-
随着固碳减排等政策的不断落实,各种废气处理方法得到不同程度的应用。在各种处理方法中,气相吸附剂由于用量少、吸附量大等优点被广泛应用。近年来,关于吸附剂的研究越来越多,如金属基吸附剂[1]、无机基吸附剂[2]以及富含多孔结构的各种活性炭[3]等。而在这些材料之中,活性炭原料来源广泛且价格低廉,制备工艺相对简单,因而被大量用于工业、农业以及日常生活中[4−6]。在制备活性炭的原料中,竹材相比其他原料(如煤质、木质、椰壳等)具有一次成林、储量庞大、生长速度快且经济效益高等优点。
大量研究以毛竹Phyllostachys edulis [7]、方竹Chimonobambusa quadrangularis [8]、瓜多竹Guadua angustifolia、金丝竹Bambusa vulgaris var. striata、青竹Phyllostachys acuta [9]及麻竹Dendrocalamus latiflorus[10]等为原料进行炭化并测试吸附性能,结果表明不同原料制成的竹炭对苯、甲苯、吲哚、挥发性有机化合物(VOC)、甲醛、氨等气态有害物质及亚甲基蓝(MB)、孔雀石绿(MG)、刚果红(CR)和结晶紫(CV)等印染废水中常见污染物有较好的吸附性能。研究者们研究了不同竹炭对不同有害气体与水污染物的吸附,但很少研究炭化工艺对其吸附有害物质的影响。
目前活性炭活化方法有物理法、化学法和物理化学法等[11]。其中,物理法具有无污染、无腐蚀、所需活化剂廉价易得等优点。张艳娟[12]以废竹材为原料,ZHANG等[13]以竹废活性炭为原料,研究水蒸气对原料进行活化并探究了活化条件对孔结构及表面化学性质的影响。化学法有产率高、所需活化温度低等优点,但所需药品腐蚀性大,环境污染严重,且残留活性炭表面活化剂难以除尽。物理化学法结合物理、化学2种方法的优点,但同时也结合了两者的缺点。许伟等[14]以竹粉为原料,经磷酸活化成型后进行水蒸气二次活化,在不同工艺下制备了柱状竹活性炭,表明制得最优活性炭的水蒸气活化温度为875 ℃,活化时间为1 h,流量为3 mL·min−1。相比而言,以上3种方法中,物理法更环保,所需助剂无害,是较理想的活化方法。
刺竹Bambusa sinospinosa作为竹种群中的重要分支,在四川等地有广泛种植,但有关刺竹的研究鲜有报道,且大量刺竹被用于低附加值(如造纸原材料、建筑脚手架等)而导致经济效益低下。本研究以刺竹炭化的低灰分竹炭为原料,通过水蒸气活化法制备刺竹活性炭,选择最优制备工艺,系统研究水蒸气活化对刺竹活性炭的影响程度,为刺竹活性炭的高附加值开发利用提供依据。
-
刺竹取自四川惊雷科技股份有限公司,该区海拔约300 m,竹长约15 m,取阴坡3年生刺竹中部制备刺竹炭。
-
称取一定量经切片后烘至绝干的刺竹装入炭化活化一体炉,设定终点温度为550 ℃,当温度达到设定温度后停止加热,保温2 h,随后取出刺竹炭原料并置于干燥器中降至室温备用。
-
采用单因素实验法,取适量制备好的刺竹炭进行活化工艺探究。根据文献[12−14]得知:竹材使用物理活化法的一般活化温度为750~950 ℃,活化时间为0.5~3.0 h,活化水蒸气量为0.20~1.00 L·h−1。因此,本研究活化参数选择温度800~900 ℃、时间0.5~2.5 h、水蒸气量0.25~0.75 L·h−1。活化温度记为A (A1~A5为800~900 ℃,25 ℃为一区间);活化时间记为B (B1~B5为0.5~2.5 h,0.5 h为一区间);活化水蒸气量记为C (C1~C3分别为0.25、0.50、0.75 L·h−1。)
-
灰分测试方法参照GB/T 36057—2018《林业生物质原料分析方法——灰分的测定》,每组样品测6个平行样,取均值。挥发分测试方法参照LY/T 1929—2010《竹炭基本物理化学性能试验方法》。强度测试方法参照GB/T 12496.6—1999《木质活性炭试验方法——强度的测定》。碘吸附值测试方法依据GB/T 12496.8—2015《木质活性炭试验方法——碘吸附值的测定》进行测定,亚甲基蓝吸附值测试依据GB/T 12496.10—1999《木质活性炭试验方法——亚甲基蓝吸附值的测定》进行测定。
-
傅里叶红外吸收光谱仪(FTIR)测试:将样品与溴化钾按质量比1∶100混合研磨,随后在红外采样器中固定压紧样品后放入仪器进行扫描。每个试样取样5次,重复实验5次,红外光谱图取其平均图谱。
X-射线衍射仪(XRD)测试:采用连续记谱扫描,Cu靶,Kα射线为辐射源;取适量粉末状样品放至试样架,设定XRD的工作电压为40 kV,管电流为30 mA,波长为0.154 06 nm,扫描速度为3°·min−1,2θ扫描范围为10°~80°。
比表面积及孔隙度分析仪(BET)测试:先称取适量样品脱气,脱气完成后,将约100 mg样品的样品管装入分析站,在杜瓦瓶中加入液氮,并将样品质量输入至分析文件中。设置测试参数,开始进行氮气(N2)吸脱附测试。
扫描电子显微镜(SEM)测试:设置SEM发射电压为15 kV,为增强样品的导电性能,在测试前对样品表面进行喷金处理,喷金电流为10 mA。
-
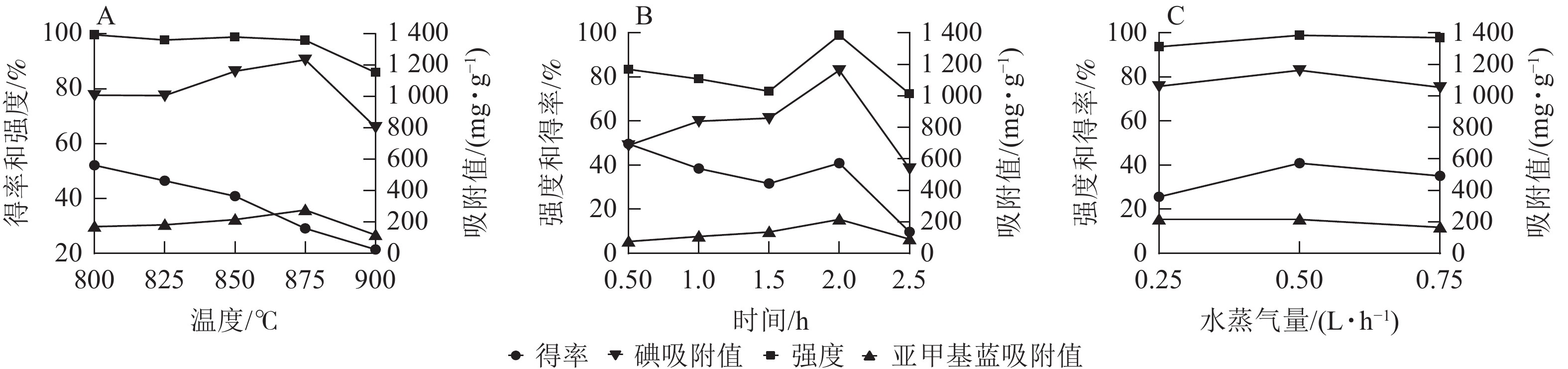
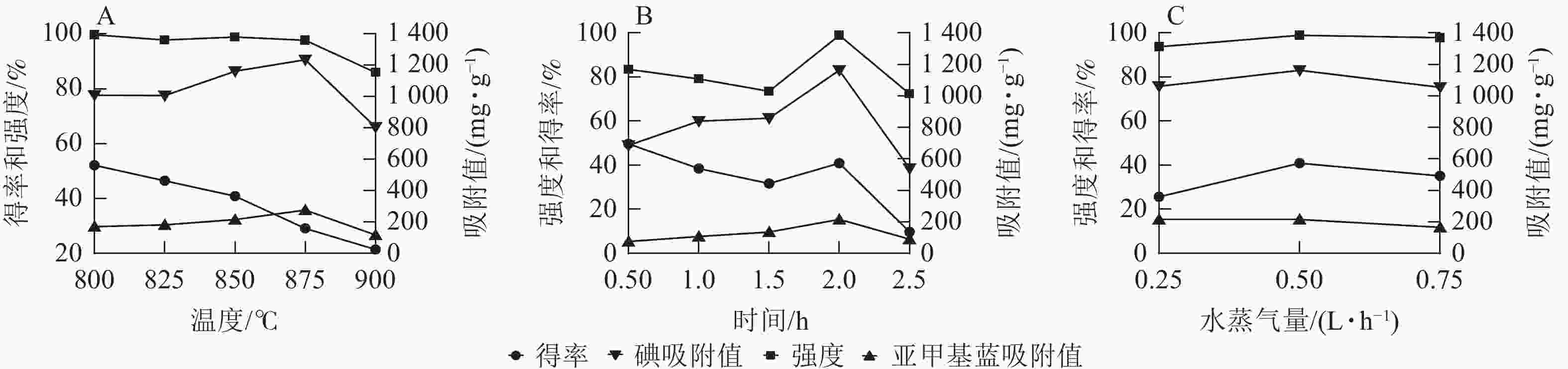
固定活化时间为2.0 h,活化水蒸气量为0.50 L·h−1,探究活化温度(800~900 ℃)对刺竹活性炭得率、强度以及碘吸附值、亚甲基蓝吸附值等吸附性能的影响。由图1A可看出:随着活化温度的升高,刺竹活性炭得率从52.12%降至21.40%,而碘吸附值与亚甲基蓝吸附值不断升高,在875 ℃时碘吸附值(1 235.03 mg·g−1)与亚甲基蓝吸附值(276 mg·g−1)达最高,但当温度达900 ℃时,碘吸附值与亚甲基蓝吸附值均下降,此时得率(21.40%)、强度(85.92%)、碘吸附值(805.00 mg·g−1)与亚甲基蓝吸附值(117 mg·g−1)最低。故选择875 ℃为最优温度。
-
固定活化温度为875 ℃,活化水蒸气量为0.50 L·h−1,探究活化时间(0.5~2.5 h)对刺竹活性炭得率、强度以及碘吸附值、亚甲基蓝吸附值等吸附性能的影响。由图1B可以看出:活化时间在0.5~2.0 h时,刺竹活性炭的碘值和亚甲基蓝吸附值在随着活化时间的增加而不断上升,2.0 h时刺竹活性炭的得率(40.85%)、强度(98.85%)、碘吸附值(1 161.60 mg·g−1)、亚甲基蓝吸附值(215 mg·g−1)均比其他活化时间的数值大。而在2.5 h时,得率、强度、碘吸附值、亚甲基蓝吸附值均比2.0 h时大幅下降。故选择2.0 h为最优时间。
-
固定活化温度为875 ℃,活化时间为2.0 h,探究活化水蒸气量(0.25~0.75 L·h−1)对刺竹活性炭得率、强度以及碘吸附值、亚甲基蓝吸附值等吸附性能的影响。从图1C可以看出:在缺水蒸气(0.25 L·h−1)时,刺竹活性炭得率及强度相比适量水蒸气(0.50 L·h−1)及过量水蒸气(0.75 L·h−1)均为最低。在过量水蒸气时,刺竹活性炭得率及强度相比适量水蒸气时有下降。与其他2组相比,适量水蒸气时碘吸附值(1 161.6 mg·g−1)与亚甲基蓝吸附值(215 mg·g−1)最高。故选择0.50 L·h−1为最优水蒸气量。
-
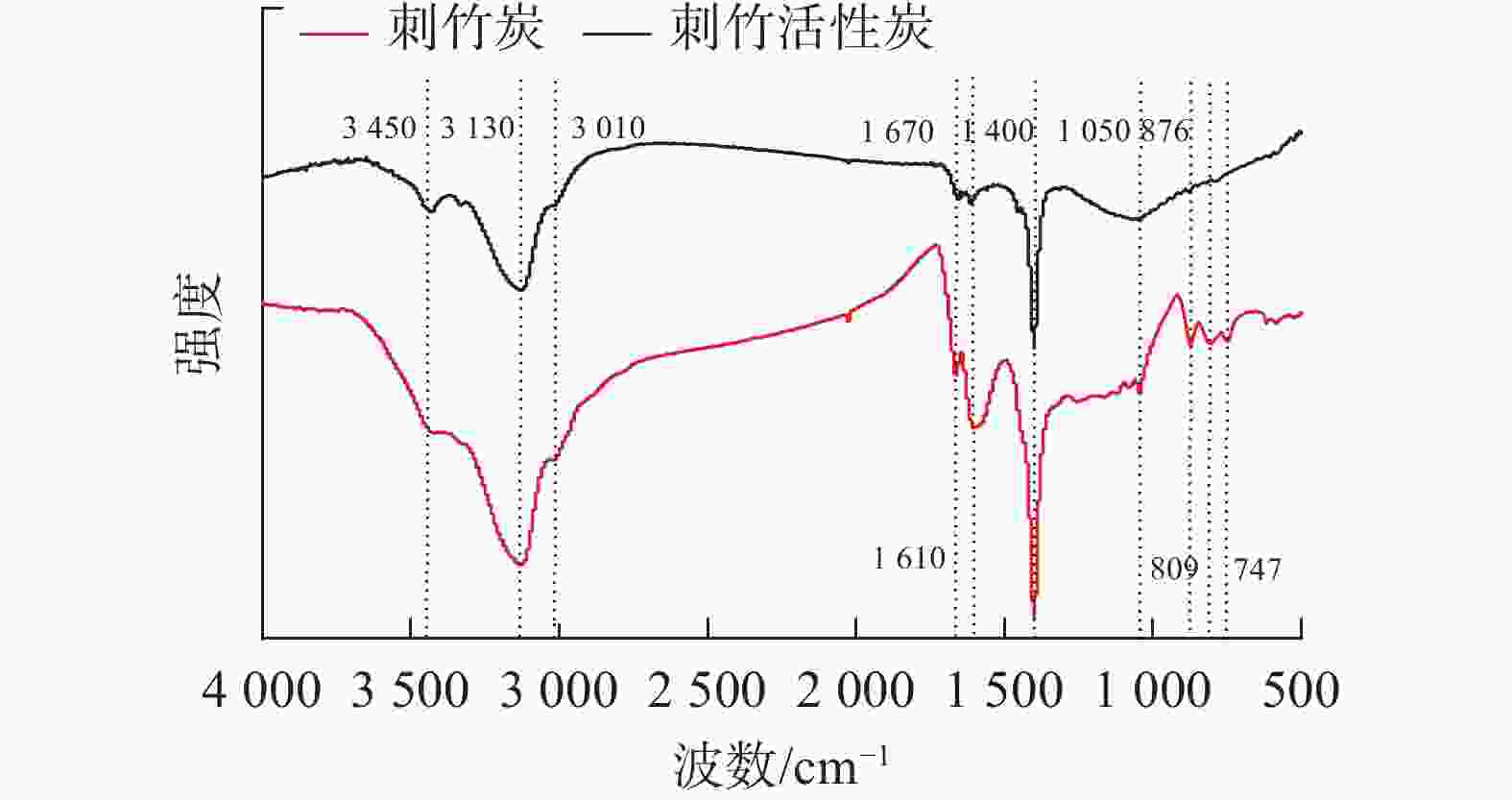
如图2所示:刺竹炭对应的红外图谱在官能团区(4 000~1 300 cm−1)有3 450、3 130、3 010、1 670、1 610、1 400 cm−1等6处吸收峰,其中3 450 cm−1处对应$ \text{—}\text{OH} $的伸缩振动峰;3 130与3 010 cm−1处对应不饱和碳上的$ \text{C}\text{—}\text{H} $伸缩振动峰;1 670 cm−1处对应C=O伸缩振动峰,可能为酮、酰胺或羧基中的C=O伸缩振动[15];1 610 cm−1处对应芳环中的C=C的伸缩振动,说明在炭和活性炭中均有苯环的存在;1 400 cm−1处对应$ {\text{—}\text{CH}}_{\text{3}} $基反对称变形振动。在指纹区(1 300~500 cm−1)有1 050、876、809和747 cm−1等4处吸收峰,其中1 050 cm−1处对应$ \text{C}\text{—}\text{O} $伸缩振动;876、809和747 cm−1等3处吸收峰是间位双取代苯的3个$ \text{C}\text{—}\text{H} $的面外弯曲振动[16],说明存在间双取代的苯基。通过图谱对比可以看到:在刺竹活性炭的红外图谱中,3 130、3 010、1 670、1 610、1 400和1 050 cm−1等6处吸收峰有不同程度的减弱,其中1 670 cm−1处峰吸收强度变弱,说明在活化过程中C=O被热解为气体或液体副产物,致使酮、酰胺或羧基中的C=O减少,1 610 cm−1处吸收强度变弱是由于高温;876、809和747 cm−1等3处吸收峰消失,说明在高温活化的过程中间双取代的苯基被烧蚀;通过电镜 (SEM)观察表明:以上主要峰形的减弱或消失说明其对应的官能团不同程度被烧蚀,是活性炭产生孔隙的来源之一。另外3 450 cm−1处刺竹活性炭的峰变强,是在红外压片时样品吸附了水所致。
-
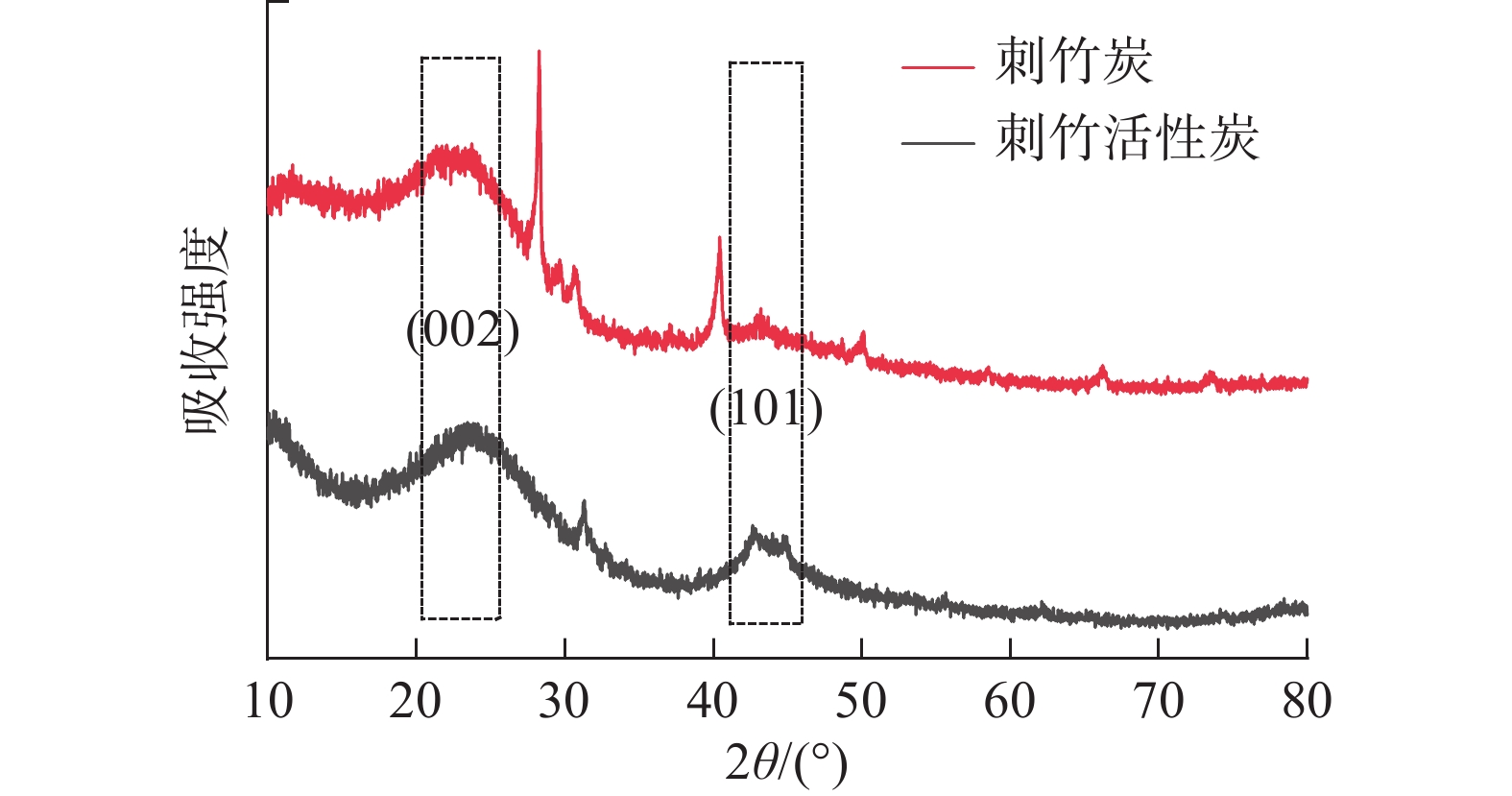
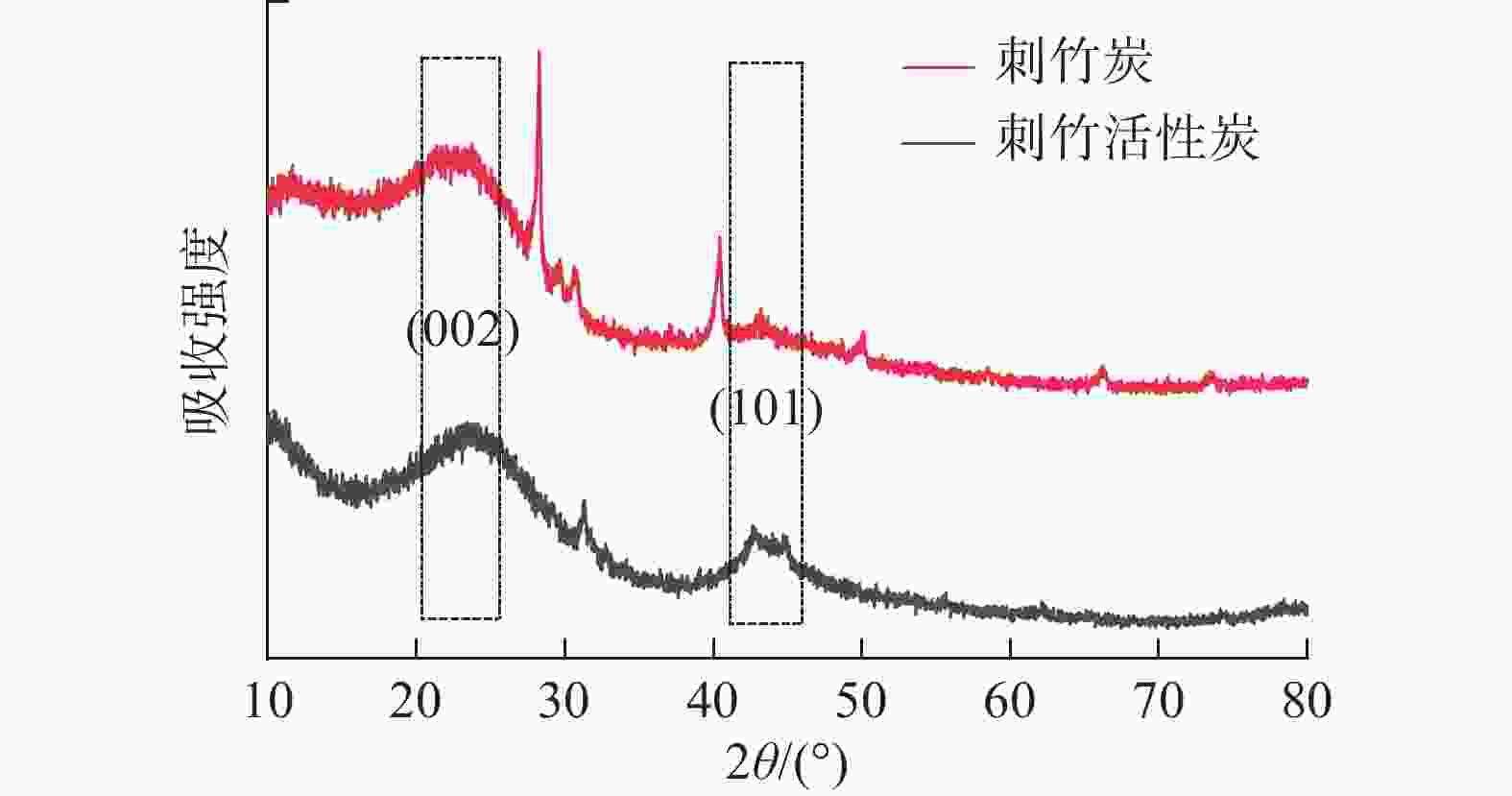
从图3可以看出:刺竹炭与刺竹活性炭在2θ=20°~30°处均有结晶峰(002)存在,说明两者均含有乱层石墨[17],刺竹活性炭在2θ=28°时尖峰消失,与刺竹炭相比,刺竹活性炭的峰向更高的角度移动、变宽。刺竹炭在2θ=40.42°处的峰与2θ=43.19°处的峰在经过活化之后均向更高角度移动,并在2θ=40°~50°内,形成石墨α轴结构(101)晶面。
-
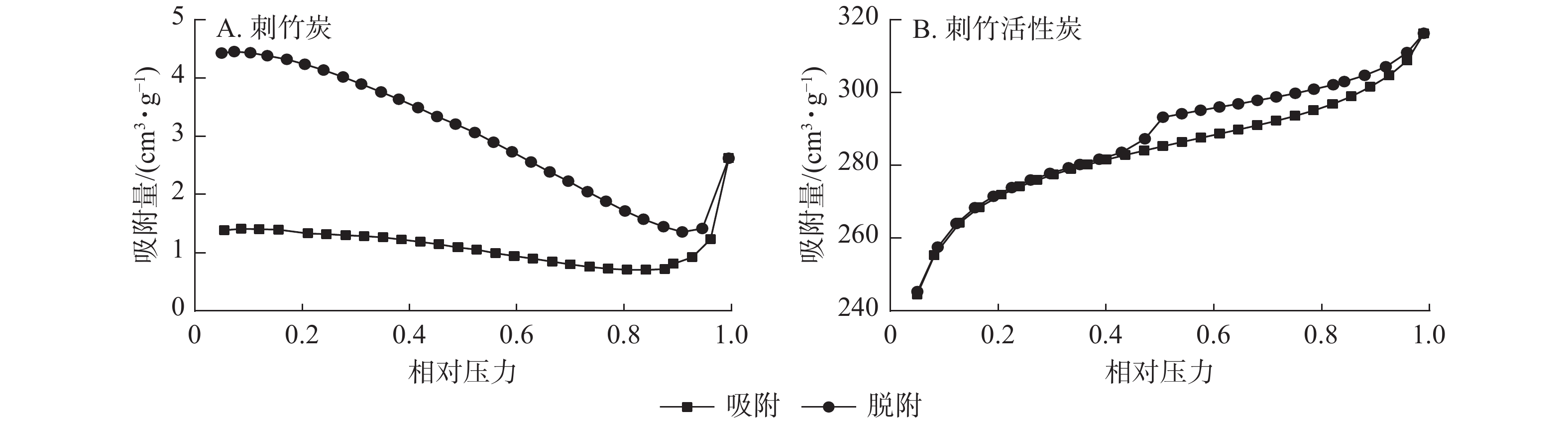
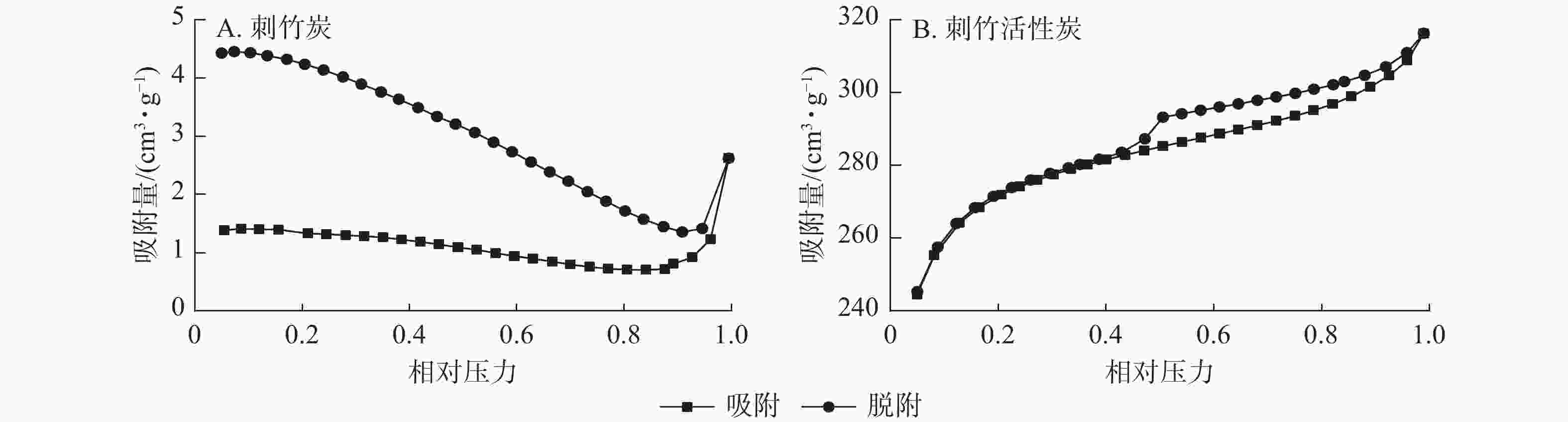
从图4A可见:刺竹炭的吸脱附曲线未闭合,这是因为刺竹炭部分未炭化完全,本身孔结构较单一,且孔径较大致使其比表面积较小所致。从图4B可以看到:刺竹活性炭的吸附支为Ⅰ型和Ⅱ型等温线的组合[18],Ⅰ型等温线表明刺竹活性炭在N2吸附过程中存在微孔填充现象,说明刺竹活性炭中含有微孔;Ⅱ型等温线表明其在吸附过程中存在大孔的物理吸附过程,说明刺竹活性炭中仍含有大孔结构;刺竹活性炭的N2吸脱附曲线并不重合,存在H4型回滞环,而回滞环的形成是因为刺竹活性炭中存在介孔,导致氮气分子在低压常压下冷凝产生毛细凝聚[19]。表明刺竹活性炭中微孔、介孔、中孔并存。
由表1可知:刺竹炭经活化后比表面积大幅提升,从3.724 m2·g−1提升至837.005 m2·g−1,增大了224倍;微孔比表面积从刺竹炭的5.902 m2·g−1提升至刺竹活性炭的756.063 m2·g−1,提升了128倍;微孔容及总孔容也有不同程度大幅提升;刺竹活性炭的平均孔径由原有刺竹炭的43.508 nm降至23.378 nm。说明经活化之后,刺竹活性炭大孔比例大幅下降,微孔与介孔比例升高。
样品 比表面积/
(m2·g−1)微孔比表面积/
(m2·g−1)微孔体积/
(cm3·g−1)总孔体积/
(cm3·g−1)微孔体积占总孔体积
百分比/%平均孔
径/nm刺竹炭 3.724 5.902 0.003 0.004 75.000 43.508 刺竹活性炭 837.005 756.063 0.388 0.489 79.346 23.378 Table 1. Pore structure parameters of B. sinospinosa charcoal and activated charcoal
-
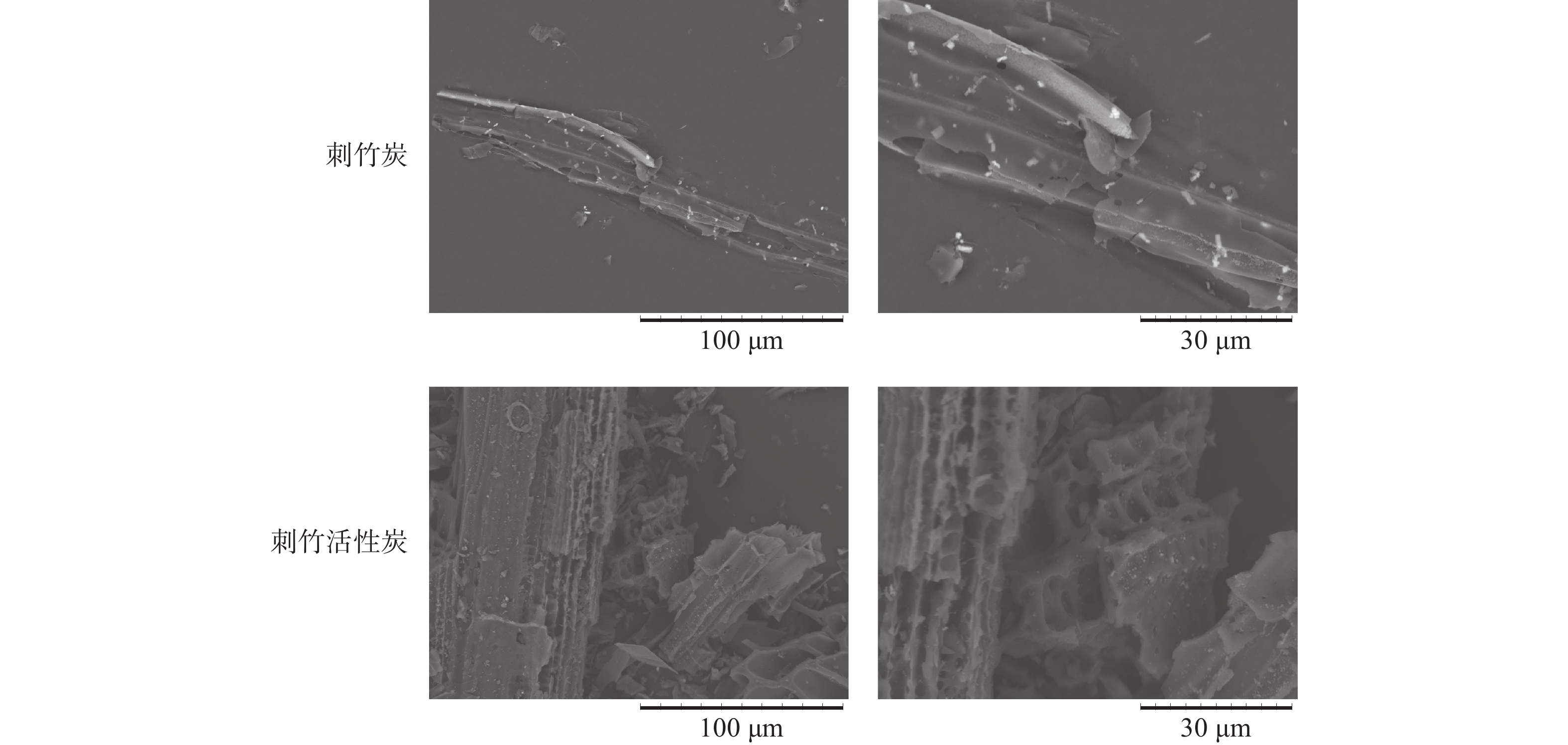
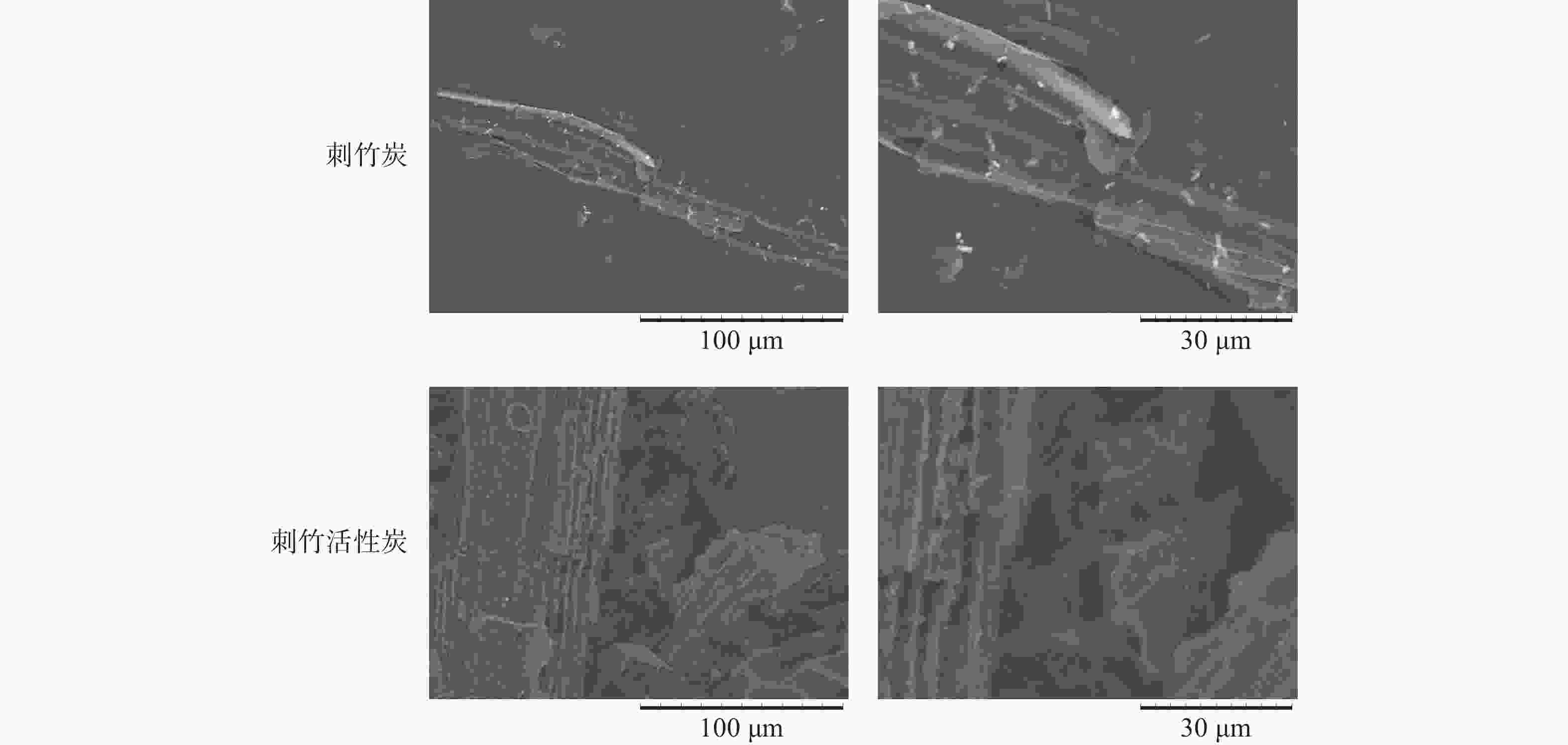
从图5可以看出:刺竹炭的维管束与基本组织较完整,表面光滑,孔隙较少,部分维管束发生破碎、坍塌。相比刺竹炭,刺竹活性炭表面仍保持微观构造的基本特征,但变得粗糙、孔隙变多,使得刺竹活性炭具有更大的比表面积以及更好的吸附能力。
-
在活化温度上,随着温度的升高,活性炭的得率与强度不断下降,碘吸附值与亚甲基蓝吸附值先升高后降低。这是刺竹木质纤维素分子量低和沸点挥发性化合物含量高导致的结果,温度越高,挥发性化合物的挥发就越快,致使得率降低[20]。随着温度升高,刺竹炭与水反应加快,空隙产生速率加快,在相同时间内空隙产生量比低温下多,因而两者的吸附值会随温度升高而增大,在875 ℃时达到峰值。但随着温度的升高,产生的空隙经受不住高温而产生坍塌致使孔隙堵塞,两者的吸附值呈下降趋势,活性炭强度也在不断下降。在孔隙产生和坍塌的过程中,刺竹炭的挥发性物质也在加速挥发,在空隙的产生和挥发性物质的挥发以及部分炭被烧失的共同作用下[21],刺竹活性炭的得率也在不断下降。
在活化时间上,随时间的延长,强度、得率、碘吸附值及亚甲基蓝吸附值先升高后降低,这是因为随着活化反应的持续进行,刺竹炭与水蒸气充分接触发生反应,使得刺竹炭表面逐渐形成大量微孔结构[22],在2.0 h时,碘吸附值与亚甲基蓝吸附值达最大;随着时间延长,活化反应进一步进行,微孔的烧失速率逐渐超过其产生速率,导致碘吸附值与亚甲基蓝吸附值下降;而微孔的烧失使得孔径结构坍塌,进而导致刺竹活性炭强度下降,得率降低。
在活化水蒸气量上,缺水蒸气时,刺竹炭在高温下只能与有限的水蒸气反应产生少量微孔,而其余部分高温条件下未能与水蒸气发生反应产生孔隙结构,使得活性炭内部结构无孔隙结构支撑,刺竹活性炭强度和得率低。过量水蒸气时,刺竹炭活化造孔所需的水蒸气已与其发生了反应,而多余的水蒸气被通入反应炉内,附着在已被活化完全的刺竹活性炭上,在高温条件下水蒸气进入空隙致使孔隙坍塌,故得率和强度一定程度下降。这一结论与苏垚[23]的研究结果一致。适量水时,通入水蒸气恰好与刺竹炭完全反应,造孔效率达到最高,4个参数均为最大,说明适量水蒸气最适宜刺竹炭的活化。
故得出刺竹炭最优活化工艺为:活化温度875 ℃、活化时间2.0 h、活化水蒸气量0.50 L·h−1。与已有研究结果[24]相比,该工艺制备的刺竹活性炭碘值与亚甲基蓝吸附值更高,说明本研究的活化工艺相对更优。
-
红外图谱得出刺竹炭与刺竹活性炭相比峰型差异不大,但在3 130、1 670、1 400、876、809、747 cm−1等处的峰强度变弱甚至消失,这是由于活化时的高温将官能团及其化学键不同程度烧蚀所致。
X-射线衍射图谱分析显示刺竹炭的乱层石墨结构相比刺竹活性炭较多,在刺竹活性炭的图谱中可以观察到2θ=28°时尖峰消失,这是由于经过高温活化之后,刺竹活性炭石墨化度和结晶度降低的原因[25],而且与刺竹炭相比,刺竹活性炭的峰向更高的角度移动、变宽,说明经过活化之后刺竹活性炭乱层石墨的晶体变小,对应于较小尺寸的晶体[26]。相对刺竹炭,刺竹活性炭的(002)和(101)晶面对应结晶峰有右移倾向,这是因为在活化过程中,随着温度的升高,孔隙内部发生应力变化、弛豫、缺陷等导致的。
比表面积测试得出:刺竹活性炭比表面积大幅提升,微孔比表面积、微孔容、总孔容及微孔率都有不同程度的提高,比表面积和微孔比表面积以及孔容的提升是活化过程高温和水蒸气共同作用的结果,高温条件下水蒸气在孔隙中快速扩散,随着活化时间的延长,水蒸气与孔壁上游离炭充分反应,使得孔内表面不断扩大,伴随水蒸气与炭进行反应不断扩大原有孔并生成新的孔隙。刺竹活性炭在相对压力为0.4~0.9处出现H4型回滞环,表明存在微孔填充现象,存在大量微孔结构。
电镜分析得出:刺竹炭的维管束与基本组织较完整,表面光滑,孔隙较少,这是由于在炭化过程中,随着温度的升高,竹炭发生缩聚反应,维管束结构收缩,在炭化过程中挥发性成分、纤维素和木质素降解后,形成了许多由气孔组成的通道[27],且数量逐渐增多,竹炭的结构逐渐松散,机械强度降低。刺竹活性炭表面粗糙,表面孔隙变多,这是由于刺竹炭在高温活化的过程中,部分炭化温度下未分解的物质在活化过程中逐渐分解,维管束孔壁上部分在炭化过程中未烧通的孔在高温下被烧通所致。
-
使用单因素实验法探究出了刺竹活性炭水蒸气活化的最优工艺为:活化温度875 ℃、活化时间2.0 h、活化水蒸气量0.50 L·h−1。最优工艺制备的刺竹活性炭碘吸附值为1 235.03 mg·g−1,亚甲基蓝吸附值为276 mg·g−1。与目前市售活性炭相比,吸附性能理想,在气相、液相除污等场合有较大的应用潜力。刺竹活性炭最优工艺的制备为刺竹的高附加值开发与利用提供了新的思路。
Production and adsorption properties of activated charcoal fromBambusa sinospinosa
doi: 10.11833/j.issn.2095-0756.20230307
- Received Date: 2023-05-15
- Accepted Date: 2023-12-11
- Rev Recd Date: 2023-10-16
- Available Online: 2024-03-21
- Publish Date: 2024-04-01
-
Key words:
- Bambusa sinospinosa /
- activated charcoal /
- steam activation /
- adsorption property /
- activation process
Abstract:
| Citation: | TIAN Huayu, LIU Huan, WANG Guorui, et al. Production and adsorption properties of activated charcoal fromBambusa sinospinosa[J]. Journal of Zhejiang A&F University, 2024, 41(2): 429-436. DOI: 10.11833/j.issn.2095-0756.20230307 |









 DownLoad:
DownLoad: