-
水体富营养化是目前全球普遍面临的环境问题之一。近年来,城市废水与工业废水的大量排放,含有高浓度氮、磷元素的污水汇入周边水域,严重影响了中国几大重要水域的水质,部分湖泊总氮浓度甚至超过富营养化临界值的10倍以上[1]。大面积的水体富营养化不仅危害人体健康,破坏生态平衡,还影响水体景观。相对于易造成二次污染的化学修复,以植物修复技术为主的生物修复因无污染和低成本,能够与绿化环境及改善景观相结合,在水体富营养化的防治中应用广泛[2]。其中,兼具生态价值和观赏价值的水生观赏植物既能净化富营养化水体[3],还能构成优美的水体景观,受到越来越多的关注。
目前,关于单种水生植物对富营养化水体修复的研究较多[4],但由于富营养化水体的污染程度和原因不同,不同植物对不同营养盐的去除也存在差异性,应用单种植物可能存在净化效果不稳定的问题。组合种植水生植物有利于实现不同植物间的优势互补,并可提高景观质量,且合理的水生植物混合栽种可使群落更加稳定、景观季相变化更为丰富。RODRIGUEZ等[5]发现:2种特定水生植物混合栽植可以提高去污效率,MOORE等[6]也认为:未来植物修复富营养水体研究时,应参考天然水生植被的混合种群。COLEMAN等[7]研究发现:灯心草Juncus effusus、香蒲Typha orientalis、水葱Scirpus validus等3种植物混合种植净化废水的效果比单独种植好。周玥等[8]选取不同生活型湿地植物黄菖蒲Iris pseudacorus、香蒲、浮萍Lemna mino和金鱼藻Ceratophyllum demersum,研究其单种及组合种植对低、高质量浓度富营养化水体的净化效果,认为香蒲+浮萍+金鱼藻对总氮去除效果较单种好;香蒲+浮萍+金鱼藻和黄菖蒲+浮萍+金鱼藻对低质量浓度污水中总磷去除率较单种高;黄菖蒲+浮萍+金鱼藻对低质量浓度污水中化学需氧量净化效果最佳,并且优于单种植物。因此,研究水生植物科学配置和组合种植十分必要。
本研究以适应性较强的挺水、浮水、沉水3类水生观赏植物为对象,设置不同的种植组合,比较其在不同富营养化程度的水体中的生长情况以及对总磷、总氮、氨氮的去除及降低化学需氧量等水体的净化效果,为科学搭配水生观赏植物,净化富营养化水体提供科学依据。
-
水生植物选取主要遵循以下原则[9]:①具有较好的去污净化效果;②能够较好地适应被污染的水体环境,耐性较强;③有一定的观赏价值;④优先选择乡土植物;⑤具有较大生物量的大型水生植物。基于以上原则,本研究选用6种水生植物:挺水植物千屈菜Lythrum salicaria、黄菖蒲Iris pseudacorus、水葱,浮水植物荇菜Nymphoides peltatum、睡莲Nymphaea tetragon ,沉水植物金鱼藻。
-
试验地点为南京市栖霞区龙潭街道太平村南京农业大学龙潭荷花基地,全程在避雨棚内进行。试验期间日最高气温为27~36 ℃,日最低气温为20~28 ℃。将供试植物根系洗净,先在自来水中培养7 d,然后种植于直径55 cm的无孔花盆中。种植土取自该基地的园土,土壤的初始pH约为6.93,有机质为20.13~21.76 g·kg−1,底泥厚约 10 cm。加入20 L自来水,待土充分吸收水分后标记水位,蒸发消耗的水每天用自来水补充到原水位高度。设置6种植物组合处理(表1),无植物的为对照组(ck)。于2020年4月下旬上盆,每盆种植1株挺水植物,1株浮水植物,15 g沉水植物。
编号 种植组合 对照组(ck) 无植物 QXJ 千屈菜+荇菜+金鱼藻 QSJ 千屈菜+睡莲+金鱼藻 HXJ 黄菖蒲+荇菜+金鱼藻 HSJ 黄菖蒲+睡莲+金鱼藻 SXJ 水葱+荇菜+金鱼藻 SSJ 水葱+睡莲+金鱼藻 Table 1. Planting combination treatment
根据GB 3838—2002《地表水环境质量标准》规定的劣五类水水质[10]的标准,采用氯化氨、硝酸钾、磷酸二氢钾、尿素、葡萄糖人工配制低、中、高3种不同富营养化程度的水体。其中氨氮∶硝态氮∶有机氮的质量比为1∶1∶1。具体参数见表2。每个组合设置3个质量浓度梯度,每个梯度设置3个平行组。
富营养
化程度总氮/
(mg·L-1)总磷/
(mg·L-1)氨氮/
(mg·L-1)化学需氧量/
(mg·L-1)低 2.00 0.20 0.67 20.00 中 10.00 1.00 3.34 50.00 高 20.00 2.00 6.67 100.00 Table 2. Parameters of the test water
-
隔7 d于9:00—10:00用注射器于每盆的不同方位、不同水位(0、10、20 cm)分别取水样100 mL,用于总氮、总磷、氨氮、化学需氧量等水质指标测定。于取样前一天补充蒸发、蒸腾和取样所消耗的水分以保持水位。试验周期为35 d。试验开始前及结束时测定株高、分枝、叶片数量等植物形态指标。
-
①用卷尺测量植物株高;统计植物分枝/叶片的数量; 用天平称量试验前后的沉水植物生物量(鲜质量)。 株高、分枝/叶片数、沉水植物生物量的相对增长量(Δa):Δa=ai−a0。其中:ai和a0分别为试验结束时开始前的株高、分枝/叶片数量、沉水植物生物量。②水样的测定方法参考《水与废水监测分析方法》(第4版)[11]。总磷采用过硫酸钾消解-钼蓝比色法检测;总氮采用碱性过硫酸钾消解-紫外分光光度法检测;氨氮采用纳氏试剂法,格林凯瑞水质检测仪检测;化学需氧量用粉末试剂法,格林凯瑞水质检测仪进行检测; 溶解氧采用雷磁JPBJ-609L便携式溶解氧测定仪现场测定;pH采用便携式pH计现场测定。
总氮、总磷、氨氮、化学需氧量去除率(降低率,R)的计算方法[12]:R=(C0−Ci)/C0×100%。其中:C0和Ci分别为总磷、总氮、氨氮、化学需氧量的初始质量浓度和第i天质量浓度;
计算植物降低总磷、总氮、氨氮、化学需氧量的效果时应排除底泥自身吸附的影响,公式如下:ΔR=R1−R0。其中:R0和R1分别为无植物组和有植物组的总磷、总氮、氨氮、化学需氧量去除率。
-
采用SPSS 25.0对数据进行统计分析,用GraphPad Prism 8作图。
-
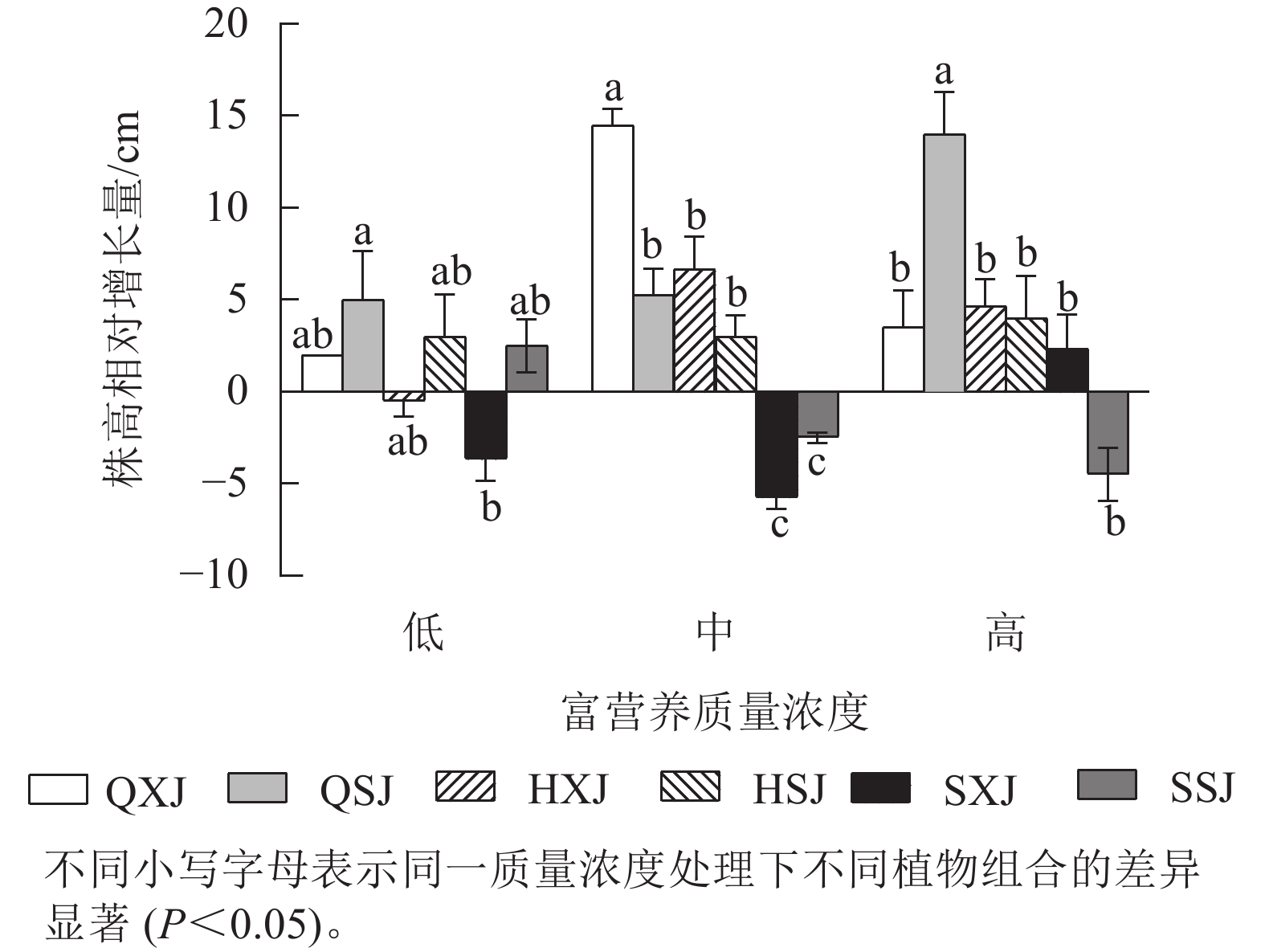
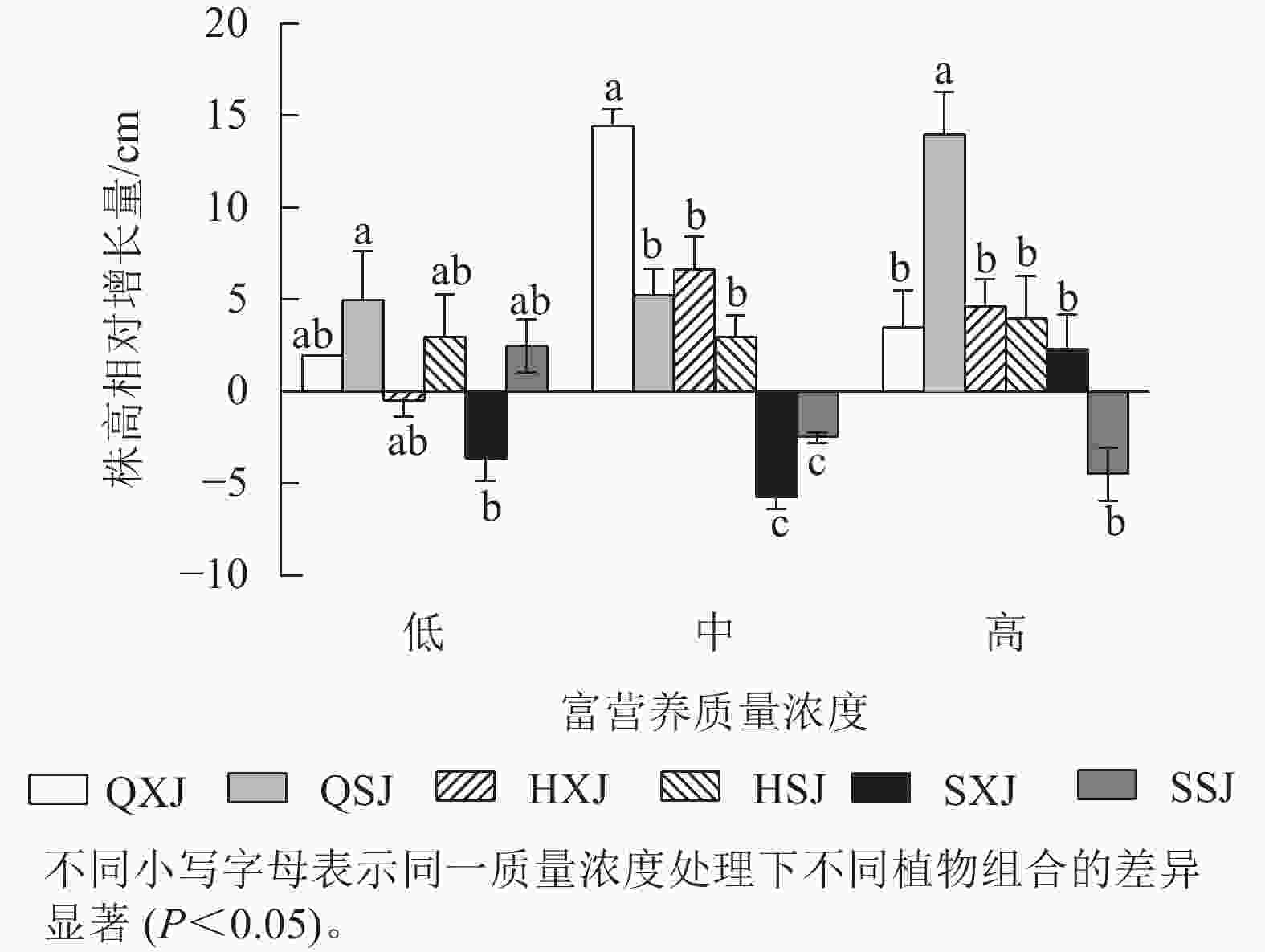
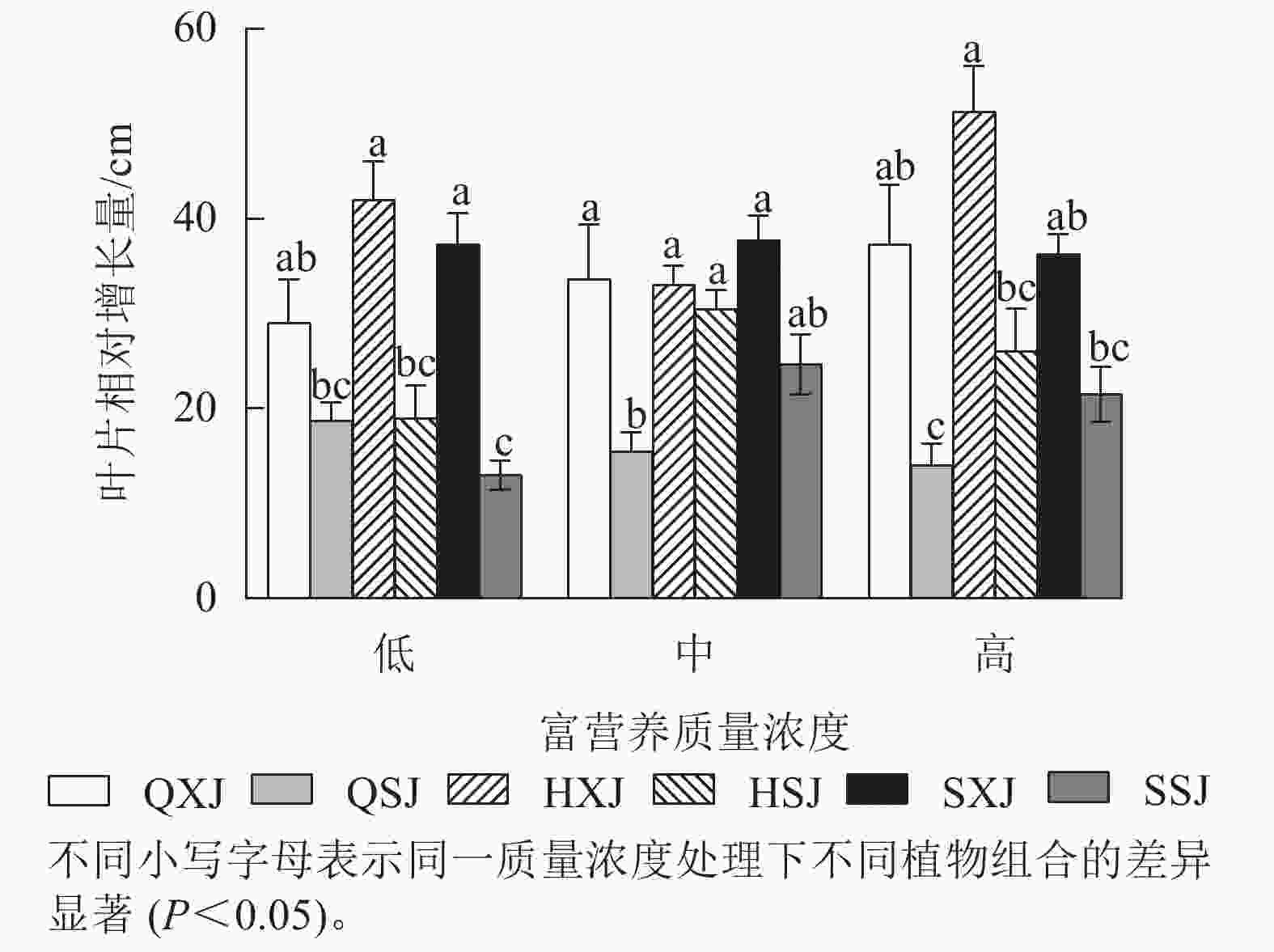
如图1所示:在不同质量浓度富营养下,挺水植物千屈菜和黄菖蒲的株高相对增长量均大于水葱。在低质量浓度下,同种挺水植物与睡莲组合时株高增量比与荇菜组合时大;中质量浓度下,QXJ组合中千屈菜的株高增量显著高于其他处理组的挺水植物(P<0.05)。
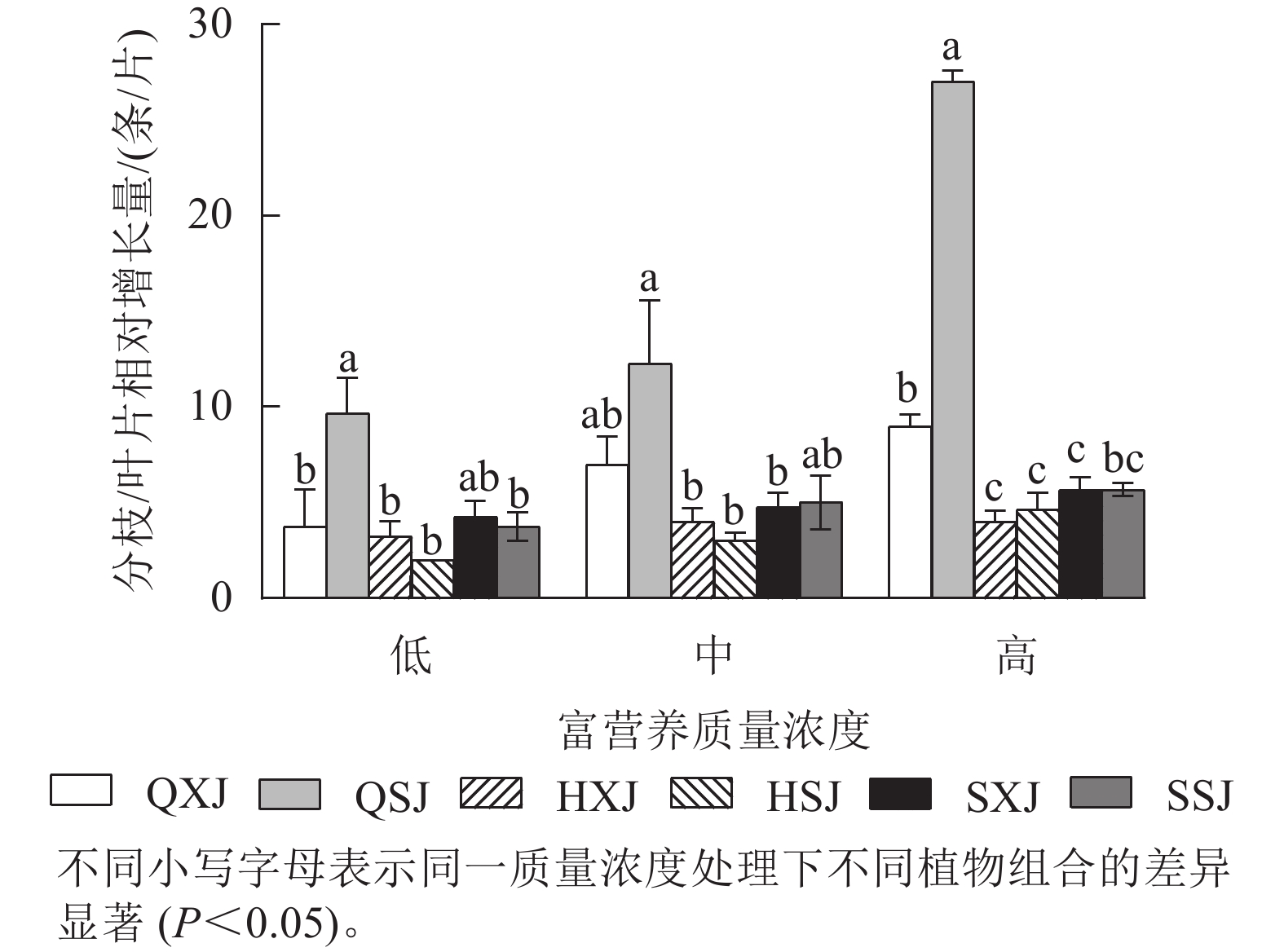
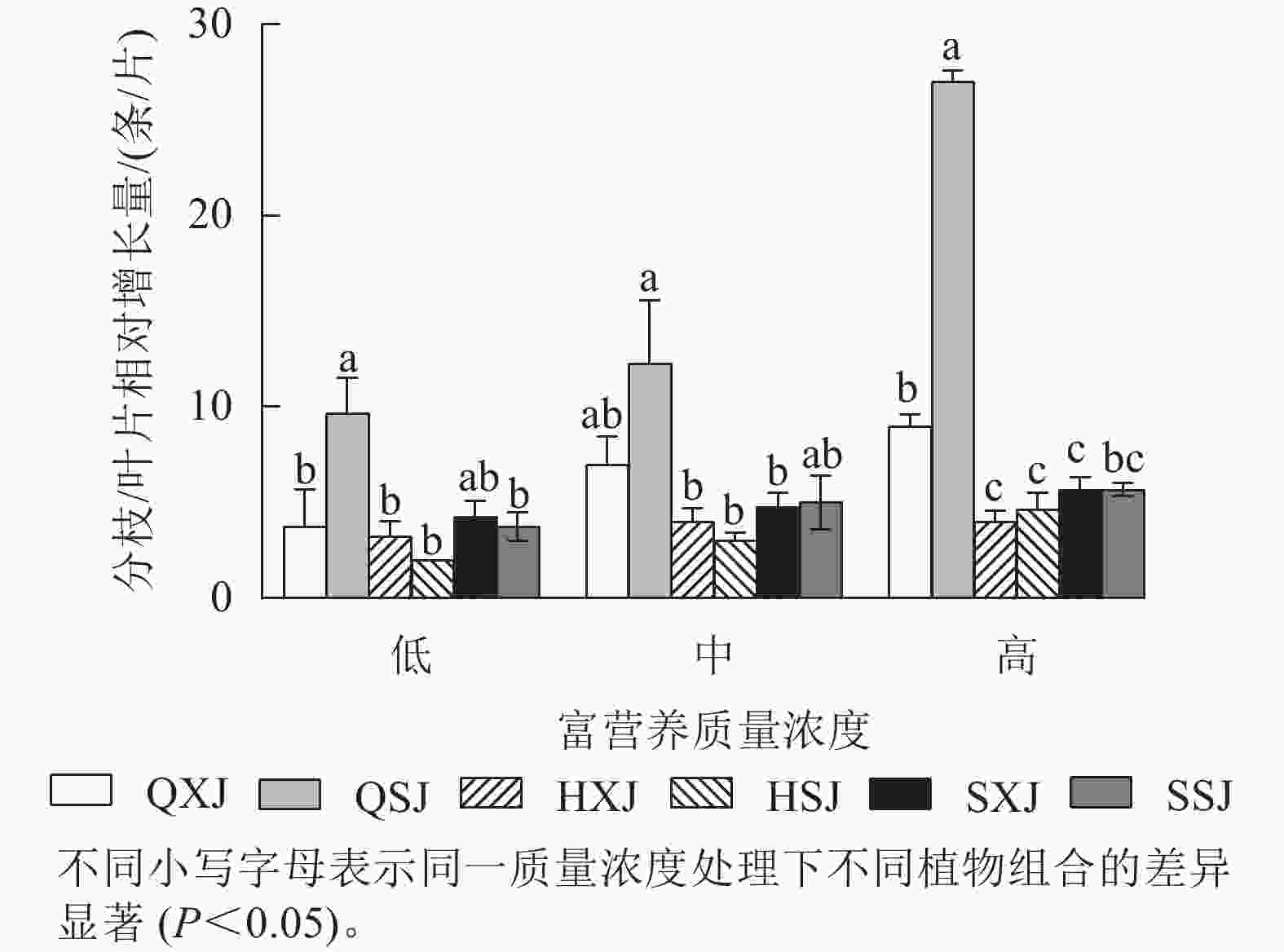
如图2所示:3种质量浓度富营养处理下,千屈菜的分枝相对增长量大于黄菖蒲和水葱。千屈菜在与睡莲组合时的分枝增量比与荇菜组合时大,尤其是在低、高质量浓度下差异显著(P<0.05),说明千屈菜在高质量浓度下长势较好,最好的组合是QSJ。3种质量浓度处理下,HXJ中黄菖蒲的叶片相对增长量基本保持不变;而HSJ中黄菖蒲的叶片相对增长量随处理质量浓度升高呈增加趋势;水葱的叶片相对增长量基本持平。总的来看,3种挺水植物在中、高质量浓度富营养水体中正常增加分枝和叶片数。
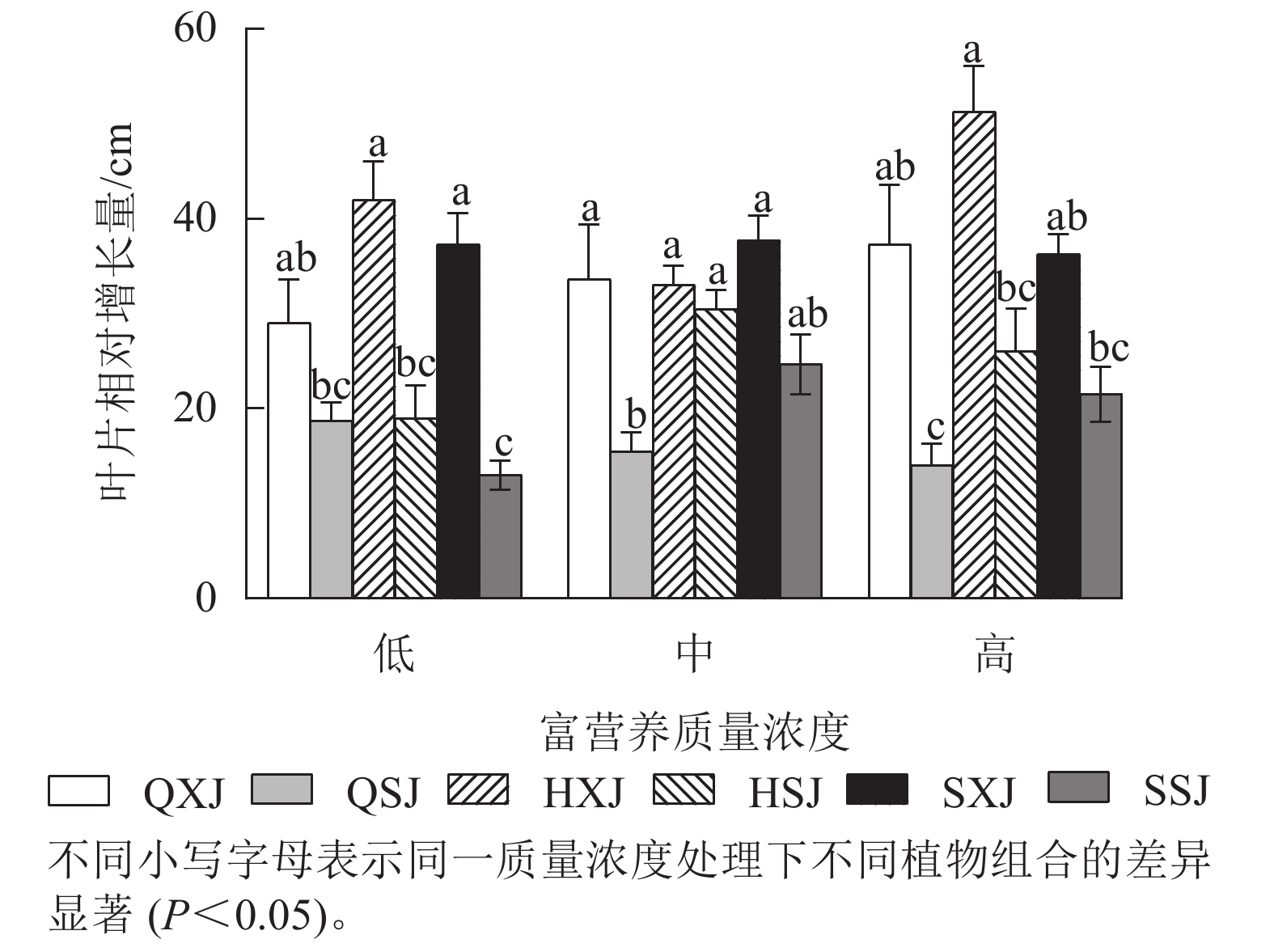
由图3所示:3种质量浓度富营养处理下,浮水植物荇菜叶片相对增长量大于睡莲,尤其是HXJ组中的荇菜,叶片增量较大,同种浮水植物与黄菖蒲组合时,浮水植物叶片增量均较大,如中质量浓度处理HSJ组的睡莲叶片增量显著大于QSJ组(P<0.05)。总的来看,荇菜在高质量浓度下叶片数相对增长量较大,叶片数增长速度较快;睡莲的叶片数则在中、高质量浓度中增长速度较快。
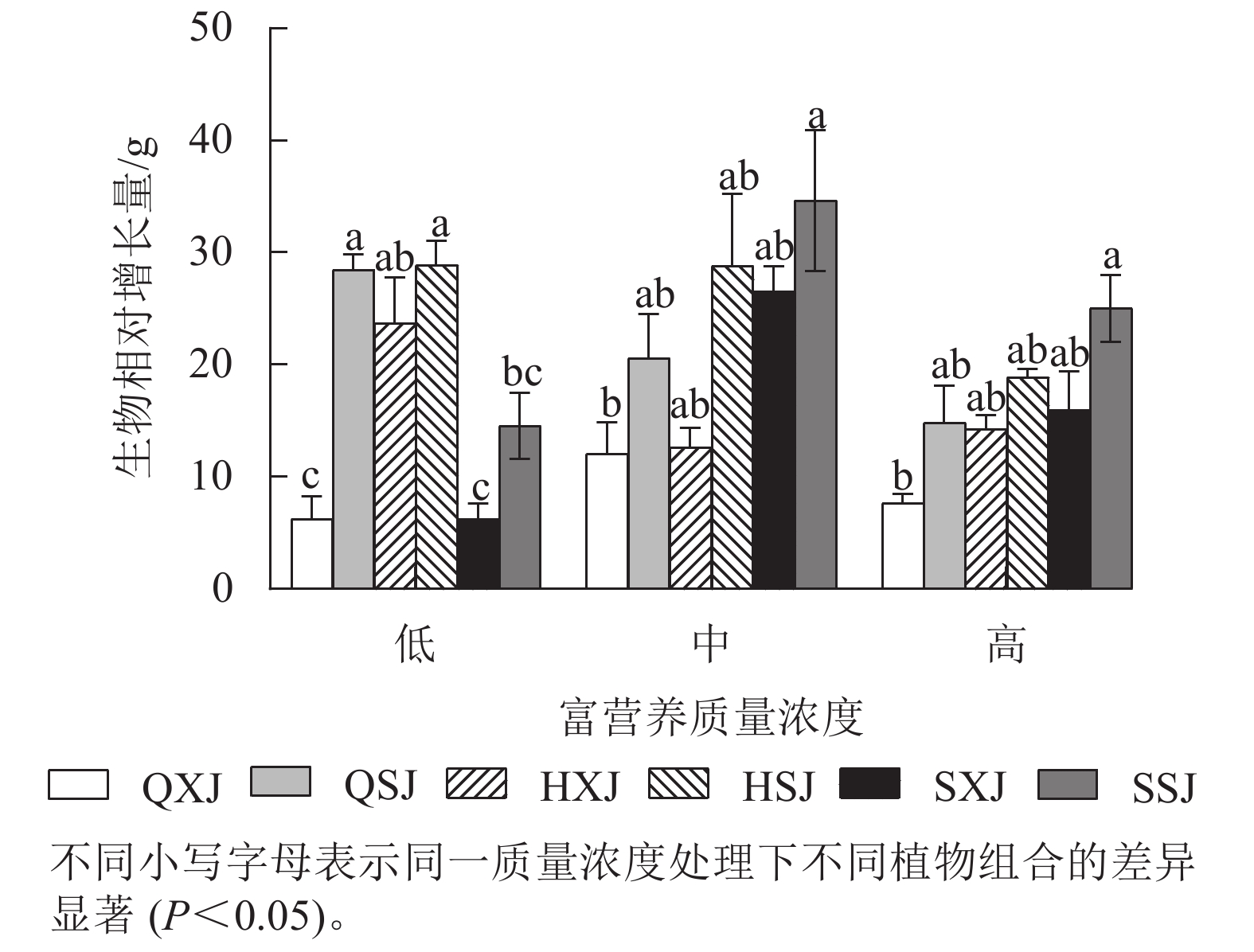
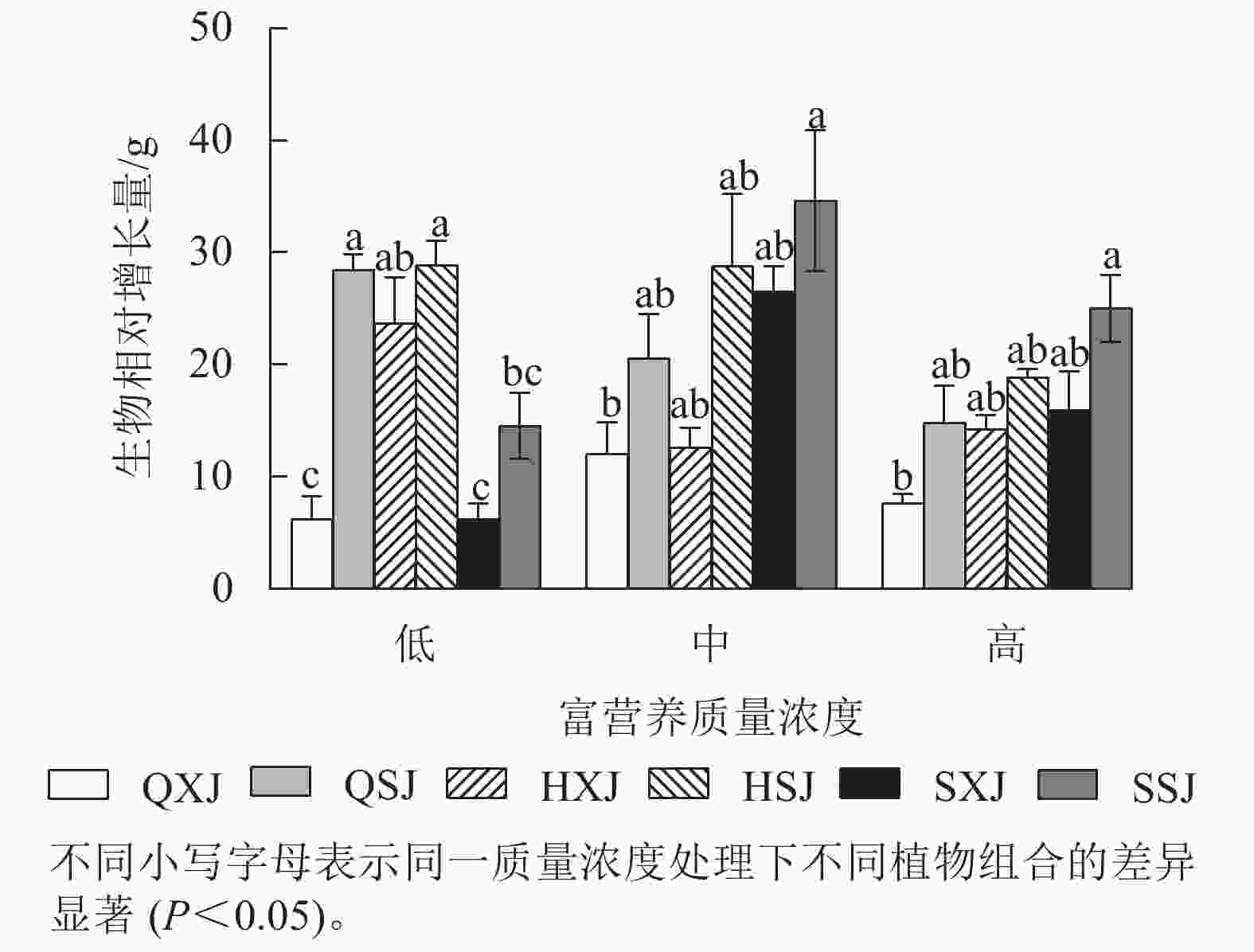
由图4所示,低质量浓度富营养处理下,沉水植物金鱼藻与黄菖蒲+睡莲、与千屈菜+睡莲组合时生物量相对增长量显著大于与千屈菜+荇菜、与水葱+荇菜以及与水葱+睡莲组合(P<0.05)。中、高质量浓度下,金鱼藻在与水葱+睡莲、黄菖蒲+睡莲组合时生物量增量最大,与千屈菜+荇菜组合时最小,差异显著(P<0.05)。3种质量浓度处理下,挺水植物相同时,金鱼藻与睡莲组合时的生物量增量均较与荇菜组合时大。浮水植物相同时,低质量浓度下,金鱼藻与黄菖蒲组合时生物量增量最大;中、高质量浓度处理下,金鱼藻与水葱、黄菖蒲组合时的生物量增量比与千屈菜组合时大。另外,HSJ组的金鱼藻在低、中质量浓度处理下生物量增量较高质量浓度处理组大;QSJ组的金鱼藻则在低质量浓度下生物量增量最大,随质量浓度增加有所减小;SSJ组中的金鱼藻生物量增量则在中、高质量浓度中较大。总的来看,沉水植物金鱼藻可通过与不同的挺水、浮水植物组合适应中、高质量浓度的富营养水体。
综上所述,3种质量浓度富营养处理下,3类6种供试水生植物长势均较好,挺水植物中千屈菜长势最好;浮水植物中荇菜长势优于睡莲,荇菜虽然长势快,但与其他植物组合配植时,可能会产生种间竞争;沉水植物金鱼藻具有较强的适应中高质量浓度富营养化水体的能力。
-
从图5A可看出:低质量浓度富营养处理下,各植物组合中水体的总磷质量浓度在7 d时快速下降;7 d后,除SXJ外,其他组水体总磷整体上呈缓慢上升趋势;试验结束时(35 d),植物组合处理水体的总磷由开始时0.20 mg·L−1下降至0.03~0.08 mg·L−1,对照组下降至0.12 mg·L−1。将对照组总磷去除率减去之后计算植物组合实际去除率,结果表明(表3):处理14 d之前,SSJ组合对总磷的去除率较大,而SXJ的去除率最低,可能与其前期对水体环境暂未完全适应有关;所有植物组合对总磷的去除率在28 d时达最大值,其中QXJ和HSJ去除率较大, SSJ去除率最低;至35 d时,所有组合对总磷的去除率较28 d都有所下降。
富营养化程度 t/d 不同植物种植组合处理对总磷的去除率/% QXJ QSJ HXJ HSJ SXJ SSJ 低 7 0.00±1.88 ab 0.35±2.20 ab −4.90±0.30 b 5.85±4.83 a −46.04±2.66 c 12.53±4.43 a 14 36.06±0.94 ab 32.80±2.72 b 40.78±0.76 ab 33.76±0.06 b 3.83±3.99 c 47.57±3.76 a 21 34.53±6.23 a 26.86±4.73 a 37.60±9.64 a 49.87±3.91 a 42.59±4.36 a 36.83±0.94 a 28 81.13±1.45 a 64.28±5.05 b 61.39±2.88 bc 74.59±2.21 ab 72.89±1.76 ab 49.15±0.92 c 35 16.10±1.33 b 42.93±6.68 a 31.05±3.45 ab 42.93±2.17 a 34.50±2.30 a 33.75±5.53 a 中 7 4.37±0.69 a 1.53±2.62 a 2.76±1.59 a 1.38±2..42 a 0.92±0.92 a 4.30±0.61 a 14 26.47±1.57 a 27.16±0.79 a 22.71±4.93 a 26.24±1.86 a 20.56±4.82 a 20.95±4.09 a 21 9.21±1.36 a 12.43±1.16 a 6.91±1.33 a 11.66±0.81 a 11.05±1.46 a 11.74±1.47 a 28 20.34±0.58 a 19.53±1.53 a 21.03±0.59 a 21.71±0.23 a 18.93±0.94 a 19.59±1.85 a 35 8.06±1.21 a 8.42±1.12 a 3.96±0.68 ab 8.52±0.23 a 2.15±1.34 b 7.37±0.99 a 高 7 4.45±0.41 a 6.22±0.13 a 4.83±0.72 a 5.06±0.80 a 5.99±0.27 a 4.95±1.35 a 14 6.98±0.27 a 7.21±0.13 a 6.98±0.46 a 4.99±0.31 b 6.98±0.03 a 6.29±0.13 ab 21 10.97±0.44 a 10.97±0.48 a 7.37±0.76 bc 10.13±1.07 ab 9.94±0.83 ab 6.14±0.01 c 28 12.20±0.62 a 12.50±0.31 a 10.66±0.67 a 10.36±1.38 a 10.66±1.31 a 12.04±1.77 a 35 9.30±0.60 ab 7.60±1.74 b 11.59±0.15 ab 12.20±0.70 a 7.75±1.01 b 12.35±0.81 a 说明:同行平均值后不同小写字母表示同一时间不同植物组合对总磷的去除率差异显著(P<0.05)。 Table 3. Removal of TP from water bodies by different aquatic plant combinations under different eutrophic concentration treatments
图5B所示:中质量浓度富营养化各植物组合水体总磷质量浓度在7 d时下降明显,在7~14 d时略微上升,随后下降。HXJ和SXJ组在28 d后总磷质量浓度有所增加。35 d时,各植物组合水体总磷质量浓度由1.00 mg·L−1下降为0.04~0.10 mg·L−1,对照组的总磷质量浓度则下降为0.13 mg·L−1。从植物组合总磷去除率来看(表3),14 d时出现第1个去除率高峰,此时QSJ、QXJ和HSJ的TP去除率均较高, SXJ的总磷去除率最低;28 d时出现第2个总磷去除率高峰,此时HSJ和HXJ对总磷去除率较大。35 d时, HSJ、QSJ、QXJ以及SSJ为净化总磷效果较好的植物组。
由图5C可知:高质量浓度富营养处理下,各植物组合水体总磷质量浓度在7 d时下降趋势明显;HSJ和SSJ的总磷质量浓度在7~21 d时上升,随后下降,其余组合组一直保持缓慢增长趋势。至35 d,植物组合组的水体总磷质量浓度由2.00 mg·L−1降至0.03~0.13 mg·L−1,对照组的水体总磷质量浓度降至0.28 mg·L−1。各组合的总磷去除率总体随时间延长呈上升趋势(表3),35 d时,HSJ及SSJ对总磷去除率最高,显著高于SXJ和QSJ。
相同的植物组合在不同质量浓度富营养水体中,对总磷的净化效果也不相同。就黄菖蒲+睡莲+金鱼藻组(HSJ)而言,其在低、中、高3种质量浓度处理中均有相对良好的净化总磷的效果。水葱+荇菜+金鱼藻组(SXJ)在低质量浓度处理中,对总磷的去除效果前期较差,后期才逐渐达到较为良好的效果;在中、高质量浓度富营养化水体中净化总磷效果相对较差。
-
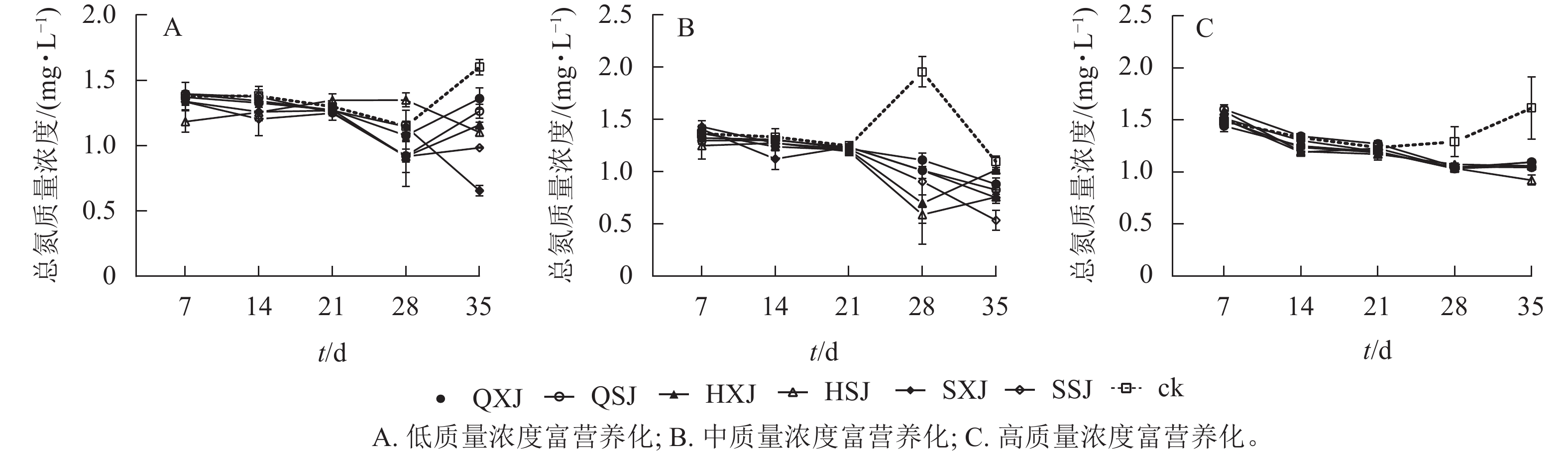
低质量浓度富营养处理下,7~28 d各植物组合水体总氮质量浓度总体上呈不同程度下降趋势,28 d后,QXJ、QSJ和HXJ的总氮质量浓度均有所增加;35 d时,所有植物组合的总氮质量浓度均低于对照组(图6A),由2.00 mg·L−1下降为0.98~1.36 mg·L−1,对照组下降至1.60 mg·L−1。从表4可知,前28 d各植物组合对总氮的去除率不高,至35 d时,各植物组合组对总氮的去除率达峰值,其中SXJ、SSJ和HSJ对水体总氮的去除率最高。
富营养化程度 t/d 不同植物种植组合处理对总氮的去除率/% QXJ QSJ HXJ HSJ SXJ SSJ 低 7 0.44±2.06 a −0.83±0.74 a −0.78±1.15 a 9.75±4.14 a 2.08±0.93 a 2.19±1.30 a 14 2.62±1.18 a 0.34±3.95 a 1.90±1.19 a 6.23±3.33 a 6.04±2.71 a 8.79±6.38 a 21 1.36±1.81 a 2.33±1.22 a 1.39±0.98 a −2.42±2.48 a 1.50±1.29 a 2.53±2.74 a 28 3.64±0.13 a 11.40±2.49 a 11.63±11.62 a −10.00±2.60 a 0.25±2.02 a 11.62±6.19 a 35 12.05±4.07 d 16.93±2.62 cd 22.10±1.04 bcd 24.95±1.41 bc 47.33±1.99 a 30.90±0.17 b 中 7 0.40±0.06 a 0.67±0.35 a −0.05±0.41 a 1.15±1.30 a −0.42±0.39 a −0.66±0.60 a 14 0.24±0.30 a 0.30±0.41 a 0.94±0.18 a 0.59±0.21 a 2.10±1.04 a 0.60±0.34 a 21 0.28±0.23 a 0.13±0.27 a 0.40±0.17 a 0.55±0.10 a 0.11±0.35 a 0.32±0.23 a 28 8.43±0.63 a 9.44±0.77 a 12.58±3.92 a 13.66±0.84 a 9.44±0.12 a 10.51±1.26 a 35 2.15±0.56 bc 2.67±0.44 bc 0.79±0.08 c 3.36±0.24 abc 3.43±0.56 ab 5.59±0.94 a 高 7 0.23±0.27 a −0.58±0.21 a −0.48±0.22 a −0.11±0.04 a −0.13±0.10 a −0.05±0.22 a 14 0.41±0.12 ab −0.06±0.06 c 0.67±0.00 a 0.71±0.04 a 0.15±0.11 bc 0.48±0.08 ab 21 0.22±0.40 a −0.17±0.15 a 0.33±0.10 a 0.05±0.13 a 0.20±0.11 a 0.24±0.22 a 28 1.29±0.13 a 1.20±0.05 a 1.08±0.06 a 1.27±0.05 a 1.29±0.09 a 1.23±0.05 a 35 2.59±0.06 b 2.85±0.04 b 2.78±0.09 b 3.47±0.24 a 2.76±0.09 b 2.60±0.12 b 说明:同行平均值后不同小写字母表示同一时间不同植物组合对总氮的去除率差异显著(P<0.05)。 Table 4. Removal of TN from water bodies by different aquatic plant combinations under different eutrophic concentration treatments
中质量浓度富营养化处理下,各植物组合水体总氮质量浓度在7 d时快速下降,随后呈相对缓慢的下降趋势,在前21 d与对照组接近;自28 d开始,植物组合的总氮质量浓度均低于对照;35 d时,植物组合水体总氮质量浓度由10.00 mg·L−1下降至0.54~1.02 mg·L−1,对照组则降至1.09 mg·L−1(图6B)。各植物组合对总氮去除率在前21 d较低,28 d时总氮净化率达最大值,其中HSJ、HXJ组对总氮的去除效果最高,QXJ组的总氮去除率最低;至35 d时,所有水生植物组合对总氮的净化率出现下降(表4)。
高质量浓度富营养处理下(图6C),各植物组合水体总氮质量浓度在前期剧烈下降,7 d后一直保持下降的趋势;各植物组合水体中总氮质量浓度在前21 d对与照组差异较小,自28 d开始显著低于对照组;至35 d时,植物组合水体总氮质量浓度由20.00 mg·L−1降为0.92~1.10 mg·L−1,对照组的水体总氮质量浓度为1.62 mg·L−1。从对总氮的去除率来看(表4),各植物组合仅在14 d时对总氮的去除率较大,去除率最高的为HSJ和HXJ组;35 d时,各植物组合对总氮的去除率达最大值,其中HSJ对总氮净化率最高,QXJ去除率最低。
同种植物组合在不同质量浓度富营养水体中净化总氮的效果不同。HSJ组在3种质量浓度下净化总氮效果较好,尤其在后期效果较为明显;SXJ组在低、中质量浓度富营养水体中去除总氮效果较好,但在高质量浓度下前期净化效果较差。
-
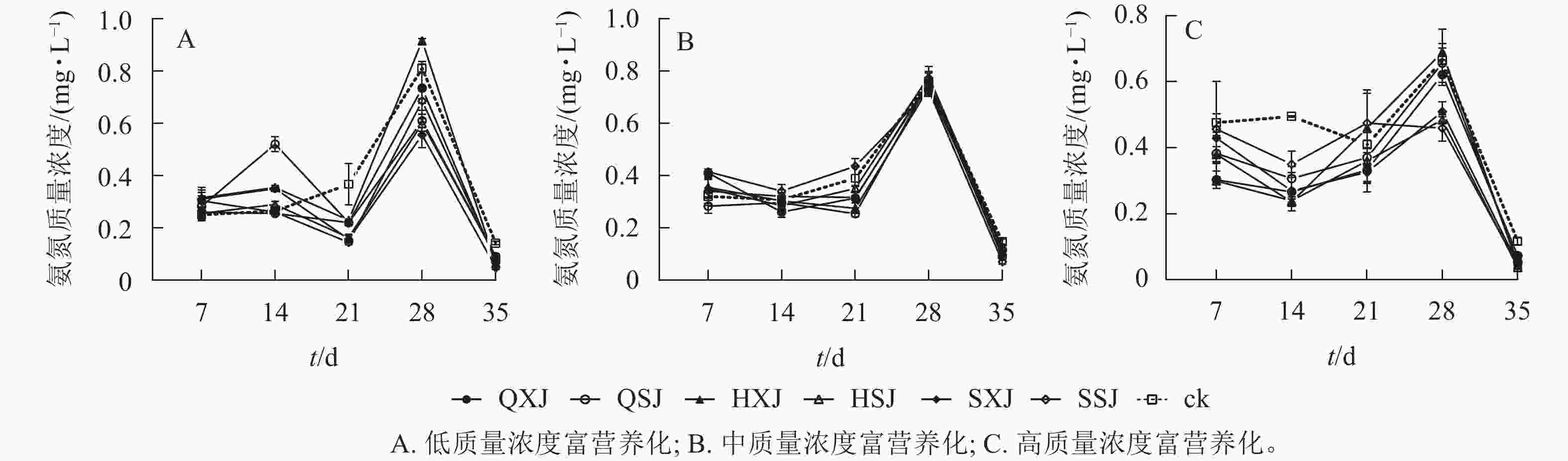
由表5可见:低质量浓度富营养处理下,7 d内各植物组合水体中氨氮质量浓度下降十分明显;在7~21 d时总体呈缓慢下降,21~28 d时,迅速上升,随后又显著下降。至35 d,植物组合组水体中氨氮质量浓度由0.67 mg·L−1下降为0.05~0.09 mg·L−1,对照组则下降至0.14 mg·L−1(图7A)。各植物组合对氨氮的去除率在前期较低,21和28 d时达到峰值,21 d时,SSJ、SXJ和HSJ组去除率较高,显著高于其他植物组合;28 d时,SXJ、HSJ、QSJ组对氨氮的去除率较高;至35 d时,SXJ组对氨氮的净化率最高(表5)。
富营养化程度 t/d 不同植物种植组合处理对氨氮的去除率/% QXJ QSJ HXJ HSJ SXJ SSJ 低 7 −1.49±4.80 a −4.98±5.87 a −9.70±3.02 a −0.75±3.88 a −8.96±5.24 a −7.96±7.77 a 14 1.50±0.43 a −38.06±4.31 d −13.43±0.43 c −3.73±1.72 ab −12.69±1.72 bc 0.75±0.00 a 21 21.89±0.86 b 21.89±1.72 b 20.40±0.86 b 30.35±1.79 a 31.34±1.99 a 33.08±1.29 a 28 11.19±15.08 ab 29.85±6.03 a −15.67±1.29 b 31.34±2.59 a 38.06±7.32 a 18.66±1.29 ab 35 10.65±0.28 ab 7.66±1.75 b 11.74±1.04 ab 7.56±1.42 b 14.03±1.29 a 9.48±0.73 ab 中 7 −2.69±0.46 a 1.10±0.87 a −1.05±1.12 a −0.90±1.04 a −0.60±1.38 a −2.84±0.09 a 14 1.40±0.17 a 0.30±0.10 a 0.10±1.90 ab 0.65±0.09 a −0.40±0.35 ab −1.00±0.17 b 21 2.30±0.53 a 4.09±0.36 a 3.44±0.61 a 1.20±0.35 ab 2.20±1.21 a −1.35±0.09 b 28 0.00±0.17 a 0.15±1.30 a 0.75±0.78 a −0.60±0.52 a 0.20±1.15 a 1.00±0.40 a 35 0.91±0.03 bc 1.68±0.10 ab 0.67±0.29 bc 0.61±0.22 c 1.50±0.25 abc 2.27±0.29 a 高 7 2.60±0.40 a 1.40±0.80 a 1.45±0.35 a 2.65±0.17 a 0.70±1.08 a 0.30±0.13 a 14 3.42±0.87 a 2.82±0.56 a 3.87±0.22 a 3.82±0.09 a 3.37±0.69 a 2.17±0.61 a 21 1.15±1.00 a 0.60±1.15 a −0.75±1.73 a 0.75±0.94 a 1.25±0.53 a −0.97±1.34 a 28 0.65±0.48 ab 0.12±0.87 ab −0.37±1.04 b 2.70±0.13 ab 2.32±0.43 ab 3.07±0.61 a 35 0.66±0.01 c 0.95±0.08 b 1.08±0.04 ab 1.19±0.03 a 1.04±0.06 ab 1.08±0.02 ab 说明:同行平均值后不同小写字母表示同一时间不同植物组合对氨氮的去除率差异显著(P<0.05)。 Table 5. Removal of NH3-N from water bodies by different aquatic plant combinations under different eutrophic concentration treatments
如图7B所示:中质量浓度处理下,各植物组合水体氨氮质量浓度在前7 d快速下降,7~21 d保持相对平稳,随后迅速增加,28 d后水体氨氮质量浓度又显著下降。35 d时各植物组合水体氨氮质浓度由3.34 mg·L−1降至0.09~0.12 mg·L−1,对照组为0.15 mg·L−1。多数植物组合在前期对氨氮的去除率较低(表5),后期才先后发挥对氨氮的净化效果,21 d时出现氨氮去除高峰,此时QSJ的去除率最高。35 d时,SSJ和QSJ对氨氮的净化效果最好。
高质量浓度富营养处理下,各植物组合水体氨氮质量浓度前期均略微下降,随后迅速增长,28 d后又明显下降。35 d时,植物组合处理水体氨氮质量浓度由6.67 mg·L−1降至0.04~0.05 mg·L−1,对照组则为0.12 mg·L−1(图7C)。各植物组合在14 d时形成第1个去除氨氮的高峰,之后对氨氮的去除率有所下降;SSJ、HSJ和SXJ在28 d时形成第2个氨氮去除高峰。35 d时,对氨氮的去除率最大的为HSJ组,最小的为QXJ组(表5)。
比较同种植物在不同质量浓度富营养处理对氨氮的去除效果可知:HSJ组在高质量浓度处理下能维持较为良好的净化氨氮效果。QSJ组在低质量浓度处理下处理前期对氨氮的去除十分缓慢,后期净化效果较好;中质量浓度处理下能保持良好的净化效果;高质量浓度处理下,前期去除效果较差,35 d时,恢复到较好的效果。
-
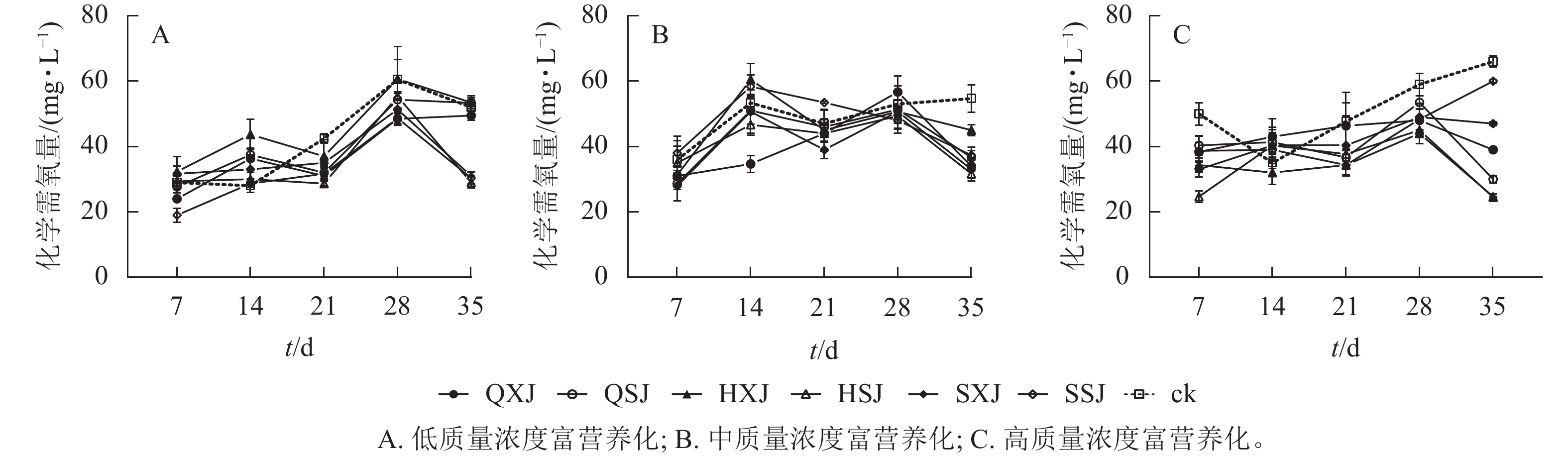
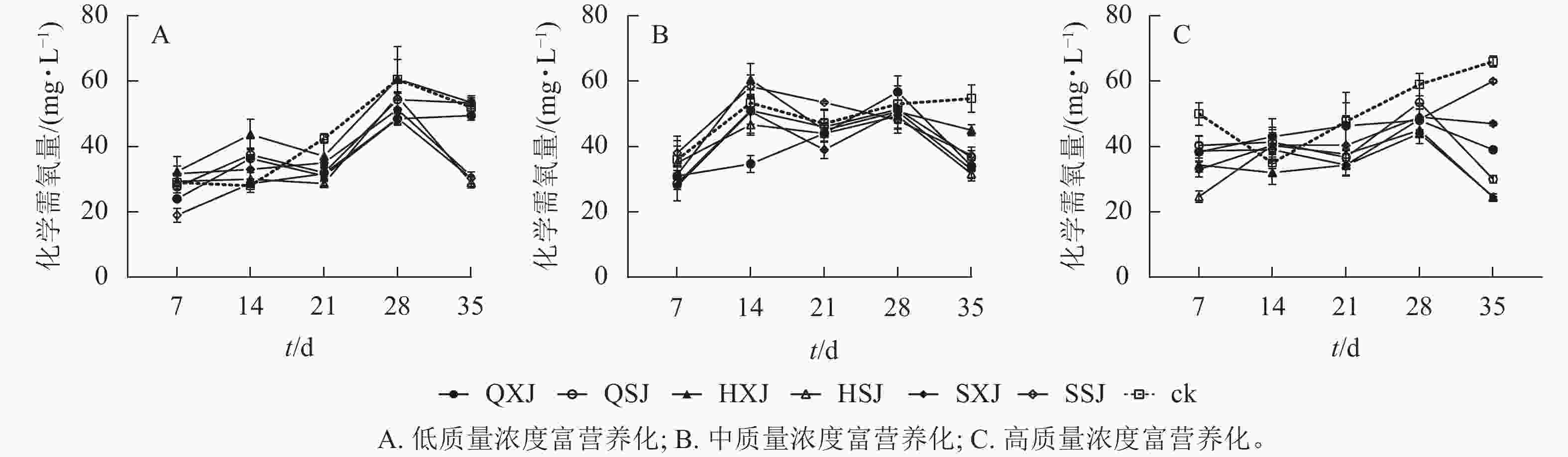
低质量浓度富营养处理下,各植物组合处理水体化学需氧量质量浓度在前28 d随时间延长而增加,随后HSJ、SSJ、SXJ和HXJ组的化学需氧量质量浓度下降,保持平稳(图8A)。至35 d时,低质量浓度处理下植物组合水体化学需氧量质量浓度由20.00 mg·L−1增加至29.00~53.33 mg·L−1,此时,对照组则增加至52.00 mg·L−1。SSJ在处理前期对化学需氧量去除率最高;HSJ、SSJ和SXJ在35 d时达到对化学需氧量去除率的高峰,且显著高于QXJ、QSJ和HXJ组(表6)。
富营养化程度 t/d 不同植物种植组合处理对化学需氧量的去除率/% QXJ QSJ HXJ HSJ SXJ SSJ 低 7 25.00±5.00 ab 6.67±9.28 ab −16.67±23.15 b −1.67±12.02 ab −13.33±11.67 b 50.00±10.41 a 14 −41.67±3.33 a −47.50±10.10 ab −78.33±23.33 b −10.00±10.00 a −25.00±22.55 a −3.33±6.01 a 21 57.50±15.28 a 52.50±11.55 a 27.50±25.98 a 69.17±3.33 a 37.50±7.64 a 54.17±8.33 a 28 60.00±7.22 a 30.83±12.02 a −0.83±30.05 a 25.83±4.41 a 45.83±16.91 a 59.17±10.93 a 35 12.50±7.22 b −6.67±4.41 b −7.50±10.10 b 115.00±8.67 a 105.00±0.00 a 108.33±16.07 a 中 7 10.00±6.11 a 14.67±2.40 a 9.33±0.67 a 2.00±16.17 a 16.00±9.24 a −4.00±7.57 a 14 37.33±5.21 a 4.67±8.33 ab −14.33±9.81 b 13.33±6.36 ab 5.33±13.28 ab −10.00±7.06 b 21 6.00±5.29 a 2.00±5.03 a 4.00±12.70 a 6.00±4.62 a 16.00±5.29 a −13.00±0.58 a 28 −7.33±9.96 a 3.33±11.39 a 4.67±10.67 a 7.33±0.67 a 4.67±6.96 a 10.00±8.00 a 35 41.33±3.46 ab 36.00±6.36 ab 19.33±3.46 b 46.00±2.91 a 43.33±7.02 a 34.67±2.91 ab 高 7 11.67±2.85 ab 9.67±2.91 b 15.67±2.19 ab 25.33±1.76 a 17.00±2.31 ab 11.33±4.67 b 14 −8.00±5.51 a −6.33±2.85 a 3.00±3.61 a −5.33±4.84 a −5.33±5.55 a −4.00±1.00 a 21 1.33±6.96 a 11.00±3.76 a 13.33±1.20 a 10.00±1.20 a 7.33±9.02 a 13.33±3.38 a 28 11.00±3.46 a 5.50±2.02 a 15.00±1.15 a 14.00±4.16 a 10.00±4.04 a 10.67±4.81 a 35 27.00±0.58 c 36.00±1.15 b 41.33±0.88 a 41.50±0.29 a 19.00±0.58 d 6.00±0.58 e 说明:同行平均值后不同小写字母表示同一时间不同植物组合对化学需氧量去除率差异显著(P<0.05)。 Table 6. Removal of COD from water bodies by different aquatic plant combinations under different eutrophic concentration treatments
从图8 B可看出:中质量浓度富营养处理下,各组合水体化学需氧量质量浓度在前7 d下降明显,在7~28 d时总体呈上升趋势,28 d后迅速下降。至35 d时,中质量浓度富营养处理下,植物组合组水体化学需氧量质量浓度由50.00 mg·L−1下降至31.67~37.33 mg·L−1,对照组则降为54.67 mg·L−1。从表6可以看出:14 d时QXJ组的化学需氧量净化效果较好;各植物组合在35 d时达到化学需氧量去除率的最大值,此时HSJ和SXJ对化学需氧量的去除率最高,HXJ去除率最低。
由图8C可知:高质量浓度富营养处理下,所有种植组合的化学需氧量质量浓度在处理前期下降较为迅速,在7~28 d期间缓慢增加,随后除SXJ及SSJ组外,其余组合下降明显。试验期间,各植物组合的水体化学需氧量质量浓度由100.00 mg·L−1降至24.67~60.00 mg·L−1,对照组降至66.00 mg·L−1。进一步对植物组合的化学需氧量去除率进行分析(表6),所有植物组合对化学需氧量的去除率在7 d时达到第1个高峰,此时HSJ组的化学需氧量净化率最大;至35 d时,SSJ组对化学需氧量的净化效率最低,且除SSJ组外,其他植物组合达到化学需氧量去除率最大值,其中化学需氧量去除率最高的为HSJ和HXJ组(表6)。
HSJ组在低质量浓度富营养处理下对化学需氧量的净化在后期效果较好;在中、高质量浓度富营养处理下对化学需氧量去除效果整体较好。XSJ组在低质量浓度下能较好地净化水体中化学需氧量。中质量浓度富营养下,SSJ组在前期对化学需氧量的去除率一直处于较低水平,后期才发挥较好效果;而在高质浓度富营养下的后期对化学需氧量的去除效果较差。
-
本研究发现:同一挺水植物在不同组合中长势不一致,可能存在植物间的竞争关系,如低质量浓度富营养化水体中,与荇菜组合的挺水植物整体上长势都弱于与睡莲组合的挺水植物,且3种质量浓度富营养处理中的沉水植物也存在类似情况。另外,荇菜生长速度过快,优于睡莲,快速覆盖水面,挤占了同一组合中其他植物的生存空间,导致含荇菜的植物组合中的挺水植物、沉水植物整体上都弱于与睡莲组合的挺水植物和沉水植物,如千屈菜和金鱼藻在千屈菜+荇菜+金鱼藻组合中长势明显弱于其在千屈菜+睡莲+金鱼藻中的长势。吴中华[13]认为:荇菜与较弱的竞争对手共存时,往往在水面通过叶片快速生长形成覆盖层,以获取更多的生态位和营养空间。所以需考虑各植物生物学特性,避免种间竞争。水葱在低质量浓度富营养下长势较好,而黄菖蒲作为块茎类挺水植物具有较强的净水能力[14],其在中、高质量浓度富营养化水体中比低质量浓度中长势更好,发挥效用更明显。这可能与黄菖蒲在低质量浓度富营养化水体中不能获得足够的氮、磷而生长受限制有关[15]。
黄菖蒲+睡莲+金鱼藻组对不同质量浓度富营养化水体中总磷、总氮、化学需氧量均有较优的去除效果,在高质量浓度富营养化水体中,还对氨氮净化效果良好。各植物组合在前28 d对化学需氧量的去除效果没有显著差异,推测与水体中植物和微生物之间的相互作用有关,王兴华等[16]研究也表明:植物微生物协同处理比微生物或植物单独处理能更有效降低污水中化学需氧量的浓度。在本研究中,各组合的植物在生长过程中为水中微生物提供较好的生存环境和营养物质。微生物在生长过程中又能把水中的氮、磷等转变为植物更容易吸收的盐类。浮水植物睡莲净化营养盐的能力较强,挺水植物黄菖蒲的磷去除率高,两者组合适用于净化重度污染水体、氮污染形式主要为氨氮且污染较严重的富营养化水体[15−19],能更好地发挥两者净化营养盐的优势。
供试水体中营养盐总氮、总磷、氨氮、化学需氧量质量浓度在前7 d迅速下降,但大部分水生植物组合对以上营养盐的去除率较低,后期其去除率有所上升。相关文献表明:由于生物化学作用较慢,水生植物生长相对缓慢而减少了前期对水柱中的氮、磷吸收和同化,因此试验前期营养盐的净化以基质吸附等物理作用为主,后期以植物吸收等生物化学作用为主[20]。在本试验后期对照组的总磷质量浓度均明显下降,该结果与杨澜等[21]的研究结果类似,推测为长时间放置后对照组盆内水分蒸发或水中磷元素被土壤吸附所致。
就各水生植物的景观特性而言,合理地利用植物的花色进行色彩搭配显得尤为重要[22]。挺水植物中千屈菜夏、秋季节开花,花红紫色,花朵繁密;黄菖蒲春季开花,花黄色,花大且姿态优美;水葱夏季开花,秆直立高达,花药线性。浮水植物荇菜夏季开花,花黄色;睡莲春、夏季开花,花淡橘色,花型优美且大[23],对周围环境的净化作用较强,能吸附空气中有毒物质,具康养价值[24]。综上所述,千屈菜+睡莲+金鱼藻组能到达春、夏、秋3个季节有花可观,景观时序性丰富;黄菖蒲+睡莲+金鱼藻组中睡莲春、夏季开花,能达到两季有花可观赏,景观时序性较丰富。千屈菜+睡莲+金鱼藻组与黄菖蒲+睡莲+金鱼藻组景观应用价值较高。
综合上述分析可知:黄菖蒲+睡莲+金鱼藻组在不同质量浓度富营养化水体中均有较为良好的净化效果,对总磷、总氮、化学需氧量综合净化能力较强,且具有较高的景观应用价值。
本研究植物组合数量上均为1株挺水植物、1株浮水植物、15 g沉水植物。但研究表明:组合中不同植物数量的配比对于水质净化效果不同[25],可对植物组合的数量配比从景观效果以及净化效果进一步研究。微生物在污水处理时有着重要作用,可进行不同植物组合与微生物协同作用的研究,为受损湿地生态系统修复提供科学依据。
Purification effects of different combinations of ornamental aquatic plants on eutrophic water
doi: 10.11833/j.issn.2095-0756.20220446
- Received Date: 2022-07-05
- Accepted Date: 2022-11-20
- Rev Recd Date: 2022-11-16
- Available Online: 2023-05-22
- Publish Date: 2023-05-20
-
Key words:
- aquatic plant /
- eutrophication /
- plant combination /
- water restoration
Abstract:
| Citation: | CHI Minghong, LIU Min, XU Yingchun, et al. Purification effects of different combinations of ornamental aquatic plants on eutrophic water[J]. Journal of Zhejiang A&F University, 2023, 40(3): 636-647. DOI: 10.11833/j.issn.2095-0756.20220446 |











 DownLoad:
DownLoad: