾根系生长的抑制作用缓解。镉抑制了水禾的叶绿素,叶绿素a比叶绿素b对镉更敏感,受到镉的影响更大;镉胁迫下,水禾的光合作用受到抑制,随着处理质量浓度增加,或处理时间延长,抑制作用主要由非气孔限制引起。镉胁迫导致水禾叶片产生和积累大量ROS而无法及时清除,引发膜脂过氧化作用增强;水禾的抗胁迫能力有一定限度,高质量浓度镉长期处理下水禾的抗胁迫能力显著降低。长期镉胁迫下,抗氧化酶在清除ROS、防御氧化伤害上发挥的作用有限,水禾的抗胁迫能力较弱。









 DownLoad:
DownLoad: