-
园林绿化废弃物包括树木、花草等植物在生长过程中的自然凋落物或者人为修剪的植物残体[1],主要成分有木质素、纤维素和多糖等[2]。其中,木质素由于组分种类多样,结构复杂且无规则,降解比较困难[3-5]。堆肥是一种较好的降解园林绿化废弃物的方式[6],堆肥过程中多个微生物群体共同作用,分泌木质素降解相关酶系而使园林绿化废弃物中的木质素降解[7]。因此,通过研制微生物菌剂,使分泌木质素降解酶酶系的菌株迅速构建优势群落,能有针对性地加快园林绿化废弃物中木质素的降解,提高堆肥效率和质量[8-9]。但是,新菌剂的制备需要在选定目标菌株的条件下,对菌剂制作涉及的培养基配方和发酵条件进行优化。优化过程主要包括试验设计、数学建模和优化设计3个部分[10]。合理的试验设计能用较少的试验数据进行建模,从而获取各因素范围内的最优解。用于发酵条件优化的方法多为响应面法[11-12],但胡欣颖等[13]研究发现:人工神经网络算法比响应面法在预测实验结果方面更准确,误差更小。目前,运用人工神经网络算法对木质素降解菌发酵条件进行优化的研究鲜有报道。因此,本研究拟从北京市植物园的腐叶土和朽木中筛选木质素降解菌,对其进行鉴定,并通过单因素试验对菌株的培养时间、接种量和培养基配方(碳源和氮源)进行优化;采用均匀试验结合Python实现人工神经网络建模与优化,寻找菌株最佳发酵条件,为园林绿化废弃物中木质素的降解提供高效菌剂。
HTML
-
腐叶土和朽木采集于北京市植物园。PDA培养基:称取200.00 g土豆,去皮去芽切成小块后加蒸馏水微沸30 min,保留滤液并用蒸馏水补足1 L,制成马铃薯浸汁;葡萄糖 20.00 g,蛋白胨15.00 g,琼脂 20.00 g,pH自然[14]。PDA-苯胺蓝培养基:称取0.10 g苯胺蓝溶于1 L PDA培养基中,pH自然。PDA-愈创木酚培养基:量取0.1 mL愈创木酚溶于1 L PDA培养基中,pH自然。PDB液体培养基:马铃薯浸汁(同PDA培养基),葡萄糖 20.00 g,蛋白胨15.00 g,pH自然。基本发酵培养基:葡萄糖10.00 g·L−1、蛋白胨5.00 g·L−1,酵母粉3.00 g·L−1、酒石酸铵10.00 g·L−1、五水合硫酸铜0.25 g·L−1、氯化钠1.00 g·L−1、pH自然[15]。所有培养基均121 ℃高压蒸汽灭菌 20 min。
-
将采集的样品捣碎[16],称取10.00 g加入装有90 mL无菌水的三角瓶中,在28 ℃、200 r·min−1下震荡摇匀后静置1 h。取上清液1.0 mL稀释成不同质量浓度梯度(10−3~10−7 g·L−1)的溶液,取不同质量浓度稀释液0.1 mL,加入PDA培养基中涂布均匀,28 ℃下培养5~7 d,观察菌落形态,用平板划线分离法纯化菌株。将得到的纯菌株以点接法接到PDA-苯胺蓝平板和PDA-愈创木酚平板中,用苯胺蓝平板褪色圈法和愈创木酚平板变色圈法确定该菌株是否具有降解木质素的能力。
-
观察PDA-苯胺蓝平板上菌体形态特征,并挑取少量菌丝于显微镜下拍照记录。菌株送往北京睿博兴科生物技术有限公司进行内转录间隔区(ITS)测序,测序结果与Genebank数据库中已知的真菌序列BLAST检索对比,采用 Mega 5.0 软件与相近种菌株构建系统发育树[17]。
-
将菌株接种至装有100.0 mL PDB液体培养基的三角瓶中,在IS-RDD3台式恒温振荡器中以30 ℃、200 r·min−1培养3 d,制得种子液。
-
木质素过氧化物酶、锰过氧化物酶和漆酶活性的测定参照田林双[18]的方法。3种木质素降解相关酶酶活性总和标记为总酶活。生物量的测定用称干质量法[19]。
-
试验数据采用Excel 2007和SPSS 22.0进行处理。发酵条件优化用单因素方差分析法,平均值多重比较用LSD最小显著性差异法(P<0.05)。均匀试验利用人工神经网络[20]建模与优化(基于深度学习框架Pytorch[21])。将本实验目标建模为回归任务,并采用SmoothL1损失函数[22]以平滑训练过程。训练过程中,采用k-折交叉验证(k-fold cross-validation)和自适应矩估计(Adam)算法[23]优化神经网络。
1.1. 材料
1.2. 方法
1.2.1. 菌株的分离与筛选
1.2.2. 菌株的鉴定
1.2.3. 种子液的制备
1.2.4. 测定指标
1.2.5. 数据统计与分析
-
PDA-苯胺蓝平板上褪色圈的出现表示该菌株具有分泌锰过氧化物酶和木质素过氧化物酶的能力,PDA-愈创木酚平板上显色圈的出现表示该菌株具有分泌漆酶的能力[24]。由表1可知:筛选得到的22株菌(分别命名为Q01~Q22)中,共有10株菌出现褪色圈或/和显色圈。其中:Q01、Q02、Q09和Q11既能出现褪色圈又能出现显色圈,说明这些菌株具备分泌3种木质素降解酶的能力。根据褪色圈和显色圈的出现时间及直径可知(表1),Q01于48 h时在PDA-苯胺蓝平板上出现褪色圈,12 h时在PDA-愈创木酚培养基上出现显色圈,在72 h时显色圈最大,因此,选定菌株Q01为目标菌株。
菌株 苯胺蓝褪色结果 愈创木酚显色结果 12 24 36 48 72 12 24 36 48 72 h Q01 − − − + ++ + ++ +++ ++++ ++++ Q02 − − − − + − − − + ++ Q06 − − − − − − − − − + Q09 − − − − + − − − − + Q11 − + + ++ +++ − − − + + Q12 − + ++ +++ +++ − − − − − Q14 − + + ++ ++ − − − − − Q17 − + + + + − − − − − Q19 − + + + + − − − − − Q22 − + + ++ ++ − − − − − 说明:−表示不褪色(或不显色);+、+ +、+ + +、+ + + +表示 褪色(或显色)圈逐渐增大 Table 1. Coloring and decoloring results of different strains on selective mediums
-
菌株Q01在PDA-苯胺蓝平板上的菌落形态为白色圆形,菌丝为致密的短绒状,向四周扩展,紧贴平板生长(图1A、图1B);前期生长较慢,1~2 d有菌丝长出,此后生长较快,3~5 d铺满整个平板。显微形态可见,菌丝较细长,有分支(图1C),孢子呈球状或柱状(图1D),菌丝可观察到隔膜和锁状联合(图1E,如箭头所示)。
-
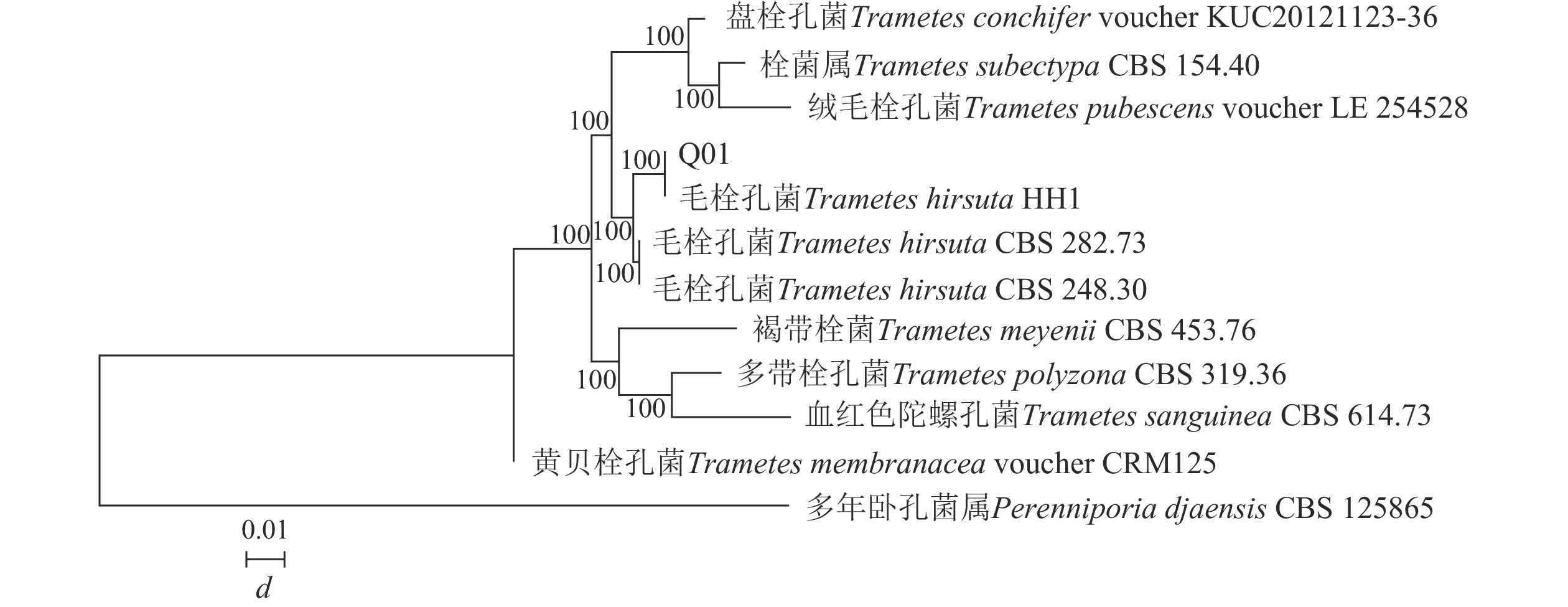
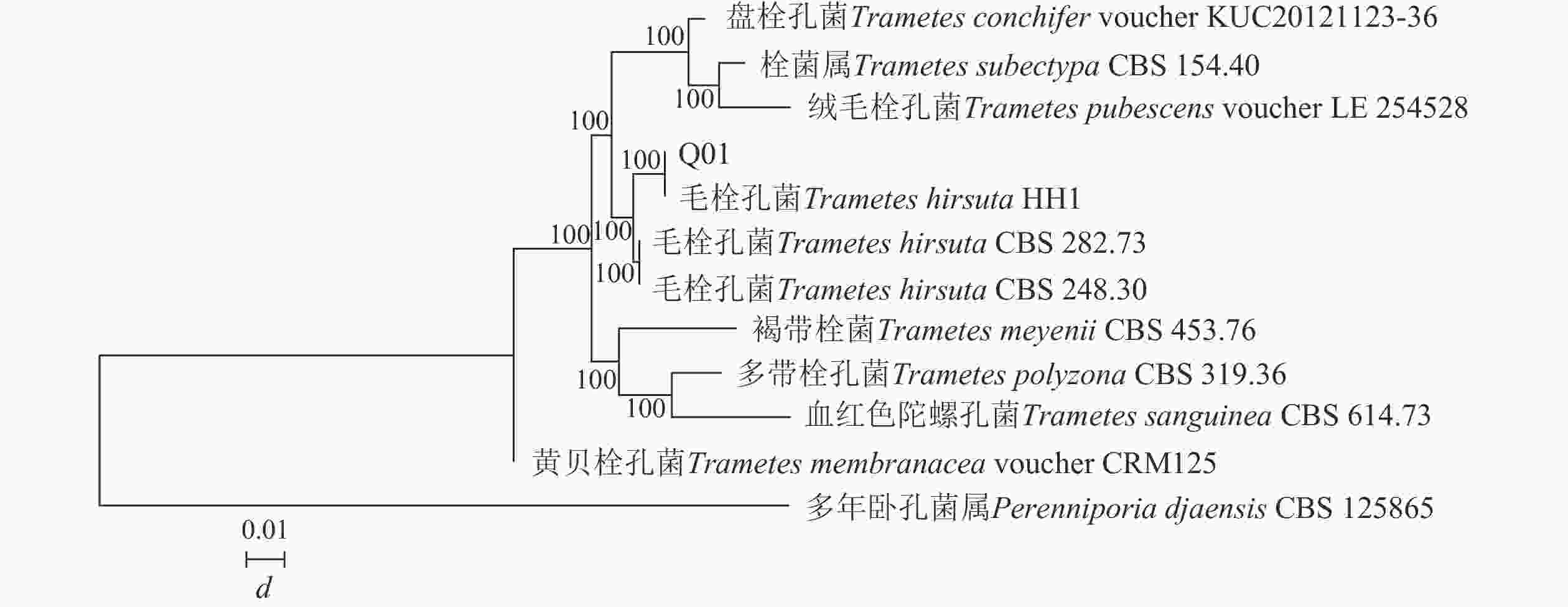
构建菌株Q01系统发育树(图2)可知:菌株Q01与栓菌属Trametes真菌的同源性相似度最高,达到100%。结合形态特征可确定菌株Q01为栓菌属真菌。
-
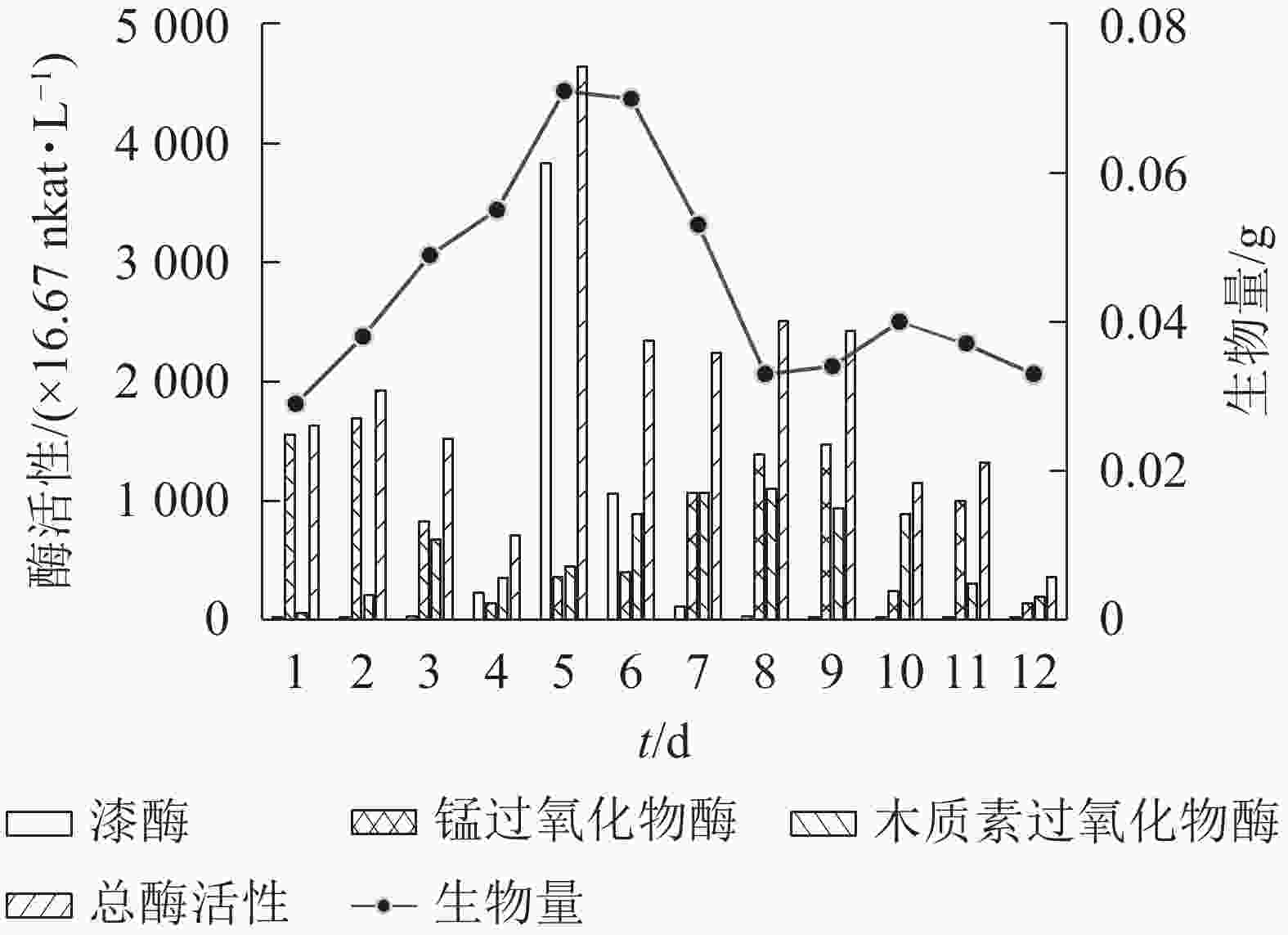
分别在12瓶100 mL基本发酵培养基中接种体积分数为12.5%的菌株Q01种子液,于30 ℃、200 r·min−1下培养,每24 h取出1瓶,测量菌株木质素降解相关酶活性和1 mL菌液中生物量的干质量。由图3可知:3种木质素降解相关酶的酶活性达到最高值时间不同,其中漆酶活性在培养5 d时达最高(3 835.25×16.67 nkat·L−1),锰过氧化物酶活性在培养2 d时达最高(1 690.60×16.67 nkat·L−1),木质素过氧化物酶活性在培养8 d时达到最高(1 096.88×16.67 nkat·L−1)。3种木质素降解相关酶在木质素降解中具有同样重要的作用[25],但不同木质素降解酶活性最高值时间不同,因此以3种木质素降解相关酶的总酶活性作为确定培养时间的依据。随着木质素降解,真菌生物量逐渐增多[26]。图3可知:菌株Q01的总酶活性与生物量均在培养5 d时最高,因此选择培养时间为5 d进行后续研究。
-
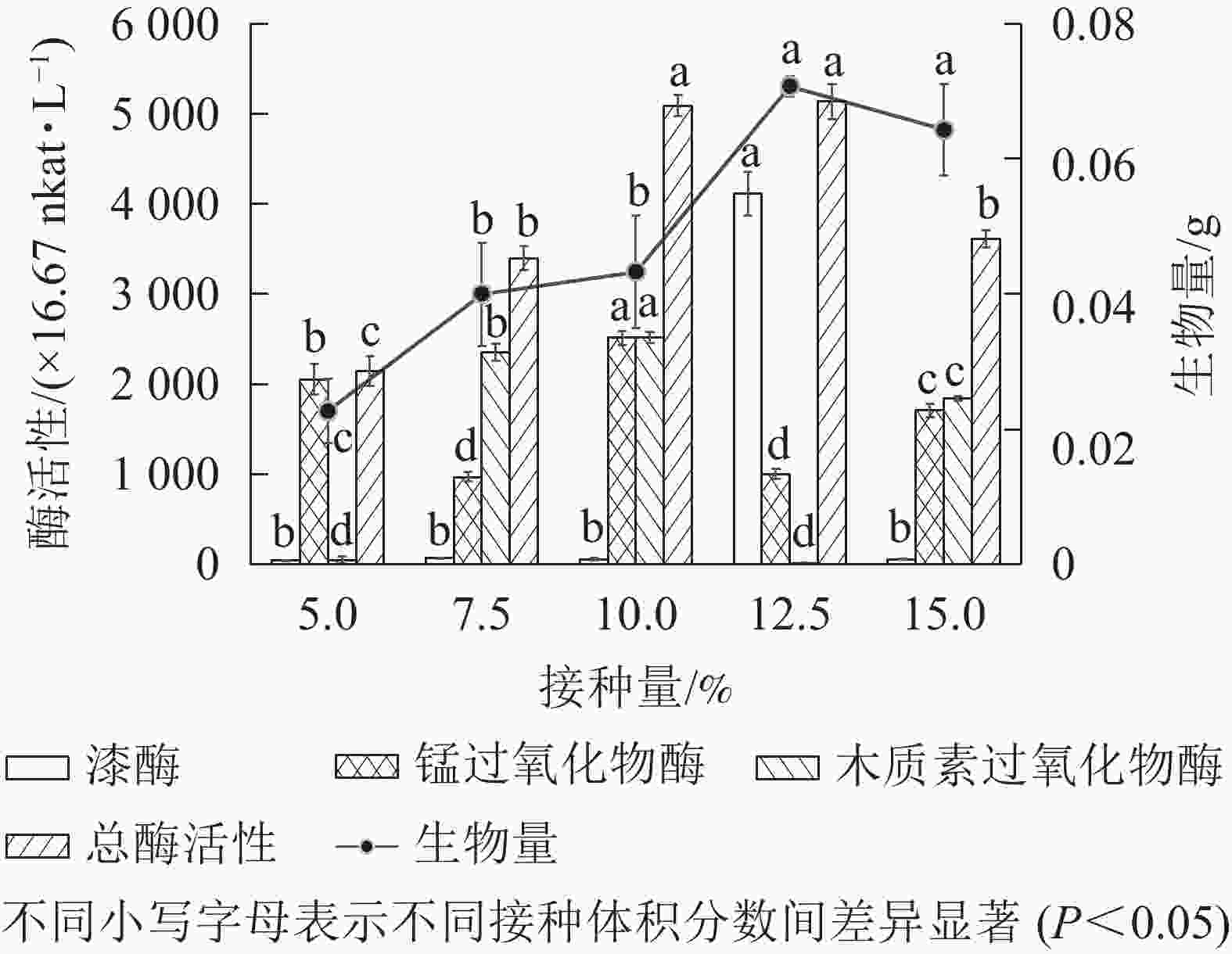
分别在100 mL 基本发酵培养基中接种体积分数为5.0%、7.5%、10.0%、12.5%和 15.0%的种子液,于30 ℃、200 r·min−1下培养5 d,测定菌株木质素降解相关酶活性和菌株生物量。由图4可知:总酶活性在菌液体积分数为12.5%时最高,达到5 129.80×16.67 nkat·L−1。随菌液体积分数增加,菌株生物量呈先上升后下降趋势,体积分数为12.5%时达到最高。与缪晓磊[19]对竹林毛栓菌Trametes sp. 接种量优化的结果一致。可见菌株的繁殖速度受接种量影响,当接种量较少时,菌株需要更多时间适应环境后繁殖生长;而接种量较多时,大量菌株接触新环境,迅速繁殖,使液体培养基黏稠不透气,溶解氧下降,影响菌株的后续生长[19]。因此,综合总酶活性和生物量的变化,选取体积分数12.5%为最适接种量。
-
以基本发酵培养基为对照,分别用 10.00 g·L−1蔗糖、麦芽糖、木质素磺酸钠和可溶性淀粉替代对照中10.00 g·L−1的葡萄糖,接种体积分数为12.5%,于30 ℃、200 r·min−1下培养5 d。由图5可知:以葡萄糖为碳源时,菌株Q01漆酶活性达3 675.23×16.67 nkat·L−1,显著高于其他碳源(P<0.05);认为简单的糖有利于菌体生长[27],可以缩短漆酶的生产时间,与刘宇等[15]结果相似。但就总酶活性和生物量而言,以木质素磺酸钠为碳源对菌株Q01生长和木质素降解相关酶分泌效果最好,最显著(P<0.05),其中总酶活性为6 474.16×16.67 nkat·L−1,总生物量为0.065 g。可能原因是来源于木质素的木质素磺酸钠能刺激菌株Q01分泌木质素过氧化物酶和锰过氧化物酶,并促进细胞快速生长,使生物量最高,与熊乙[28]研究结果相似。综合考虑各碳源生产成本及对菌株Q01生长的促进效果,选择木质素磺酸钠为菌株Q01的液体培养基碳源。
-
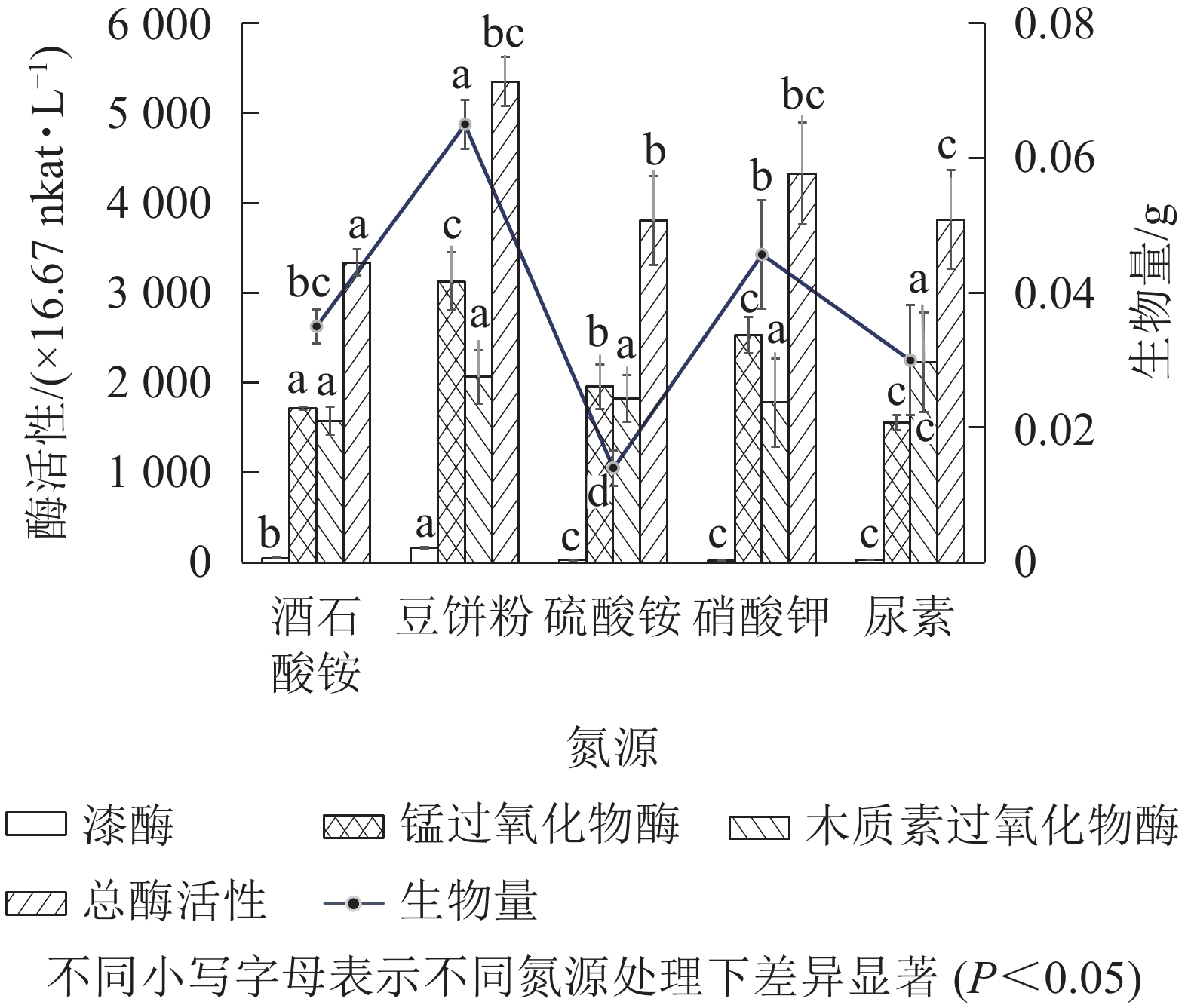
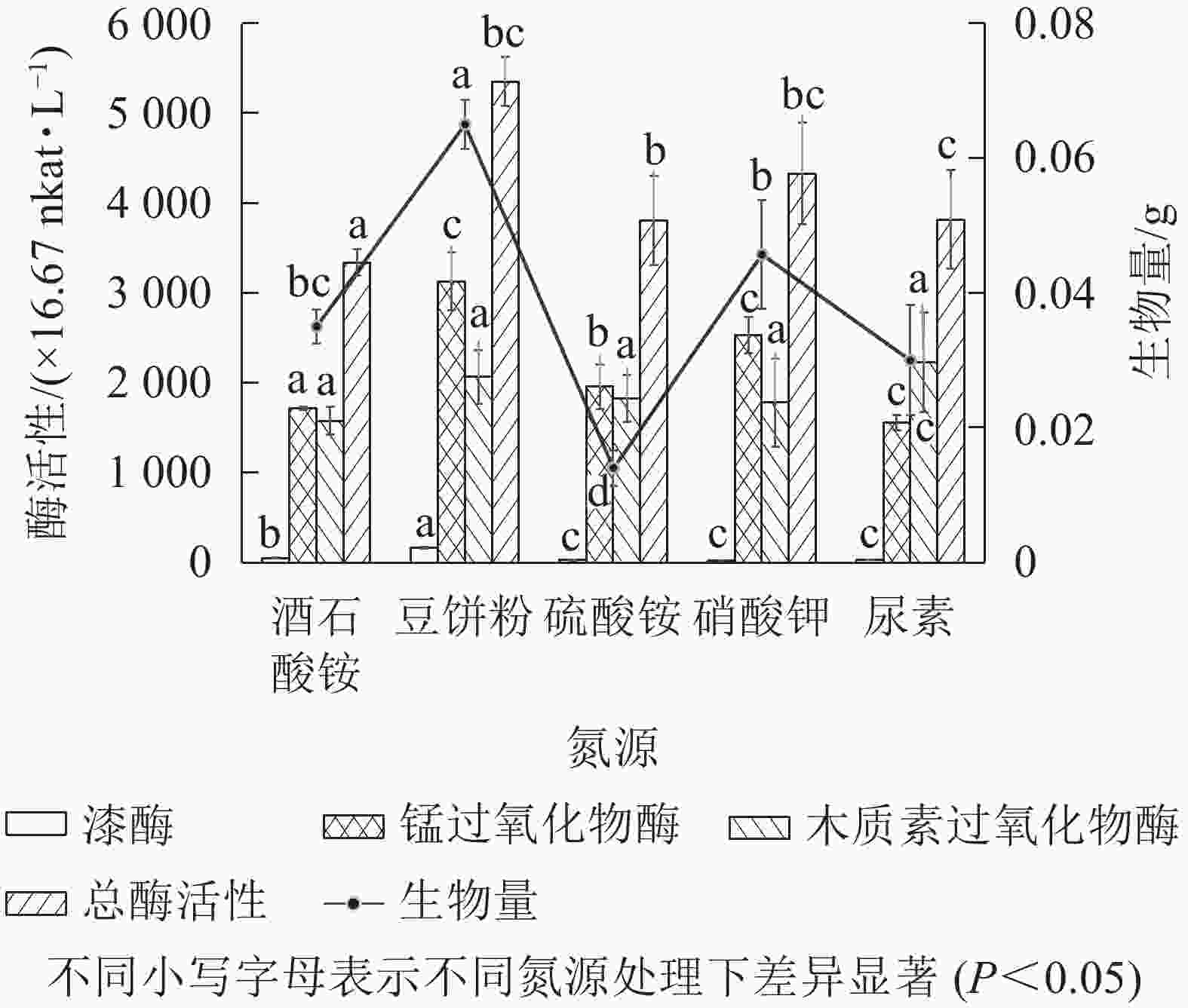
以基本发酵培养基为对照,有效含氮量按1.52 g·L−1进行换算,分别用7.00 g·L−1的豆饼粉、6.50 g·L−1尿素、14.30 g·L−1硫酸铵、11.00 g·L−1硝酸钾替代对照组中的酒石酸铵,发酵培养条件为30 ℃、200 r·min−1,培养5 d。由图6可知:以豆饼粉为有机氮源发酵时,菌株Q01总酶活性最高、生物量最大,与其他无机氮源差异显著(P<0.05)。豆饼粉营养丰富,含大量碳水化合物、蛋白质及少量异黄酮类物质和可溶性多糖,可作为发酵氮源及生长因子。有研究表明[29]:在培养某些真菌时,培养基中添加豆饼粉有利于生物量的积累。综合考虑各氮源生产成本及对菌株Q01生长的促进效果,选择豆饼粉作为菌株Q01的液体培养基氮源。
-
均匀试验设计6因素5水平的10组试验,获取实测值,再通过基于SmoothL1损失函数的人工神经网络模型的训练及自适应矩估计(Adam)算法寻优,得到仿真值。对比实测值和仿真值(表2)可知:3种酶活性误差均小于10.0%,生物量的仿真值基本与观察值相同,可知该模型预测值可信赖。
试验号 木质素
磺酸钠/
(g·L−1)蛋白胨/
(g·L−1)酵母粉/
(g·L−1)豆饼粉/
(g·L−1)五水合
硫酸铜/
(g·L−1)氯化钠/
(g·L−1)锰过氧化
物酶活性/
(×16.67 nkat·L−1)木质素过氧
化物酶活性/
(×16.67 nkat·L−1)漆酶活性/
(×16.67 nkat·L−1)总酶活性/
(×16.67 nkat·L−1)生物量/
g实测值1 6.00 5.00 3.00 11.00 0.20 1.50 4 296.20 1 520.43 219.19 6 035.83 0.037 仿真值1 6.00 5.00 3.00 11.00 0.20 1.50 3 186.76 2 888.58 218.40 6 293.73 0.037 实测值2 8.00 10.00 1.00 11.00 0.25 1.25 4 200.35 3 790.22 252.62 8 243.18 0.055 仿真值2 8.00 10.00 1.00 11.00 0.25 1.25 4 190.01 3 799.79 288.91 8 278.71 0.049 实测值3 10.00 2.50 4.00 9.00 0.35 1.00 4 095.77 3 605.59 318.60 8 019.96 0.048 仿真值3 10.00 2.50 4.00 9.00 0.35 1.00 4 094.32 3 712.88 282.19 8 089.38 0.048 实测值4 12.00 7.50 1.00 9.00 0.15 0.75 4 601.21 5 278.06 313.29 10 192.56 0.014 仿真值4 12.00 7.50 1.00 9.00 0.15 0.75 4 607.23 4 178.73 318.24 9 104.20 0.024 实测值5 14.00 12.50 4.00 7.00 0.20 0.50 5 263.50 5 028.28 399.44 10 691.23 0.059 仿真值5 14.00 12.50 4.00 7.00 0.20 0.50 5 290.88 4 799.66 366.29 10 456.83 0.062 实测值6 6.00 2.50 2.00 7.00 0.30 1.50 4 453.06 3 746.77 178.82 8 378.66 0.123 仿真值6 6.00 2.50 2.00 7.00 0.30 1.50 4 446.86 4 033.08 306.97 8 786.91 0.092 实测值7 8.00 7.50 5.00 5.00 0.35 1.25 3 999.91 4 387.53 186.13 8 573.57 0.042 仿真值7 8.00 7.50 5.00 5.00 0.35 1.25 4 018.98 3 644.46 276.89 7 940.33 0.047 实测值8 10.00 12.50 2.00 5.00 0.15 1.00 3 215.62 4 322.37 363.36 7 901.34 0.050 仿真值8 10.00 12.50 2.00 5.00 0.15 1.00 4 289.80 3 890.43 295.93 8 476.16 0.050 实测值9 12.00 5.00 5.00 3.00 0.25 0.75 5 411.65 3 822.80 432.66 9 667.11 0.063 仿真值9 12.00 5.00 5.00 3.00 0.25 0.75 5 287.09 4 905.21 374.46 10 566.76 0.063 实测值10 14.00 10.00 3.00 3.00 0.30 0.50 5 120.37 4 356.31 385.32 9 862.00 0.060 仿真值10 14.00 10.00 3.00 3.00 0.30 0.50 5 241.27 4 754.60 362.81 10 358.68 0.061 Table 2. Uniform test design and results
-
根据模型预测得到最优发酵培养基组成并进行实验验证。由表3可知:优化后的发酵培养基酶活性和生物量,实测值与预测值误差约为3.0%;结合图3可知:优化前锰过氧化物酶活性为357.29×16.67 nkat·L−1、木质素过氧化物酶活性为445.27×16.67 nkat·L−1、总酶活性为4 637.81×16.67 nkat·L−1、生物量为0.071 g;即在优化培养基上生长的菌株Q01,其生物量比优化前提高1.27倍,锰过氧化物酶活性提高31.71倍,木质素过氧化物酶活性提高19.12倍,总木质素酶酶活性提高4.38倍。
试验号 木质素磺酸钠/
(g·L−1)蛋白胨/
(g·L−1)酵母粉/
(g·L−1)豆饼粉/
(g·L−1)五水合硫酸铜/
(g·L−1)氯化钠/
(g·L−1)锰过氧化物酶活性/
(×16.67 nkat·L−1)木质素过氧化物酶活性/
(×16.67 nkat·L−1)漆酶活性/
(×16.67 nkat·L−1)总酶活性/
(×16.67 nkat·L−1)生物量/
g预测值 14.00 12.30 5.00 3.00 0.12 0.53 11 727.39 8 795.36 505.56 21 028.31 0.093 7 实测值 14.00 12.30 5.00 3.00 0.12 0.53 11 328.73 8 514.41 484.73 20 327.87 0.090 0 Table 3. Optimization results of artificial neural network
2.1. 木质素降解菌的筛选
2.2. 菌株的鉴定
2.2.1. 菌株Q01的形态特征及显微观察
2.2.2. 菌株Q01的ITS序列分析及系统发育树
2.3. 液态发酵条件的优化
2.3.1. 培养时间的优化
2.3.2. 接种量的优化
2.3.3. 培养基碳源的优化
2.3.4. 培养基氮源的优化
2.3.5. 均匀试验结合Python实现人工神经网络建模与优化
2.3.6. 优化后菌株木质素降解相关酶活性及生物量
-
通过苯胺蓝平板褪色圈法和愈创木酚平板变色圈法筛选出目标菌株Q01。经鉴定,菌株Q01为栓菌属Trametes真菌。实验证明人工神经网络模型预测结果可值得信赖。根据单因素试验和人工神经网络算法结果,确定菌株Q01的最优发酵条件:培养时间5 d,温度30 ℃,转速200 r·min−1,接种菌液体积分数为12.5%,培养基配方为木质素磺酸钠14.00 g·L−1、蛋白胨12.30 g·L−1、酵母粉5.00 g·L−1、豆饼粉3.00 g·L−1、五水合硫酸铜0.12 g·L−1、氯化钠0.53 g·L−1、pH自然。
菌株Q01在优化后的发酵条件下制得的液体菌剂具有高酶活性和高生物量的特点,可促进园林绿化废弃物堆体初始微生物的数量增长,提高木质素降解相关酶的酶活性,加快木质素的降解。












 DownLoad:
DownLoad: