-
在全球气候变暖的背景下,干旱事件愈渐频发。干旱影响植物的生长代谢,随着干旱强度的增加,轻度干旱导致植物根系活力下降,呼吸作用降低,叶片细胞体积变小,膜系统受到破坏,光合作用受影响[1−3];重度干旱引发活性氧过度积累,产生氧化应激反应,对植物造成永久性伤害[4−5]。因此,干旱被预测为未来农业中最重要的环境压力之一[6]。植物为了减轻干旱造成的损伤,通过一系列的生理生化反应来应对,如产生大量的渗透调节物质以提高渗透电位和增强根细胞的吸水能力[7−8],激活抗氧化防御系统并刺激一些重要的细胞信号过程,如钙信号、植物激素等[9−10]。
香榧Torreya grandis ‘Merrillii’是红豆杉科Taxaceae榧属Torreya常绿乔木,雌雄异株,为第三纪孑遗植物,是榧树Torreya grandis中唯一优良栽培类型,也是中国特有的珍稀木本油料树种,具有较高的营养价值和经济价值[11−13]。随着温室效应的加剧,全球气温不断升高,夏季极端酷热天气使得高温和干旱胁迫极易发生。加之香榧种植区域多位于野外山坡,极端天气不仅限制了香榧苗木的生长发育,甚至会造成其脱水死亡,严重影响其产业发展。
雌雄异株植物是一种特殊的植物类群,在维持生态系统的稳定性和可持续性方面发挥重要作用[14]。研究表明:长期的生物进化过程和性别的特异性表达以及繁殖成本的不同[15−16],使得雌雄异株植物在逆境胁迫下表现出显著的性别差异。因此在全球干旱日益严重的背景下,研究香榧雌雄植株对干旱的生理响应差异,不仅对实际生产具有指导意义,对丰富雌雄异株植物逆境胁迫响应机制也具有重要的科学价值。因此,本研究采取不同生长阶段的香榧雌雄株枝条进行扦插处理,以获得的香榧扦插苗为研究材料,探讨干旱胁迫对香榧雌雄株膜脂过氧化程度、渗透调节物质、活性氧质量摩尔浓度和抗氧化酶活性等方面的影响。
-
于2022年6—12月,在浙江农林大学东湖校区竹子研究院驯化室进行试验,室内恒定温度为21~25 ℃。首先于2022年6、7、8月对香榧雌雄株插穗进行扦插繁殖,获得香榧雌雄株插穗的最佳扦插时间。之后通过聚乙二醇(PEG)模拟干旱处理,测定生理指标,进一步探究香榧雌雄株的抗旱差异性。
基质按V(泥炭)∶V(蛭石)∶V(珍珠岩)=1∶1∶1的比例混合后高压锅内灭菌,冷却后加入适量清水静置过夜,后装入4 cm×10 cm的无纺布袋,整齐摆入30 cm×45 cm的育苗盆中待用。
在浙江农林大学潘母岗基地,分别随机挑选5株10~12年生香榧雌雄株作为采样母树,按雌雄分组并标号。选取生长健壮、长势相对一致,且无病虫害和机械损伤的当年生枝条为插穗进行试验。先对插穗进行消毒,将其浸泡在质量分数为0.125%的多菌灵(25%有效含量)溶液中,消毒2 min;然后用去离子水冲洗3遍,用消毒过的剪刀平口剪去基部(剪去的长度为总长度的1/5,使插穗的长度为5~6 cm)的茎段、顶部的分枝(剪去的长度为分叉枝条长度的1/2)和底部1/3的叶片;再用100 mg·L−1的萘乙酸(NAA)处理20 min。扦插时,将插穗垂直插入基质内,长度约为整个茎段长的1/3,然后轻轻把插穗周围的基质压实,再浇清水以触摸基质有湿润感为宜。
-
对不同月份的香榧枝条分别在扦插3、4、5个月后,随机选取相同月份香榧雌雄株幼苗各35株,拍照并记录每株香榧的根系形态指标,包括成活率、生根率、侧根数、侧根长和根的直径。然后将香榧幼苗小心移栽进8 cm×8 cm×11 cm的方形育苗盆中,同时对过长的根部进行适当修剪。
-
在已生根5~7个月的香榧雌雄株幼苗中,挑选长势一致的香榧雌雄株幼苗各50株,各平均分成5组,并分别按照如下处理:第1组用纯净水培养8 d (干旱胁迫0 d);第2组先用纯净水培养6 d,再用PEG 6000配置质量分数为30%的PEG溶液处理2 d (干旱胁迫2 d);第3组先用纯净水培养4 d,再用质量分数为30%的PEG溶液处理4 d (干旱胁迫4 d);第4组先用纯净水培养2 d,再用质量分数为30%的PEG溶液处理6 d (干旱胁迫6 d);第5组用质量分数为30%的PEG溶液处理8 d (干旱胁迫8 d)。试验时将香榧放入一次性纸杯中,PEG溶液没过香榧根系但不超过香榧叶片,于恒温培养室培养,并每日更换培养液。以上处理组均在第8天拍照记录,随后立即将处理的香榧清洗擦干,将每株所有的叶片取下并在液氮中研磨成粉末,然后取0.1 g的叶片粉末装入2 mL 离心管中,放入液氮中速冻后转移至−80 ℃冰箱备用。
-
超氧化歧物酶(SOD)活性采用WST-8法测定,过氧化物酶(POD)活性采用可见分光光度法测定,过氧化氢酶(CAT)活性采用钼酸铵比色法测定,过氧化氢(H2O2)采用可见分光光度法测定,超氧阴离子(O2−)采用可见分光光度法测定,丙二醛(MDA)采用可见分光光度法测定,脯氨酸(Pro)采用可见分光光度法测定。上述试剂均使用苏州科铭生物技术有限公司生产的试剂盒,具体实验步骤参照对应说明书进行,每个生理指标测定重复4次。
-
采用Excel 2019对所测数据进行整理,用 GraphPad Prism 8进行图表绘制,用 SPSS 25.0对数据进行方差分析,利用最小显著差异法(LSD)进行多重比较。
-
由表1可知:6月处理的香榧雌株成活率高于7和8月;8月处理的香榧雄株成活率高于6和7月,成活率和生根率最高可达100%。香榧雌雄株扦插生根的直径、侧根数和侧根长随扦插时长的增加而增加。对香榧雌株来说,6月扦插生根的侧根数最多,为(4.45±1.37)条,7月扦插生根的直径和侧根长最大,分别为(2.29±0.53) mm和(3.76±2.43) cm。对香榧雄株而言,6月扦插生根的侧根长最大,为(4.21±2.30) cm,7月扦插生根的直径最大,为(2.45±0.67) mm,8月扦插生根的侧根数最多,为(4.22±1.57)条。
香榧性别 扦插月份 扦插时长/月 成活率/% 生根率/% 直径/mm 侧根数/条 侧根长/cm 雌株 6月 3 80.00 53.57 1.71±0.36 ef 3.67±1.44 bcdefg 2.20±0.95 cd 4 80.00 92.86 1.49±0.40 f 5.30±1.12 a 3.49±1.51 c 5 40.00 76.47 2.00±0.53 cdef 4.38±1.61 abcd 3.32±1.40 c 7月 3 68.57 66.67 2.28±0.40 abcde 2.44±0.81 ghi 2.12±1.70 cd 4 74.29 42.31 2.48±0.48 abcd 2.18±0.92 hi 2.28±1.78 cd 5 45.71 62.50 2.11±0.70 bcde 2.60±1.65 efghi 6.88±3.82 a 8月 3 71.43 28.00 2.27±0.42 abcde 1.43±0.71 i 1.47±1.21 d 4 48.57 47.06 2.13±0.66 bcde 4.00±1.41 abcd 2.64±1.80 cd 5 37.14 46.15 2.29±0.54 abcde 5.00±1.09 ab 6.68±5.13 a 雄株 6月 3 97.14 79.41 1.72±0.26 ef 3.30±1.54 defgh 3.31±1.82 c 4 82.86 82.76 1.96±0.38 cdef 3.83±1.43 bcdef 3.43±1.45 c 5 85.71 100.00 2.82±0.73 a 3.87±1.46 bcde 5.89±3.73 ab 7月 3 94.29 87.88 2.20±0.70 bcde 2.48±1.30 fghi 1.74±1.29 cd 4 94.29 100.00 2.53±0.73 abcd 3.73±1.56 bcdefg 5.07±2.46 b 5 77.14 92.59 2.63±0.57 ab 3.4±1.35 defgh 5.76±2.41 ab 8月 3 100.00 85.71 2.22±0.95 abcde 4.23±1.52 abcd 3.26±1.94 c 4 97.14 100.00 2.56±0.75 abc 3.59±1.58 cdefgh 3.29±1.89 c 5 88.57 100.00 1.92±0.59 def 4.84±1.61 abc 5.18±3.92 b 说明:直径、侧根数、侧根长为平均值±标准差,同列不同小写字母表示不同时长间差异显著(P<0.05)。 Table 1. Analysis of softwood cutting results
-
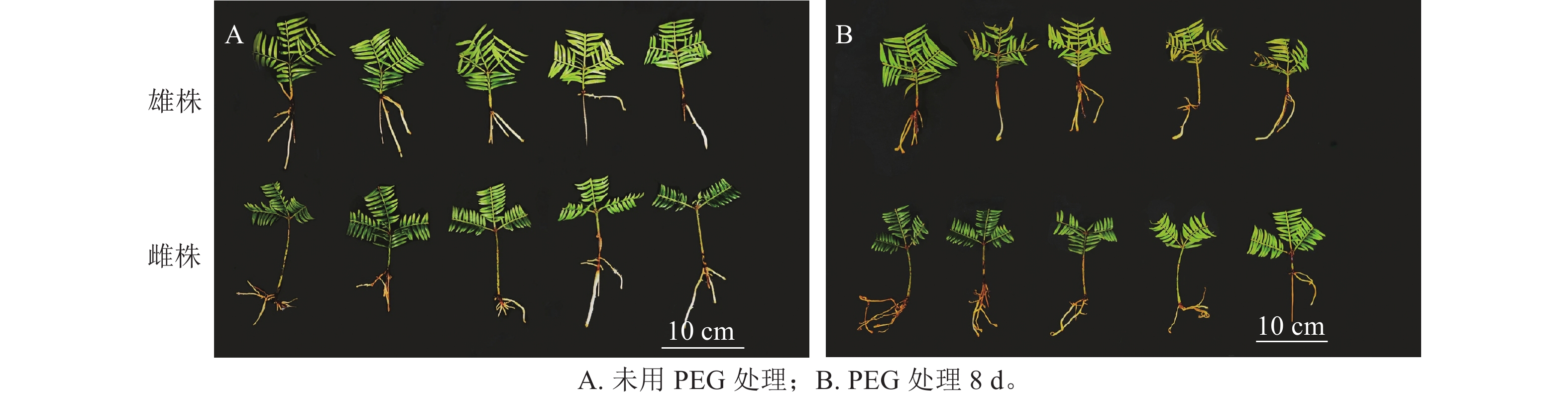
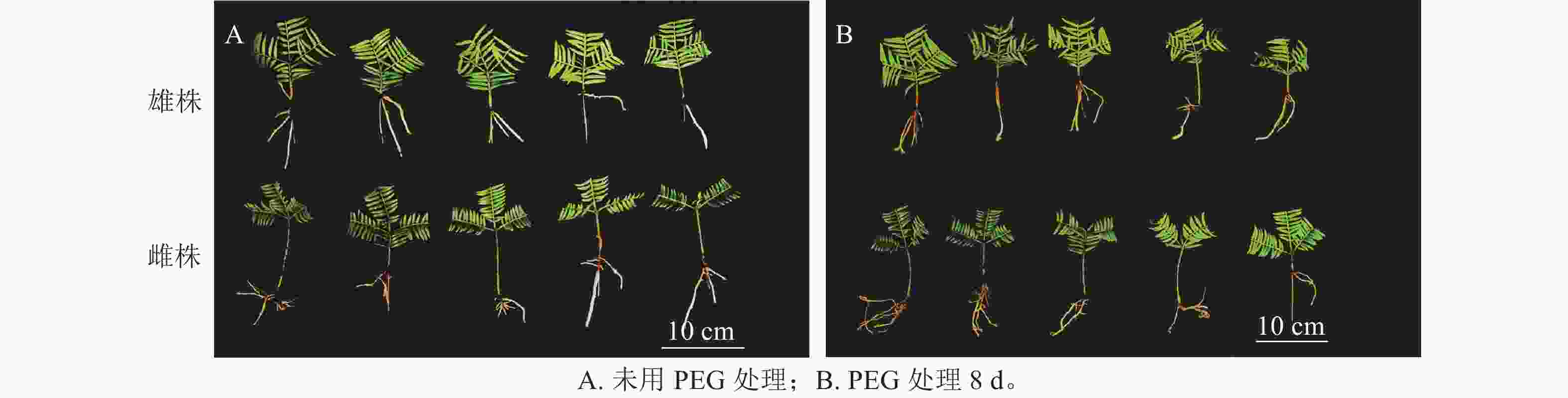
如图1所示:PEG处理香榧8 d后,雄株叶片明显发黄卷曲,表现缺水,而雌株干旱前后叶片无明显变化,说明雌株的抗旱性较强。这意味着在相同的干旱条件下,雌株的外观表现优于雄株。
-
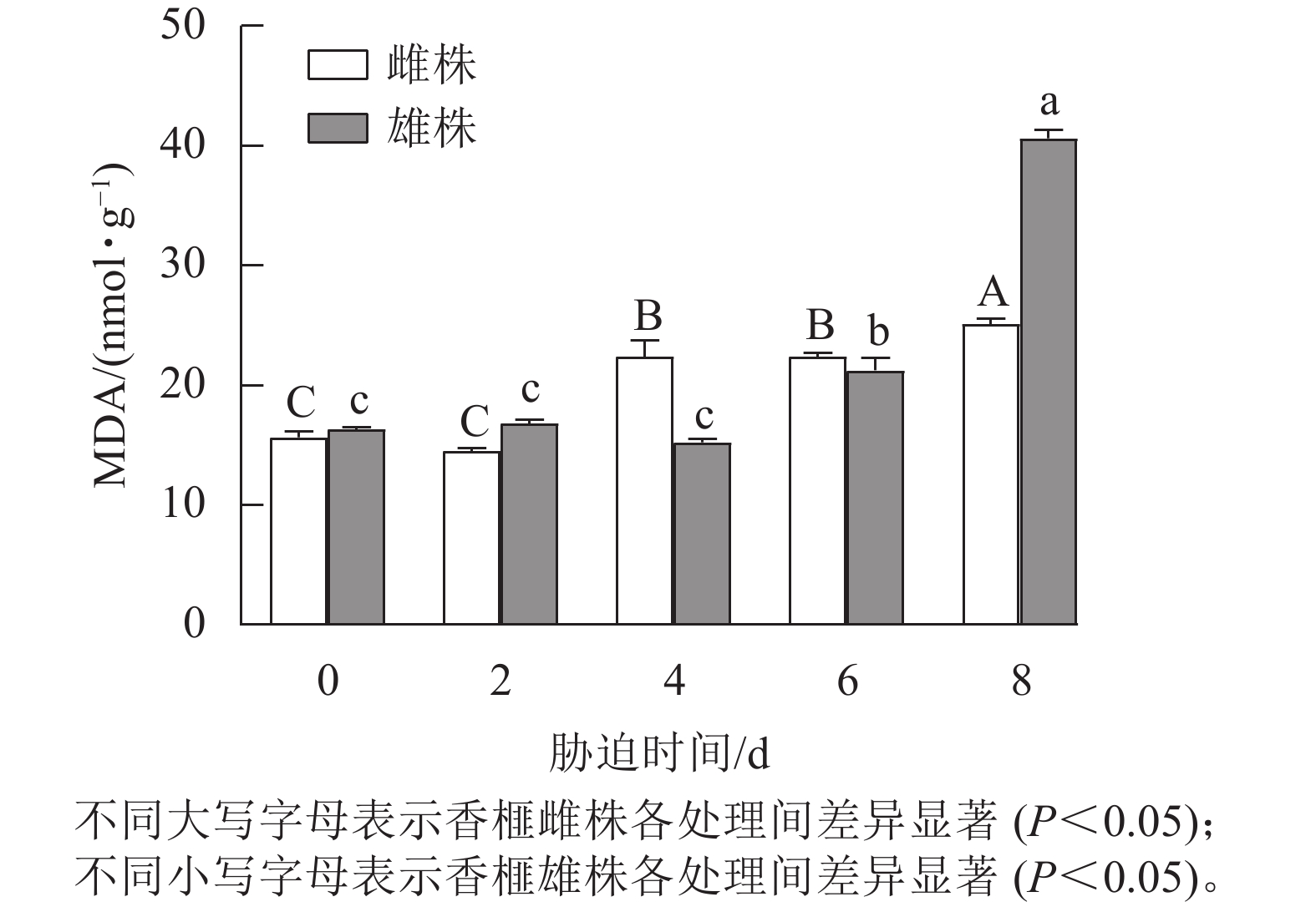
从图2可以看出:干旱胁迫显著影响香榧雌雄株扦插幼苗叶片的MDA质量摩尔浓度。随着干旱胁迫时间的延长,雌雄株MDA质量摩尔浓度呈上升趋势。处理0~2 d,香榧雌雄株MDA质量摩尔浓度无显著差异,处理4~8 d,香榧雌雄株叶片中的MDA质量摩尔浓度表现出上升趋势;干旱8 d时,雄株MDA质量摩尔浓度显著高于雌株(P<0.05)。干旱0~8 d,雌株MDA质量摩尔浓度的变化趋势较小,上升了60.36%,而雄株MDA质量摩尔浓度上升了148.65%。
-
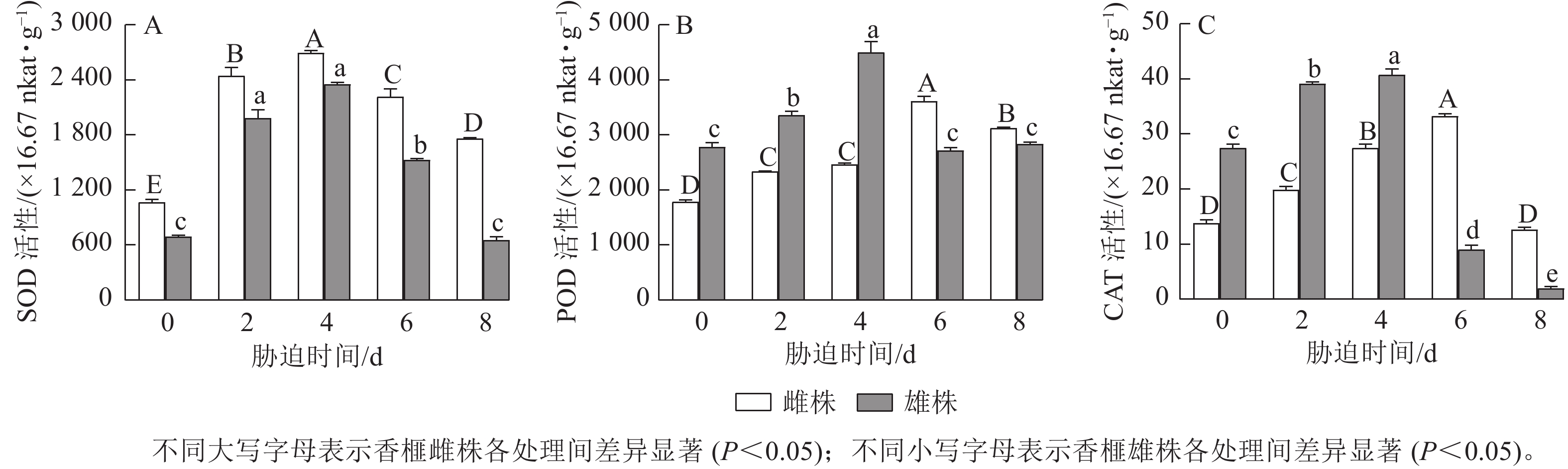
如图3A所示:干旱胁迫引起香榧雌雄株扦插幼苗叶片的SOD活性发生明显变化,不同处理时间下雌株的SOD活性均高于雄株,随着干旱胁迫时间延长呈现先升高后降低的趋势。胁迫4 d时,香榧雌雄株叶片的SOD活性达到最高值,与0 d相比分别增加了152.7%和239.72%;随后雌雄株SOD活性开始下降,胁迫8 d时,雌株SOD活性差异显著高于雄株(P<0.05),雌株SOD活性较0 d增加了65.23%,而雄株SOD活性与0 d差异不显著。
-
由图3B可知:香榧雌雄株扦插幼苗叶片中的POD活性随着干旱胁迫时间的延长呈现先增强后减弱的趋势。胁迫4 d时,雄株POD活性达到最高值,与0 d相比增加了61.49%;胁迫6 d时,雌株POD活性达到最高值,与0 d相比增加了101.88%;胁迫 8 d时,香榧雌株叶片中的POD活性显著高于雄株(P<0.05),较0 d相比,雌株增加了74.51%,而雄株POD活性与0 d相比差异不显著。
-
从图3C可以看出:随着干旱胁迫时间的延长,香榧雌雄株扦插幼苗叶片中的CAT活性呈先升高后降低的趋势。胁迫4 d时,雄株CAT活性达到最高值,与0 d相比增加了47.97%;胁迫6 d时,雌株CAT活性达到最高值,与0 d相比增加了140.3%;胁迫8 d时,香榧雌株叶片中的CAT活性显著高于雄株(P<0.05),雌株CAT活性与 0 d相比差异不显著,雄株较0 d降低了92.62%。
-
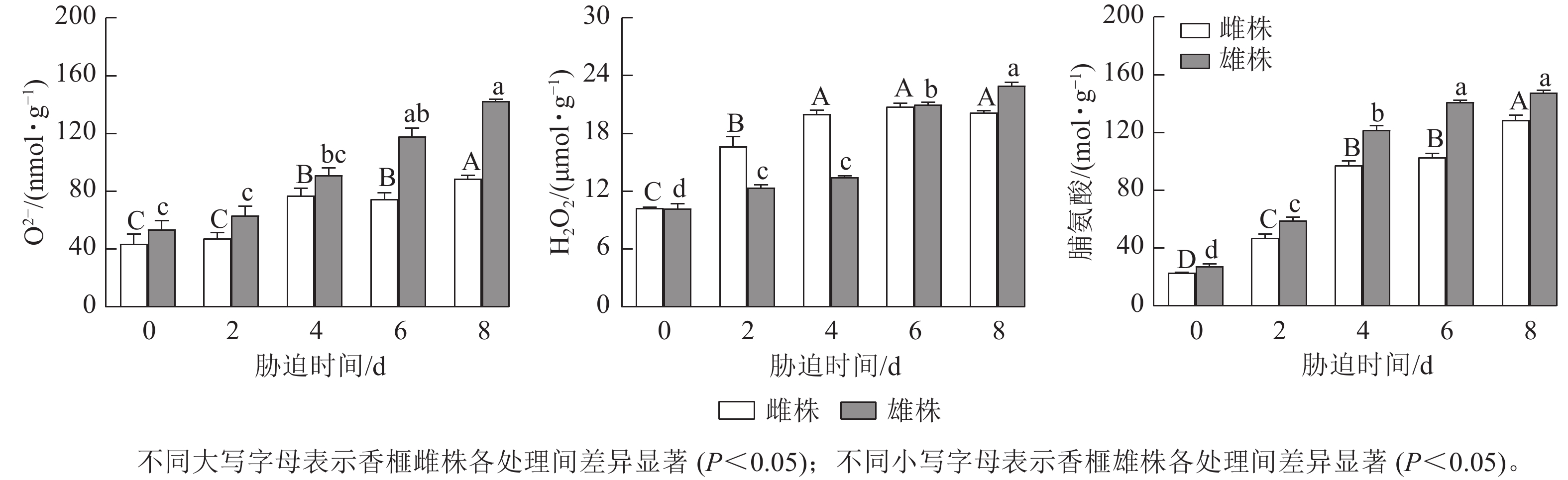
由图4可知:香榧雌雄株扦插幼苗叶片中的O2−和H2O2质量摩尔浓度随着干旱胁迫时间的延长,总体呈上升趋势。干旱胁迫0~2 d,香榧雌雄株O2−质量摩尔浓度无明显差异,干旱胁迫4~6 d,香榧雌雄株叶片中的O2−质量摩尔浓度逐渐升高;干旱胁迫8 d时,香榧雄株叶片的O2−质量摩尔浓度显著高于雌株(P<0.05),较0 d相比,雌株增加了102.83%,雄株增加了166.2%。香榧雌雄未遭受干旱胁迫时,叶片中O2−质量摩尔浓度几乎一致,干旱胁迫0~4 d时,香榧雄株H2O2质量摩尔浓度无明显变化,而雌株呈上升趋势;干旱胁迫4~8 d时,香榧雌株H2O2质量摩尔浓度趋于稳定,雄株明显升高;干旱胁迫8 d时,香榧雄株叶片的H2O2质量摩尔浓度显著高于雌株(P<0.05),较0 d相比,雄株增加了124.87%,雌株增加了96.61%。
-
干旱胁迫显著增加了香榧雌雄扦插幼苗叶片中脯氨酸(Pro)质量摩尔浓度,香榧雌雄叶片中Pro质量摩尔浓度随胁迫时间的延长呈增加趋势,但不同处理时间下雄株的Pro质量摩尔浓度均高于雌株(图4)。胁迫8 d时,香榧雌雄叶片中Pro质量摩尔浓度达最大值,雄株的Pro质量摩尔浓度显著高于雌株(P<0.05),与0 d相比,香榧雌雄叶片中Pro质量摩尔浓度分别增加了462.00%和433.38%。
-
本研究表明:夏季扦插繁殖的香榧雌株成活率较低,可能是雌雄异株之间不同的生理特性和生长习性差异所致[17]。雌雄株在生殖特性上存在差异,雌株的无性繁殖能力相对较弱,扦插时容易受到环境条件的影响。其次,雌雄株的生长习性与也存在差异,雄株在生长过程中能够更快地获取养分和水分资源,并能更好地抵抗一些外界压力,例如干旱[18]和盐碱胁迫[19]。这些特点使得雄株植物在一定程度上具备更高的成活率。本研究发现:6月扦插的香榧雌株生根率高于7和8月,而8月扦插的香榧雄株生根率高于6和7月。香榧雌雄株扦插相同天数时,雄株的成活率、生根率和侧根数均优于雌株,这与华中东青Ilex centrochinensis的扦插结果相似[20]。香榧雌雄株在不同月份的扦插效果不同,可能与不同月份的光照强度、日照时间、温度和湿度不同有关。
雌雄异株植物对环境胁迫表现出性别响应差异。有研究发现:镉胁迫对雄株南方四季杨Populus deltoides × nigra ‘Chile’负面影响更大[21],蒙古柳Salix linearistipularis雌株的耐盐碱能力强于雄株[22]。本研究结果显示:香榧雌雄叶片的SOD、POD、CAT活性和MDA质量摩尔浓度在干旱胁迫下表现出显著差异,这表明性别调节了雌雄植株对干旱胁迫的抗氧化酶响应特征。香榧雌雄株抗氧化酶活性在干旱过程中呈先上升后下降趋势,且不同处理天数之间差异显著,表明干旱胁迫下香榧抗氧化酶均能正常发挥作用。香榧雌株的SOD、POD和CAT活性在干旱处理8 d时高于雄株,而MDA质量摩尔浓度较雄株低,说明干旱胁迫中雌株表现出更好的抗氧化酶活性和膜稳定性。这与苟蓉[23]的研究结果一致。通过提高防御酶活性抵抗和适应一定程度的干旱胁迫来维持活性氧产生与清除之间的平衡[24],细胞膜的稳定对植物的生命活动起着重要的作用。
已有研究表明:干旱胁迫下植株的活性氧水平与MDA质量摩尔浓度呈极显著正相关[25]。本研究发现:持续干旱胁迫使得香榧幼苗叶片的H2O2、O2−和MDA质量摩尔浓度显著升高,且香榧雄株的H2O2、O2−和MDA质量摩尔浓度明显高于雌株。表明在干旱胁迫下,香榧雄株叶片膜系统比雌株更容易受损,雌株对干旱胁迫具有更强的适应性,这与刘牧野等[26]的研究结果一致,干旱胁迫下,野牛草Buchloe dactyloides雌株抗旱性强于雄株。
渗透调节是植物抵抗逆境的方式之一,Pro等作为重要的渗透调节物质,会在水分亏缺时主动积累以适应干旱逆境[27]。本研究发现:干旱胁迫处理后香榧Pro质量摩尔浓度随时间的延长而升高,干旱处理8 d时表现出显著的性别响应差异,且雌株Pro质量摩尔浓度高于雄株,说明香榧雄株在相同干旱胁迫下比雌株更易遭受损伤。这与马少薇等[28]的研究结果相同,干旱胁迫下黄柳Salix gordejevii雌株叶片Pro质量摩尔浓度显著低于雄株,雌株叶片保水能力较强,对水分短缺不敏感。
-
本研究表明:香榧雌株选择6月扦插,雄株选择8月扦插,可以获得较为理想的扦插效果。在干旱胁迫下,香榧雌株通过调节抗氧化酶的活性来增强抗氧化能力,降低其活性氧的积累。此外,香榧雌株在干旱胁迫下还表现出更好的细胞膜稳定性,使得香榧雌株比雄株具有更高的抗旱性。
Physiological differences in rooting and response to drought in shoots of female and male Torreya grandis‘Merrillii’
doi: 10.11833/j.issn.2095-0756.20230471
- Received Date: 2023-09-18
- Accepted Date: 2024-01-25
- Rev Recd Date: 2024-01-16
- Available Online: 2024-05-22
- Publish Date: 2024-05-22
-
Key words:
- Torreya grandis ‘Merrillii’ /
- dioecious plant /
- drought stress /
- plant physiology
Abstract:
| Citation: | WANG Xiaorong, QIU Hong, ZHANG Qixiang, et al. Physiological differences in rooting and response to drought in shoots of female and male Torreya grandis‘Merrillii’[J]. Journal of Zhejiang A&F University, 2024, 41(3): 478-485. DOI: 10.11833/j.issn.2095-0756.20230471 |












 DownLoad:
DownLoad: