-
随着染料工业的发展,染料废水的污染越来越严重,废水处理问题引起了社会各界的广泛关注[1]。刚果红是一种基于联苯胺的阴离子双偶氮染料,在生产和使用过程中易产生流失进入水体,且难以降解,对水生动植物微生物都具有毒害作用[2]。目前处理有机染料废水的有效方法主要分为物理法和化学法,具体有吸附法[3]、絮凝法、膜分离法[4-5]和光催化法[6]等。相较于其他方法,吸附法是一种更为经济有效的方法,具有成本低、可得性好、操作简单、去除效率高等优点,被广泛用于处理染料废水[7]。
木质素磺酸钠是造纸工业副产物,结构中包含的芳香族酚羟基、脂肪族羟基以及侧链的负电荷基团可有效吸附溶液中的染料离子和金属离子,同时含有磺酸基官能团,具有良好的水溶性、分散性和表面活性[8]。利用其独特结构开发天然生物质吸附剂具有环境友好、成本低廉等特点,在水处理方面得到了广泛的研究和应用[9]。单纯的木质素磺酸盐虽然表现出一定的吸附性能,但是自身吸附性能有限,对染料的亲和性不高,限制了它的广泛应用,因此,需要利用木质素磺酸盐表面丰富的官能团进行化学改性制备木质素基吸附材料,从而进一步增强其吸附性能。目前存在的改性方法主要包括酚化、磺化、曼尼希反应、羧甲基化、接枝共聚、复合改性等[10−11]。例如,谷飞[12]利用木质素磺酸钠与壳聚糖复合制备吸附材料,用于吸附阴离子染料罗丹明B和刚果红,表明随壳聚糖含量的增加,胺基数目增多,复合吸附剂对刚果红的吸附效果增加。薛蓓等[13]利用曼尼希反应将不同比例的木质素与磁性材料结合,制备了磁性木质素纳米材料,对刚果红的吸附量达234.1 mg·g−1,同时缓解了吸附材料难回收的问题。任建鹏等[14]使用聚苯胺和木质素磺酸盐复合,引入胺基、亚胺基等活性基团的同时改善了聚苯胺在溶液中易聚合的特点。目前,木质素磺酸钠用于刚果红吸附的研究大多集中在利用木质素与其他材料复合提高其吸附性能,对木质素磺酸钠直接化学改性用于刚果红吸附的研究较少。因此,本研究利用化学改性方法制备胺化木质素磺酸钠,并将其与共价有机框架聚合物复合制备吸附剂,进一步增强材料的吸附效果,为化学改性木质素磺酸钠以及共价有机框架聚合物在有机污染物吸附领域的应用提供参考。
-
将10.0 g木质素磺酸钠,0.5 g 氢氧化钠(NaOH)溶解于23.0 mL水中,加入3.0 g二乙烯三胺升温至85 ℃;缓慢滴加4.5 mL质量分数为36%的甲醛水溶液,冷凝回流条件下反应4 h;反应结束冷却至室温,滴加1.0 mol·L−1盐酸(HCl)直至无棕色沉淀析出,过滤,分别使用异丙醇和石油醚洗涤产物至滤液无色,放入干燥箱中烘干。研磨后再次抽滤,用蒸馏水洗涤至滤液无色,得到最终产物ASLS,胺值为2.34 mmol·g−1。
-
将36 mg四氟-1,4-苯醌(TFBQ)、96 mg二羟基蒽醌(DHAQ)、4 mL 1,4-二氧六环、156 µL三乙胺加入到充有氩气的10 mL反应瓶中,混合均匀,超声5 min,随后放入120 ℃烘箱反应72 h;反应结束后冷却至室温,离心,收集棕色沉淀物;随后用大量丙酮、二氯甲烷、四氢呋喃洗涤产物。用丙酮索式提取24 h后收集产物,60 ℃真空干燥后得棕色粉末为AQ-COF。称取一定质量的ASLS粉末、AQ-COF粉末放入研钵中,混合并研磨均匀,制得AQ-COF/ASLS复合材料。
-
采用Nicolet 6700傅里叶变换红外光谱仪对样品进行表征分析。具体流程如下:取1~2 mg 样品在玛瑙研钵中研磨成细粉末与干燥的溴化钾混合均匀,装入模具内,在压片机上压制成片测试,扫描范围为400~4 000 cm−1。
-
准确称量2 g样品到250 mL烧瓶中,加入50 mL乙醇,加热至沸腾1 min以除去可能存在的游离氨,冷却至室温。滴加5滴溴酚蓝指示剂并搅拌,使用0.2 mol·L−1的盐酸标准溶液滴定至黄色终点出现,记录消耗的HCl体积。总胺值(A)计算公式为:$ A=56.1VN/S $。其中:V为样品滴定所需的HCl体积(mL);N为HCl溶液的浓度(mol·L−1);S为使用的样品质量(g)。
-
使用S20206414 SU 8010冷场发射SEM,观察样品表面形貌。将样品粉末分散于少量乙醇中,超声使其分散均匀,对样品表面进行喷金处理,观察样品表面形貌。
-
配制质量浓度为200 mg·L−1的刚果红溶液,根据实验需要进行稀释或者调节pH。吸附实验的具体步骤如下:取25 mL一定浓度的刚果红溶液于锥形瓶中,分别加入一定质量的吸附剂,在磁力搅拌器上以300 r·min−1转速进行搅拌吸附。吸附一定时间后,将悬浮液在8 000 r·min−1下离心取上清液,利用分光光度计测定其吸光度,计算刚果红质量浓度,并按以下公式计算刚果红的吸附率及吸附量。
式(1)~(2)中:R为刚果红平衡吸附率;$ {c}_{0} $为初始时刚果红质量浓度(mg·L−1);${c}_{\mathrm{t}}$为吸附结束后刚果红质量浓度(mg·L−1);q为单位质量吸附剂上的吸附量(mg·g−1);V为液相体积(mL);m为吸附剂质量(g)。
刚果红起始质量浓度对吸附效果的影响实验中,刚果红起始质量浓度分别为50、100、150、200、250 mg·L−1;pH对吸附性能的影响实验中,利用0.1 mol·L−1 HCl或NaOH调节刚果红溶液pH变化为1~11。
-
取25 mL质量浓度为200 mg·L−1刚果红溶液,加入70 mg ASLS,分别在吸附时间为20、40、60、80、100、120 min时测定刚果红溶液的吸光度,计算t时刻的吸附量qt,采用准一级动力学和准二级动力学模型对数据进行拟合。方程如下所示:
式(3)~(4)中:k1为准一级动力学方程的速率常数;k2为准二级动力学方程的速率常数;t为吸附时间(min);qe为平衡吸附量(mg·g−1);qt是t时刻对刚果红的吸附量(mg·g−1)。
-
ASLS对刚果红的吸附过程分别采用Langmuir和Freundlich吸附模型[15−16]进行拟合,其等温线模型分别如下所示:
式(5)~(6)中:Ce为吸附后溶液中刚果红的剩余质量浓度(mg·L−1);qe为平衡吸附量(mg·g−1);qm为吸附剂理论最大吸附量(mg·g−1);kL为Langmuir等温线模型与吸附能有关的常数;kF为Freundlich等温线模型与吸附容量相关的常数;n为Freundlich等温线模型的经验参数。
-
将吸附饱和后的吸附剂过滤收集,在0.2 mol·L−1的NaOH溶液中解吸30 min,后用蒸馏水洗涤,重复3次,真空干燥至恒量得到再生的吸附剂,用于循环吸附实验,最后用紫外分光光度计测定吸光度,并计算吸附率。
-
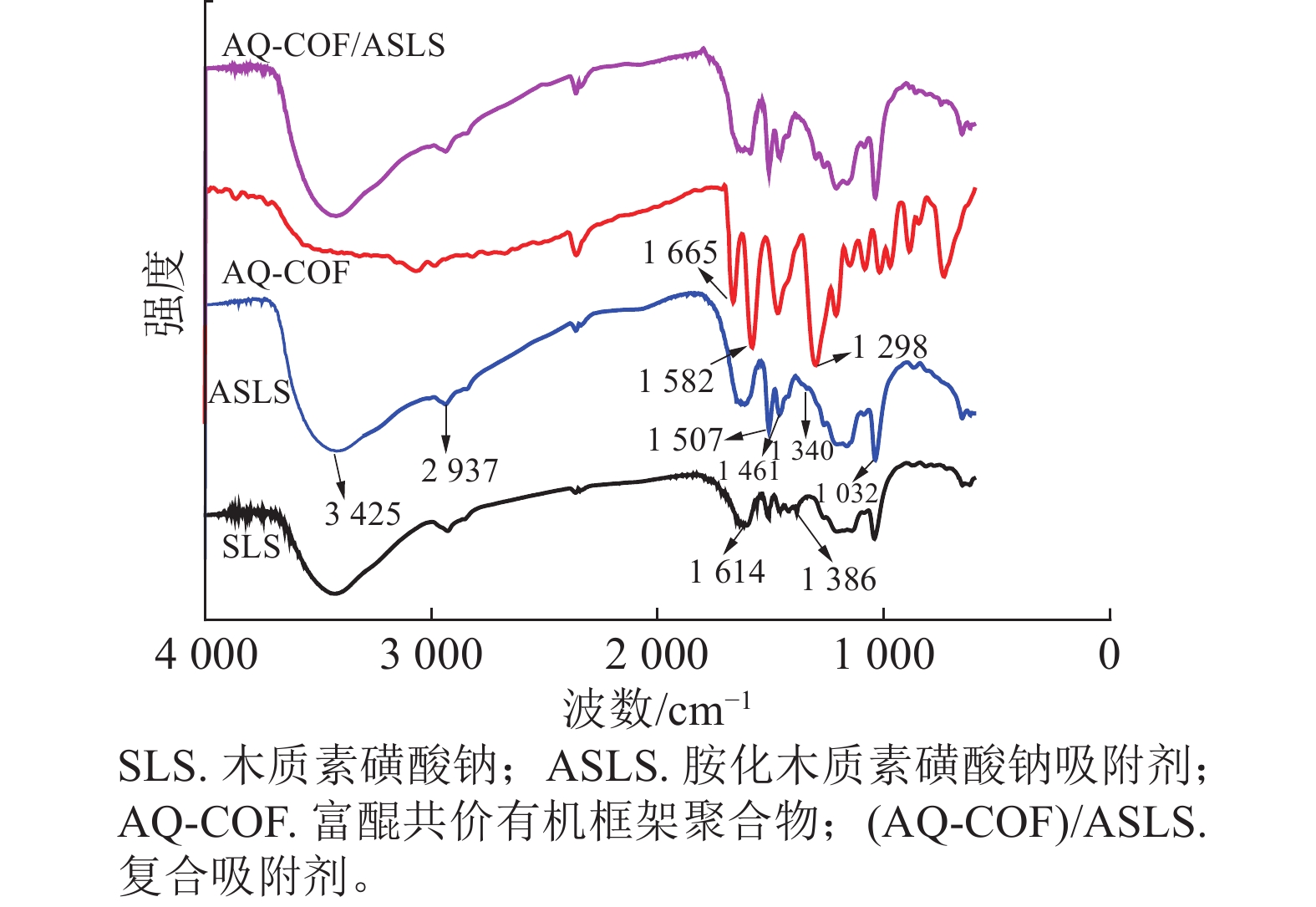
如图1所示:胺化前后SLS和ASLS都出现了典型的木质素磺酸钠的红外吸收峰。例如,3 425、2 937 cm−1处分别为木质素磺酸钠中O—H、C—H伸缩振动吸收峰,1 614、1 507 cm−1处分别为木质素磺酸钠芳环上C=C双键吸收峰和芳环骨架伸缩振动吸收峰,1 032 cm−1处为木质素磺酸钠结构中磺酸基团的特征吸收峰,说明胺化后并未改变木质素磺酸钠的骨架结构。SLS谱图中1 386 cm−1处为芳环上的C—H面内弯曲振动吸收峰,该峰在ASLS谱图上减弱,说明芳环上的氢原子被取代;同时ASLS谱图中1 461 cm−1处吸收峰增强,是因为引入了二乙烯三胺,其亚甲基上C—H面内弯曲振动峰所致。同时1 340 cm−1处出现了C—N键的伸缩振动吸收峰。以上吸收峰的出现和强弱变化说明了胺化木质素磺酸钠与甲醛、二乙烯三胺之间发生了曼尼希反应,成功引入胺基。
AQ-COF谱图中,1 665 cm−1为—C=O伸缩振动吸收峰,1 582 cm−1处为C=C吸收峰,1 298 cm−1为C—C单键伸缩振动吸收峰,说明富含醌结构的共价有机框架聚合物成功制备。AQ-COF/ASLS红外光谱图中,无明显新增特征吸收峰出现。与复合前相比,AQ-COF和ASLS的特征吸收峰均无明显变化,说明两者之间无化学反应发生,可能依靠物理相互作用复合。
-
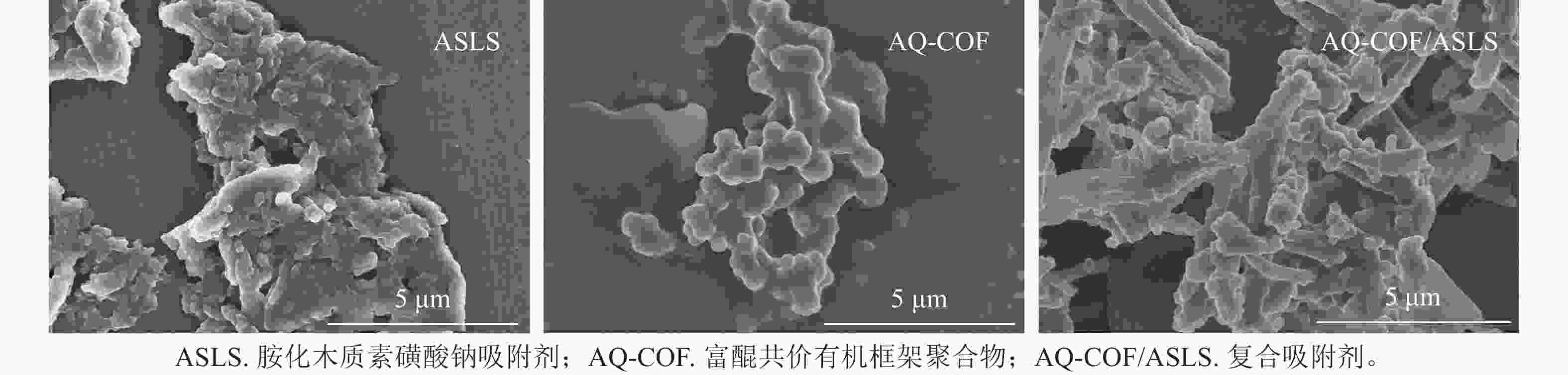
如图2所示:ASLS表面含有粗糙鳞片状结构的聚集体结构;AQ-COF为颗粒状聚集体,颗粒直径为0.5~1.0 μm;AQ-COF/ASLS中含有大量的棒状纳米结构,表面附着许多颗粒,棒状结构直径约500 nm,这种棒状结构为ASLS在AQ-COF的作用下自组装形成。表明ASLS和AQ-COF复合后表面形貌发生显著变化,ASLS被AQ-COF分散得更为均匀,提高了吸附剂的比表面积,可为吸附剂提供更多的可吸附位点。
-
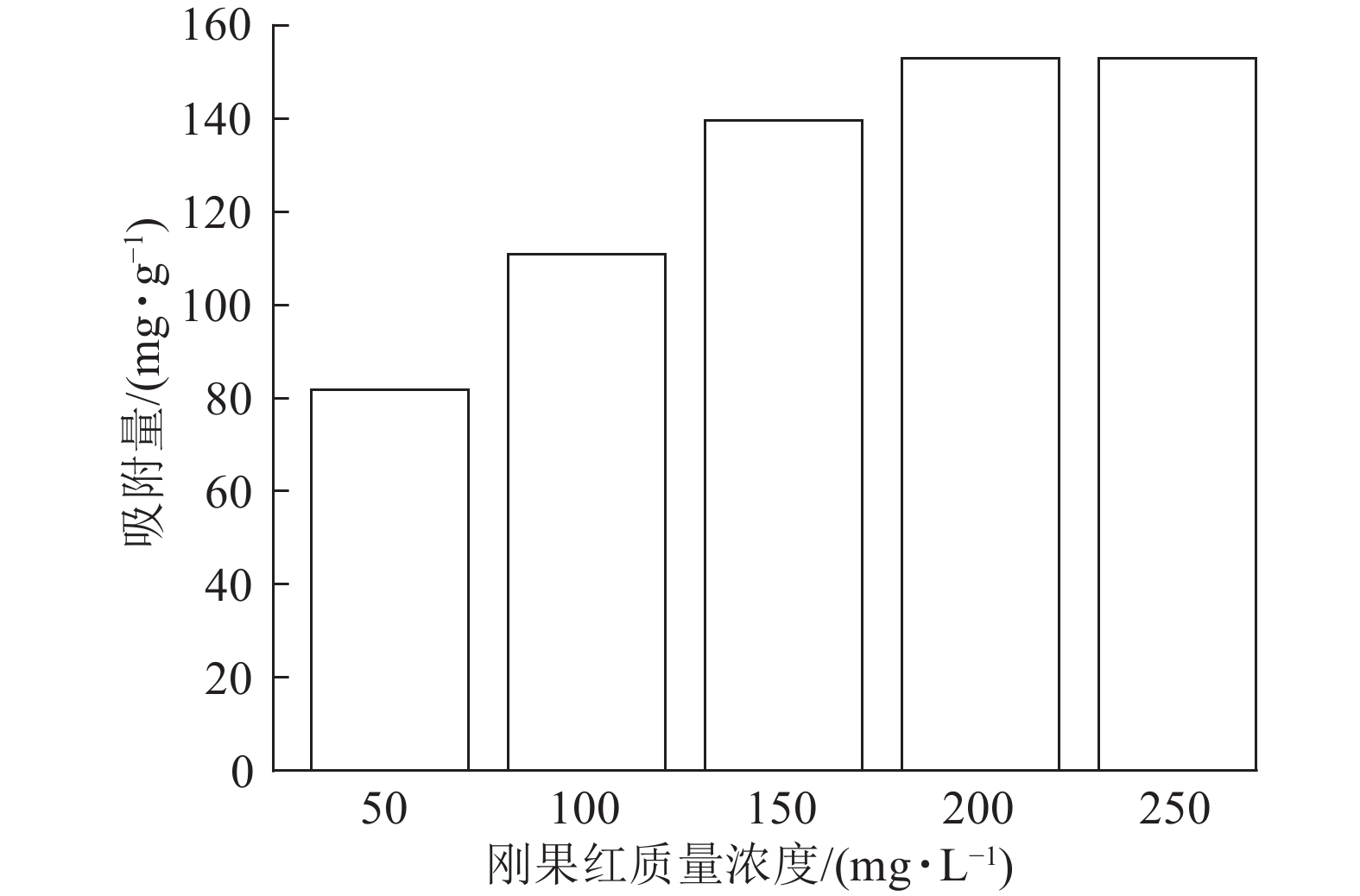
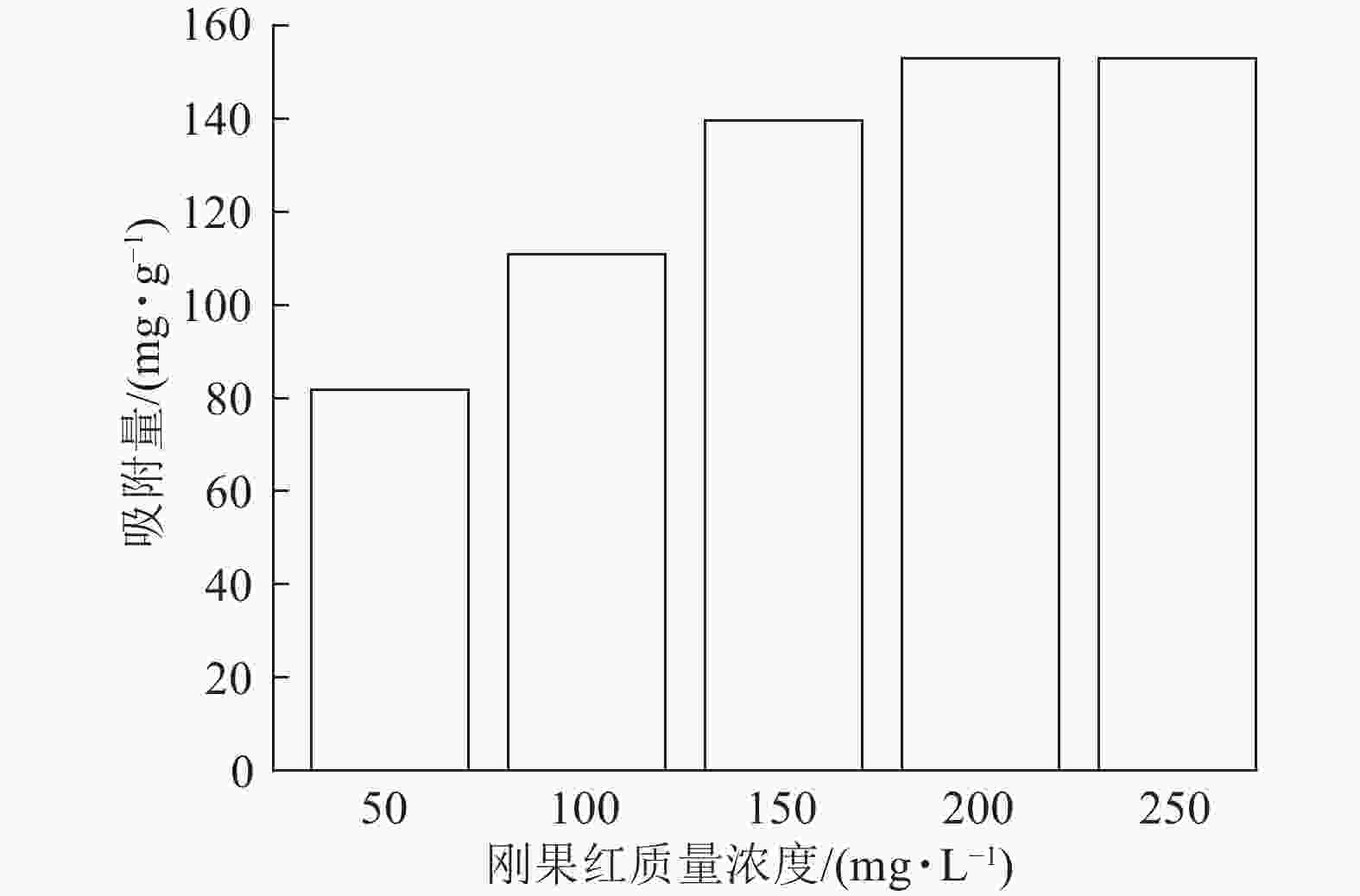
当吸附剂ASLS用量为10 mg,刚果红溶液体积为25 mL,保持刚果红初始pH不变的情况下,考察室温下刚果红初始质量浓度对吸附量的影响。从图3可以看出:随着刚果红溶液初始质量浓度的增加,ASLS对刚果红的吸附量先逐渐增加,后达到平衡吸附量。这是由于吸附通常为物质在两相界面处的接触,初始质量浓度较低时,刚果红分子与ASLS中的大量活性位点产生强烈吸附,并很快达到平衡。随着刚果红初始质量浓度的增加,刚果红与吸附剂表面的接触概率增大,活性吸附位点得到充分利用,增强了吸附效果,因此吸附量增加。当刚果红初始质量浓度达200 mg·L−1时,吸附量最大,达153 mg·g−1;继续增加刚果红初始质量浓度,更多的刚果红分子吸附在ASLS表面,使有效吸附活性中心被占据。当刚果红分子数量大于或等于吸附位点数目时,ASLS的吸附达到上限,即达到平衡吸附量,因此,继续增加刚果红质量浓度,吸附量不再上升[17]。
-
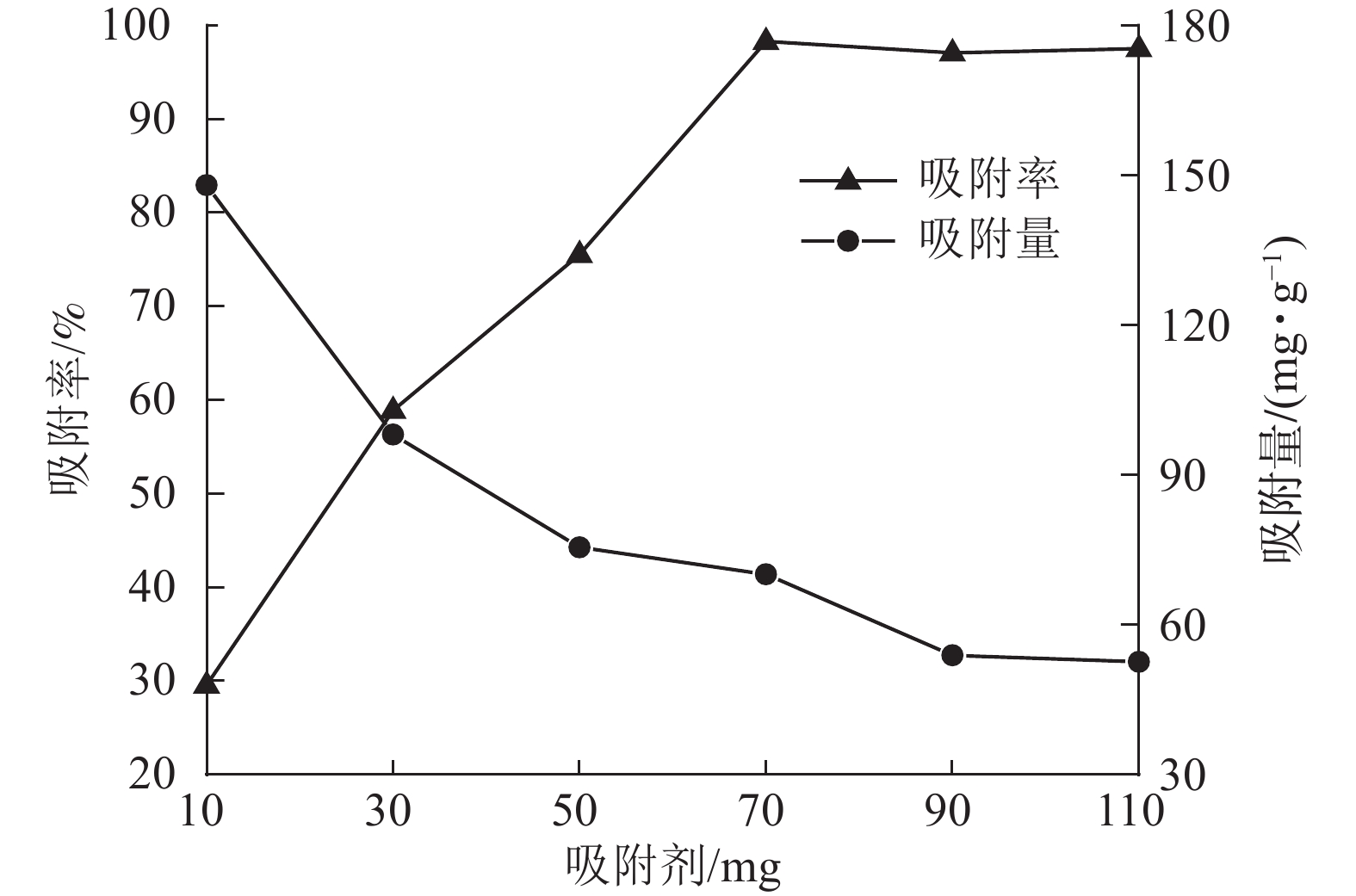
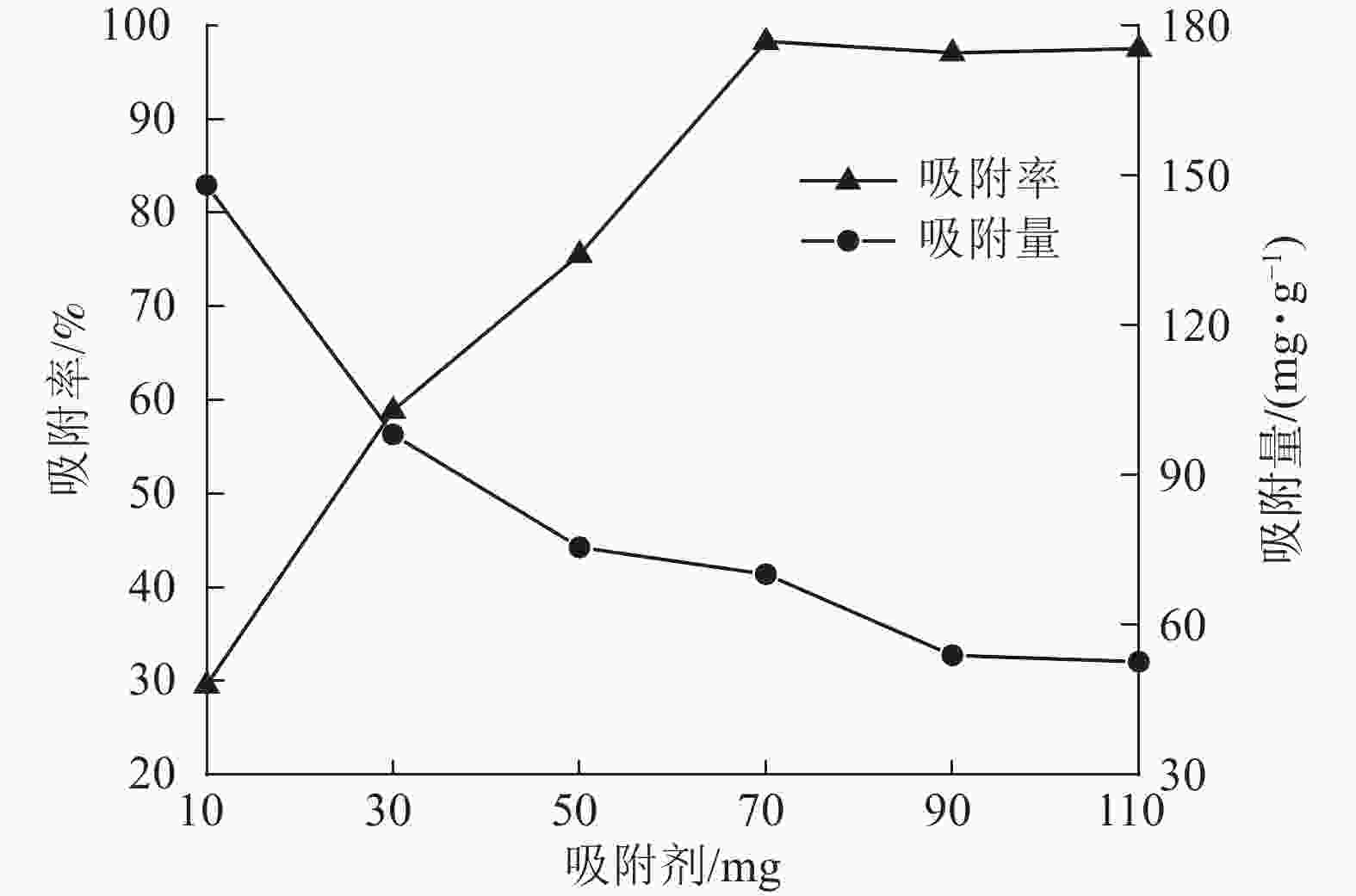
当刚果红初始质量浓度为200 mg·L−1,体积为25 mL时,保持刚果红初始pH值不变,改变ASLS用量,测定刚果红吸附量和吸附率随ASLS用量的变化。从图4可以看出:随着ASLS用量增加,对刚果红的吸附率从29.6%逐渐增加到98.3%,但单位质量的ASLS对刚果红的吸附量从148.00 mg·g−1降低到70.19 mg·g−1。这是因为随着ASLS用量的增加,吸附剂吸附面积增大,活性吸附位点数量增加,因此对刚果红的吸附率逐渐增大;但是体系中刚果红总质量浓度一定,随ASLS用量增加,部分ASLS分子间可能会发生团聚,导致部分活性位点被包埋,降低了吸附率[18],因此单位质量的ASLS对刚果红的吸附量降低。
-
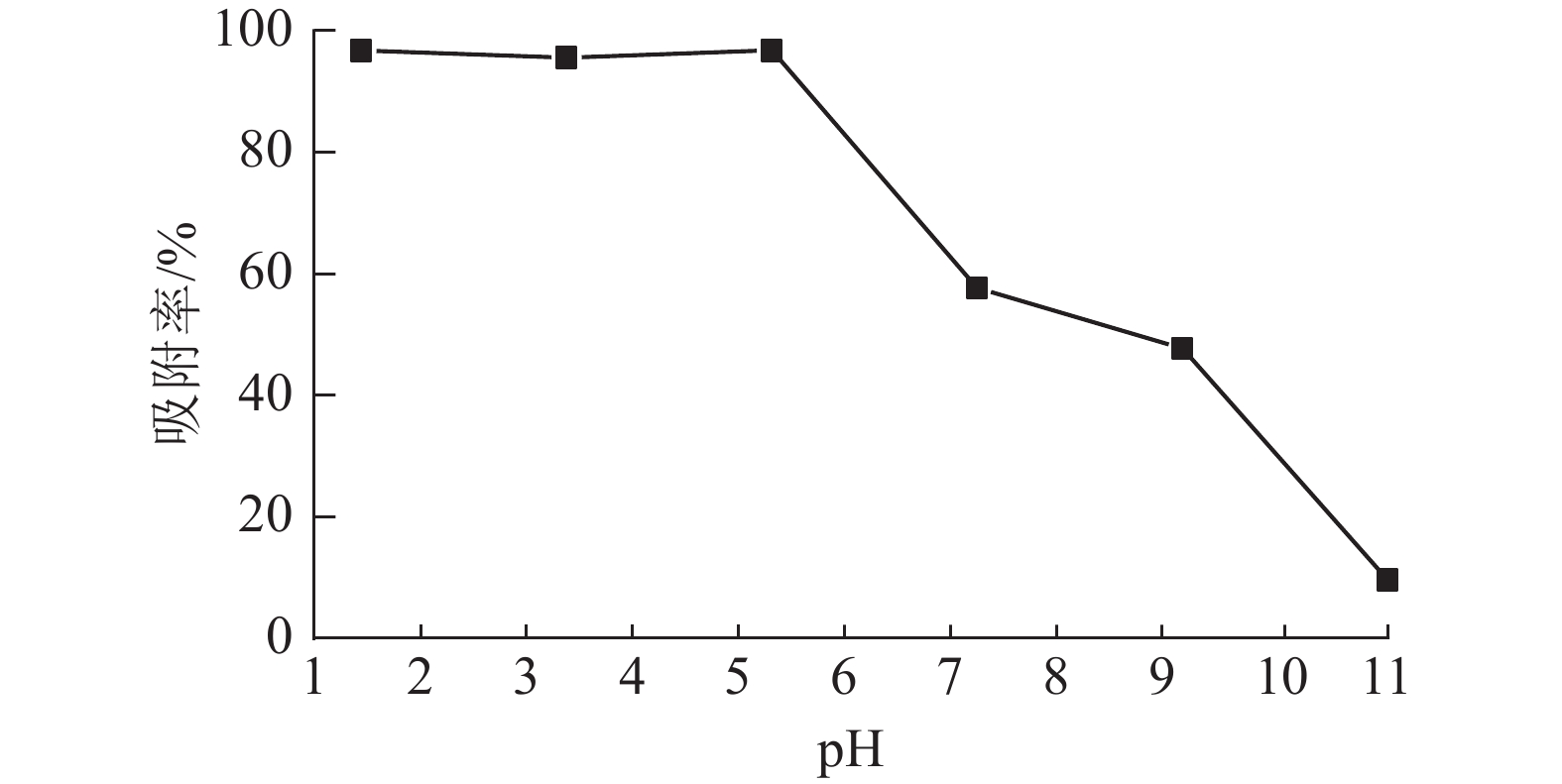
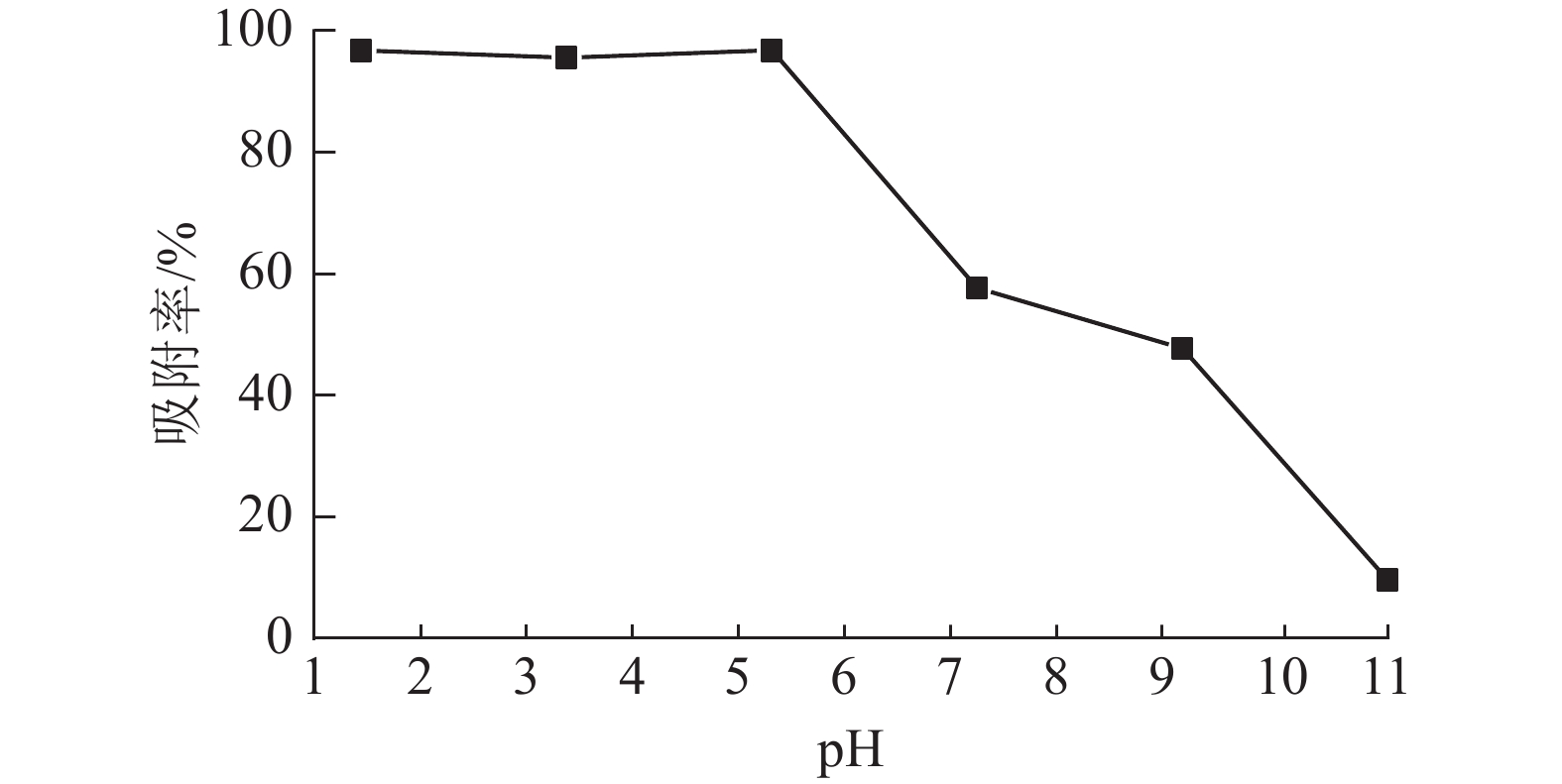
溶液pH是影响吸附材料吸附性能的关键因素之一,染料和吸附材料在溶液中的表面电荷差异对整个吸附过程起重要作用[19]。当温度为25 ℃,刚果红质量浓度为200 mg·L−1,体积为25 mL,ASLS用量为50 mg时,研究溶液pH对刚果红吸附率的影响。从图5可见:在pH低于5时,ASLS对刚果红保持了较好的吸附能力,去除率达96.8%以上;随着pH的增大,吸附效果降低,当pH大于5时,去除率下降明显。这可能是在酸性环境中,ASLS中的—NH2被质子化,形成带正电荷的${\rm{NH}}_3^+ $。刚果红是一种阴离子染料,结构中含有2个带负电的磺酸基团,可以与质子化的胺基之间形成强烈的静电相互作用,因此酸性环境中,对刚果红的去除率比较高;随着pH的升高,溶液中OH−增大,OH−与刚果红之间存在竞争,因此对刚果红的去除率逐渐降低。
-
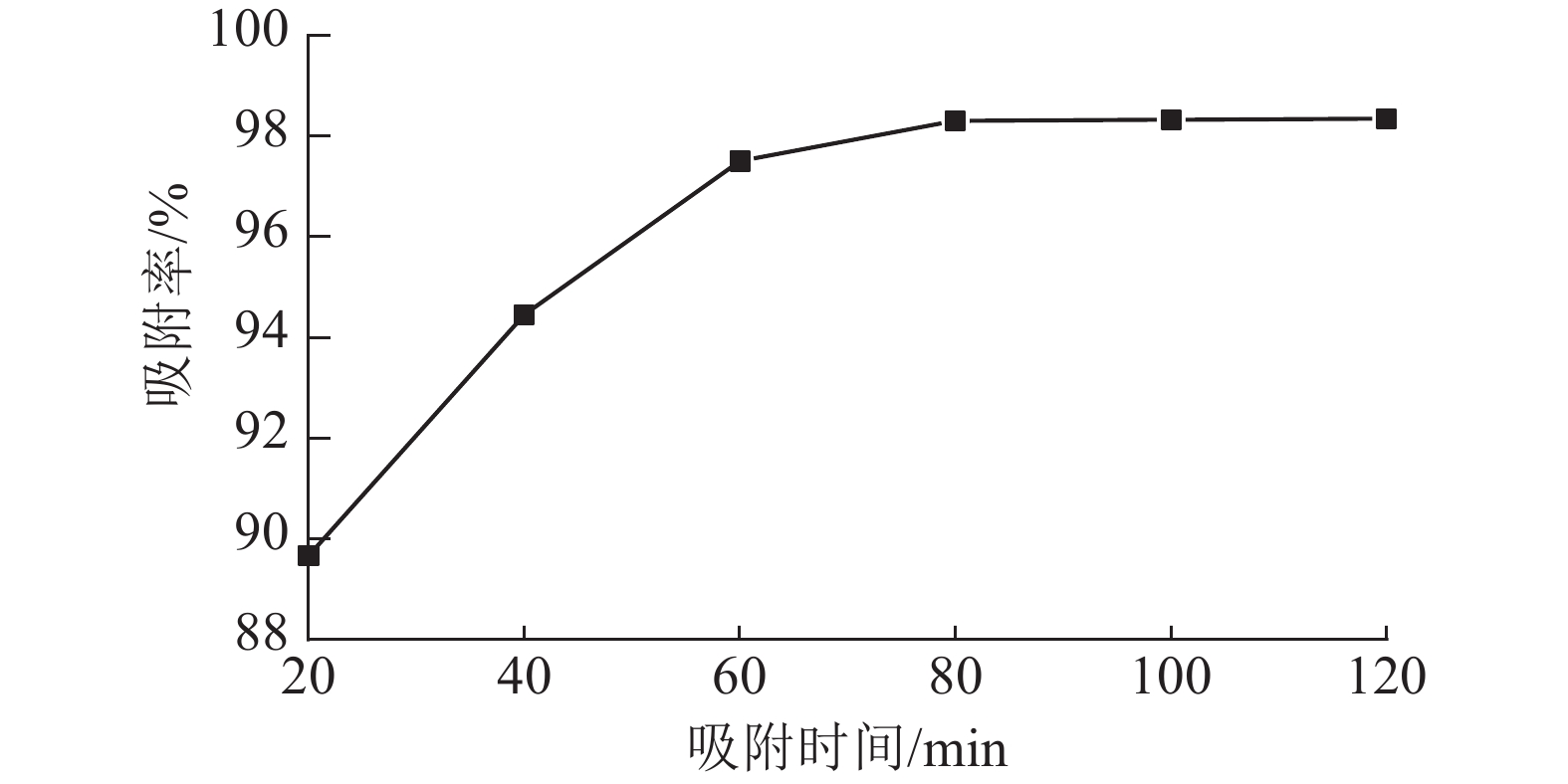
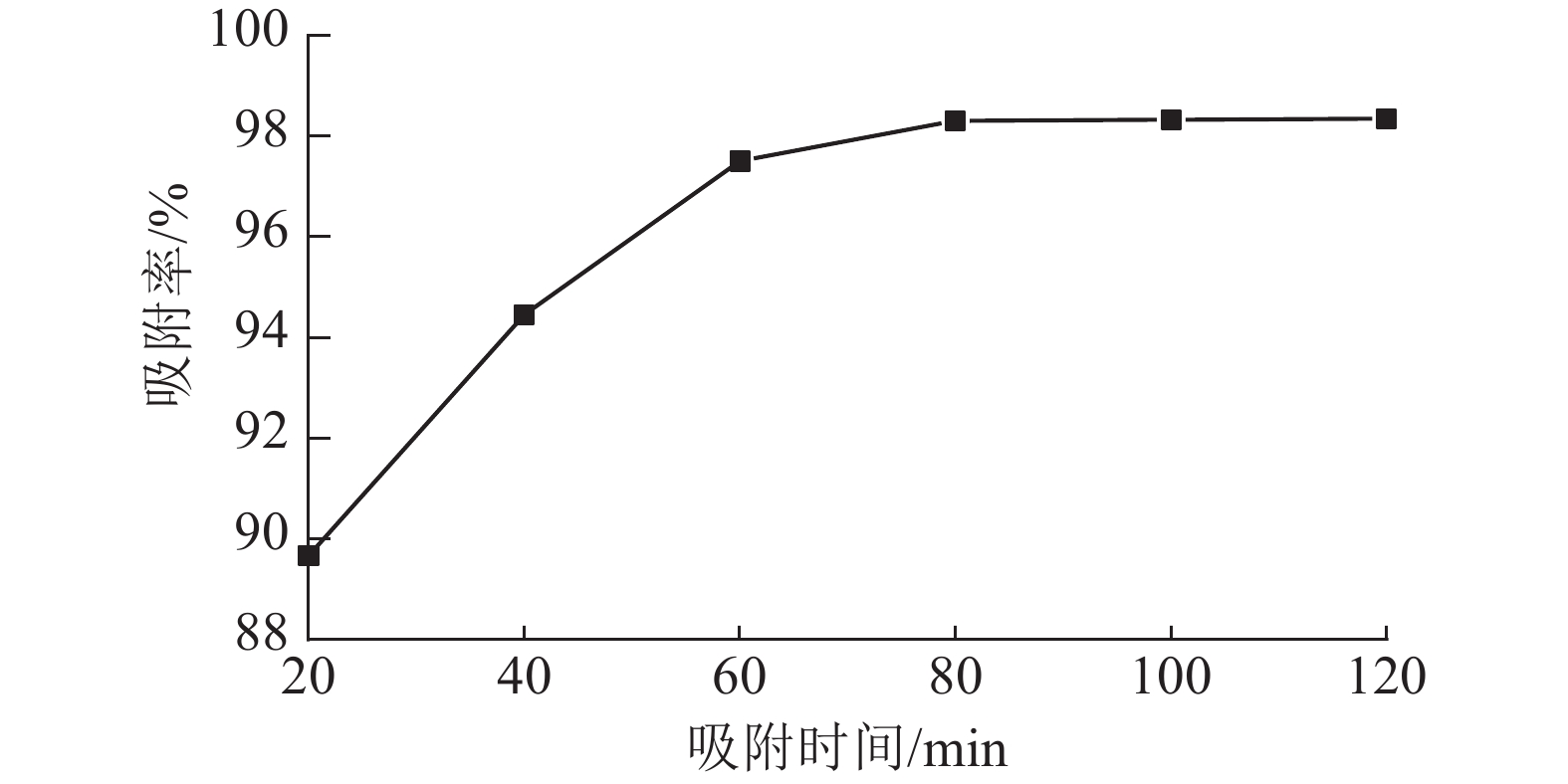
从图6可以看出:ASLS对刚果红的吸附分为2个阶段,吸附时间低于70 min时,随时间增加,吸附率增长较快;吸附时间达70 min时,吸附率接近98%;继续延长吸附时间,吸附率增加趋于平缓,80 min时达到吸附平衡。这是因为吸附初期,ASLS有较多吸附位点,且与刚果红接触良好,因此刚果红较容易被吸附;随着吸附时间延长,ASLS表面吸附位点逐渐达到饱和,因此吸附速率逐渐下降,直至达到吸附平衡状态。
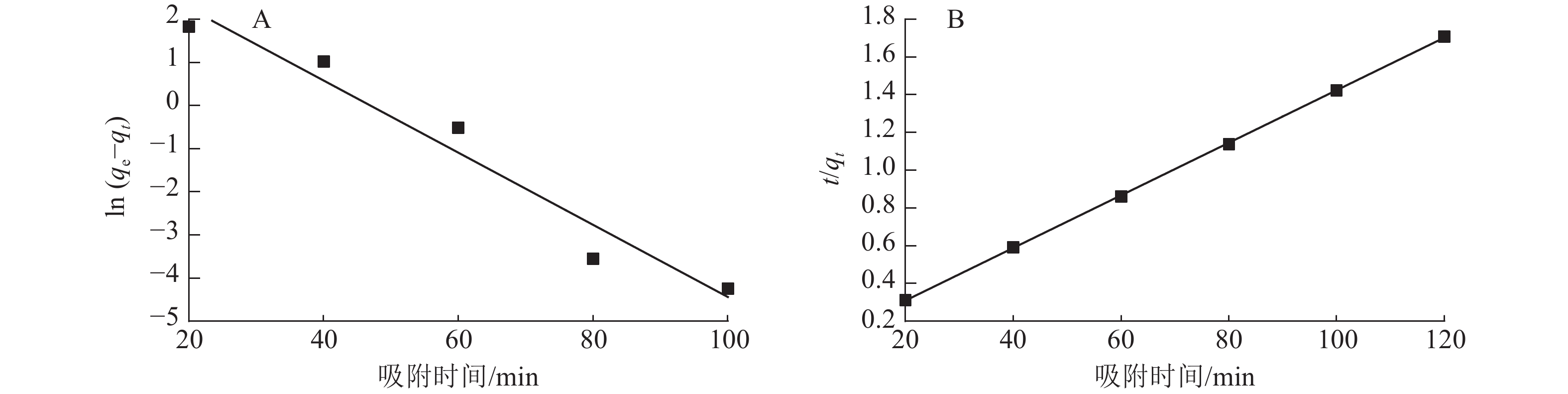
图7为准一级动力学和准二级动力学模型拟合曲线,表1为准一级动力学和准二级动力学模型参数。由表1和图7可以看出:准二级动力学模型拟合参数中的决定系数R2为0.999 9,高于准一级动力学模型(R2为0.953 0)。说明二级动力学模型可以更好地描述ASLS对刚果红的吸附过程,因为ASLS对刚果红的吸附过程主要由化学吸附控制[20]。
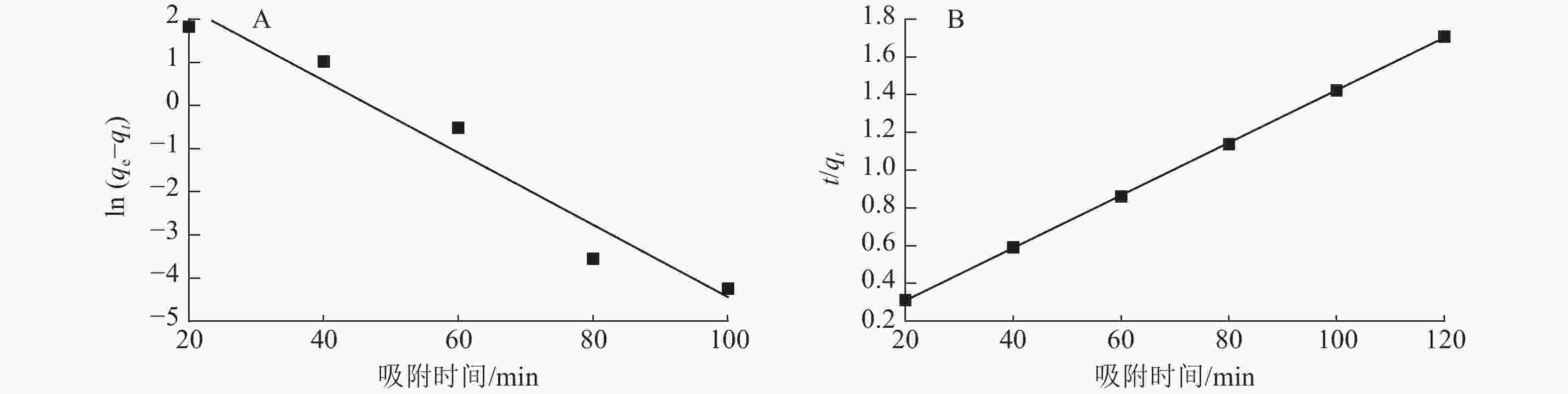
Figure 7. Quasi-first-order kinetics (A) and quasi -second-order kinetics (B) fitting of ASLS adsorption
动力学模型 qe/(mg·g−1) 速率常数(k) R2 准一级动力学 50.63 −0.083 6 0.953 0 准二级动力学 71.94 0.013 9 0.999 9 Table 1. Quasi-first-order kinetic and quasi-second-order kinetic model parameters for the adsorption of congo red adsorbed by ASLS
-
从表2可见:Langmuir模型的R2为0.990 4,大于Freundlich模型的决定系数(R2为0.958 9),说明ASLS吸附刚果红的过程遵循Langmuir模型,为单分子层吸附,其理论最大吸附量为174 mg·g−1。Freundlich模型中n为经验参数,通常1/n小于1时有利于吸附过程,1/n大于1时不利于吸附过程。本研究的1/n为0.283 1,说明吸附过程易于进行。
Langmuir方程 Freundlich方程 KL qm/(mg·g−1) R2 KF 1/n R2 0.042 173.913 0.990 4 1.280 9 0.283 1 0.958 9 Table 2. Fitting parameters for isothermal adsorption lines
-
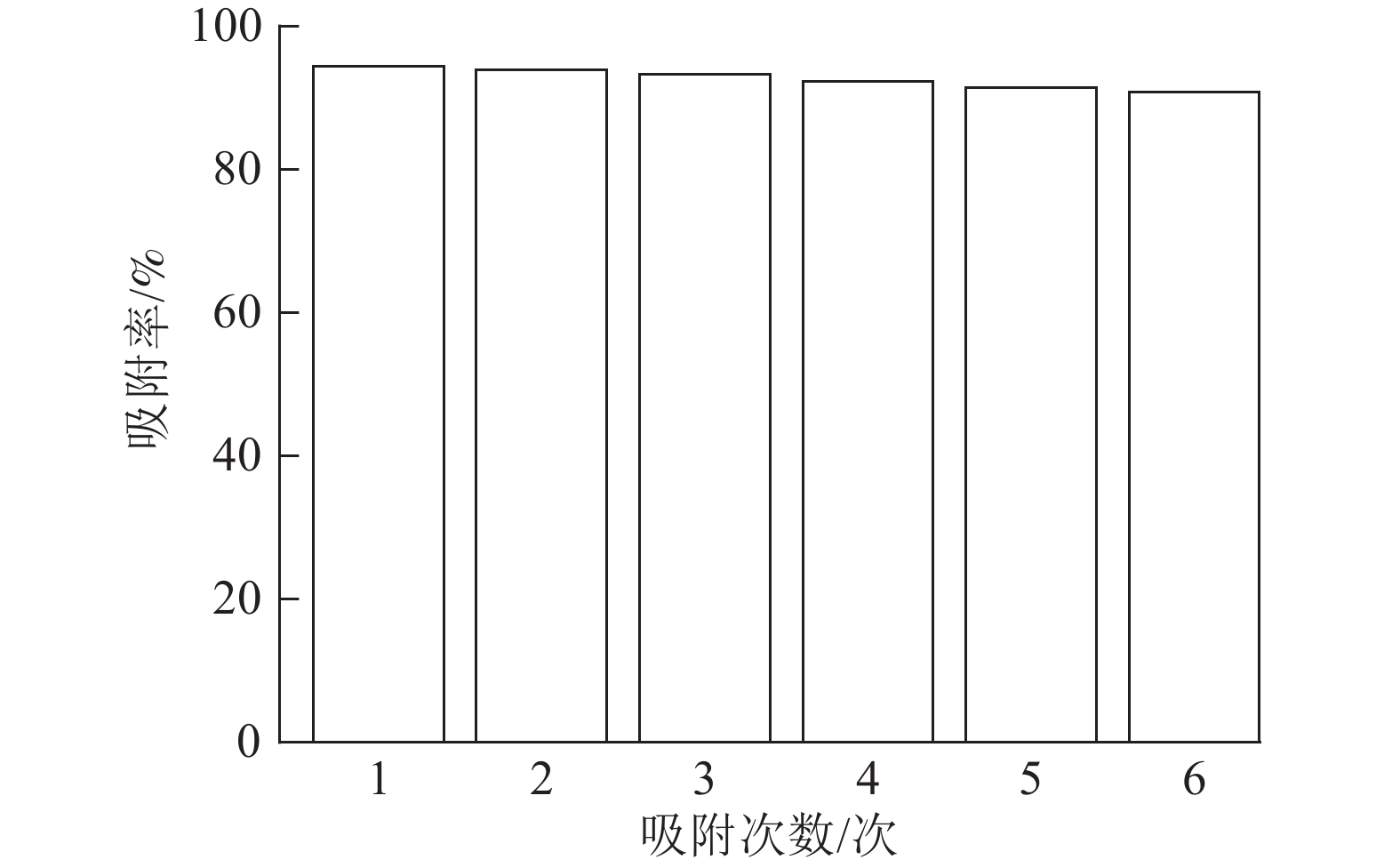
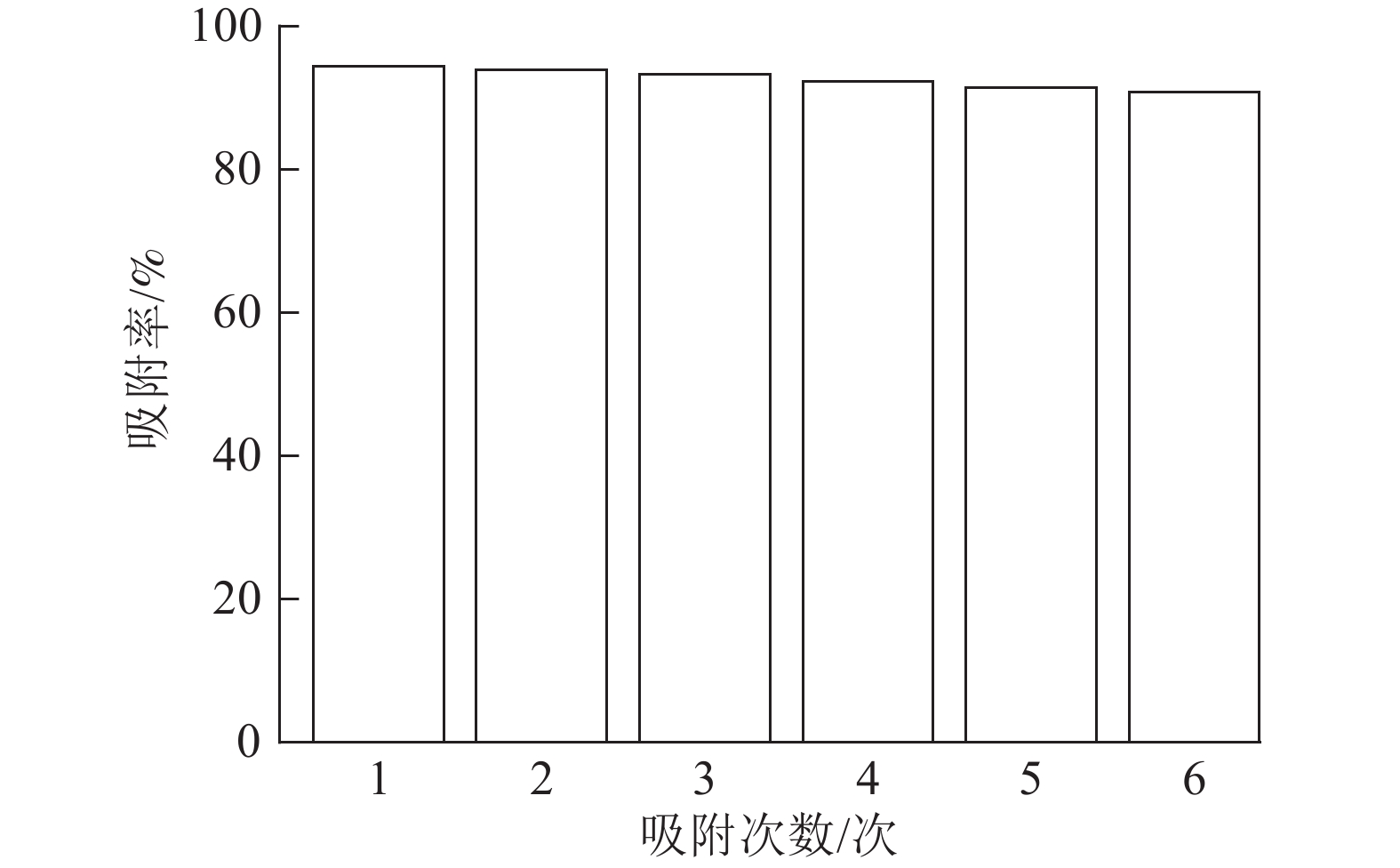
由图8可以看出:ASLS经过6次循环吸附实验后,仍然能够保持91.38%的吸附率,少量吸附效率的损失可能是由于ASLS中部分官能团的不可逆结合,导致吸附位点减少,从而导致吸附率下降。综上,说明ASLS在使用过程中具有良好的再生性能。
-
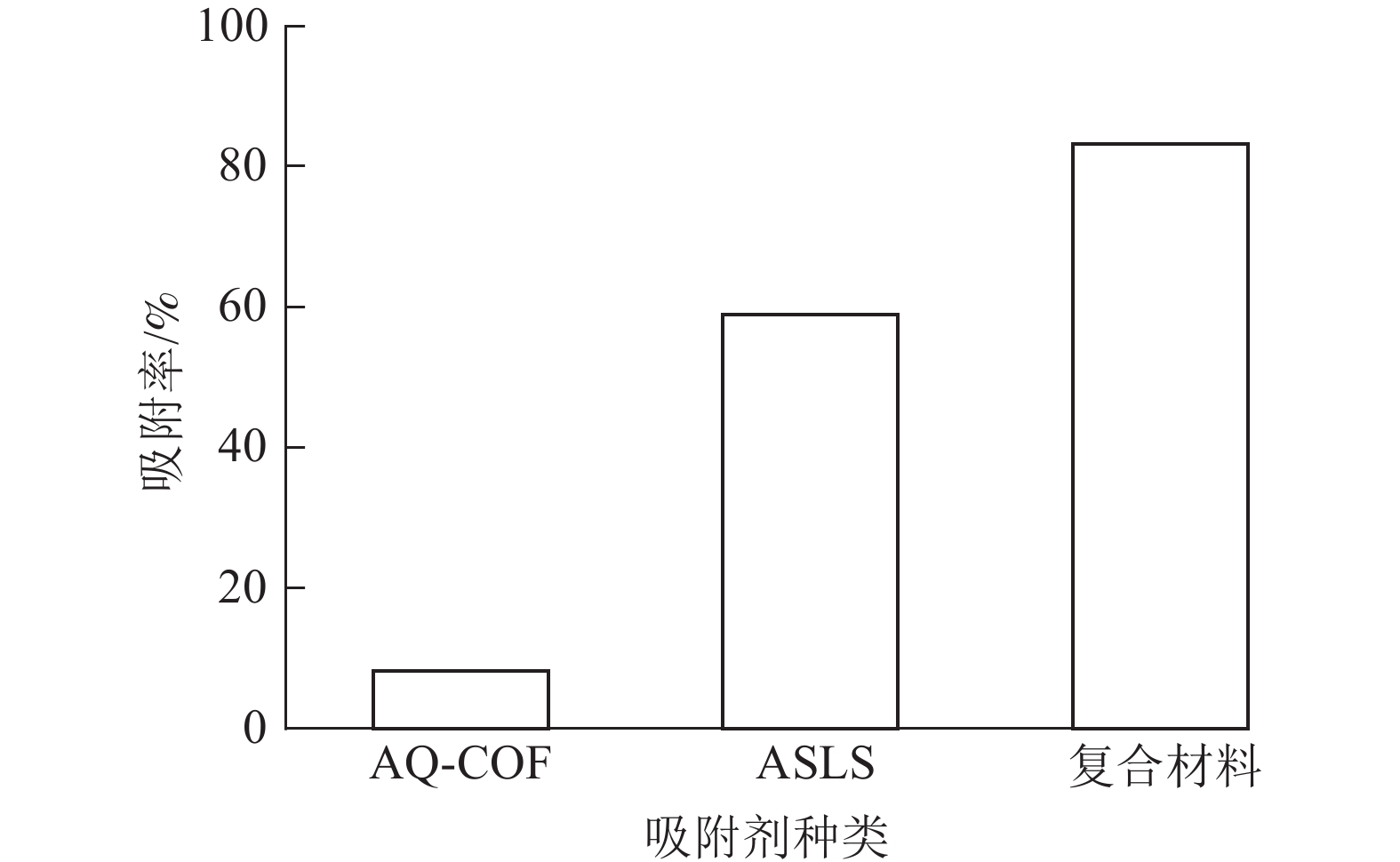
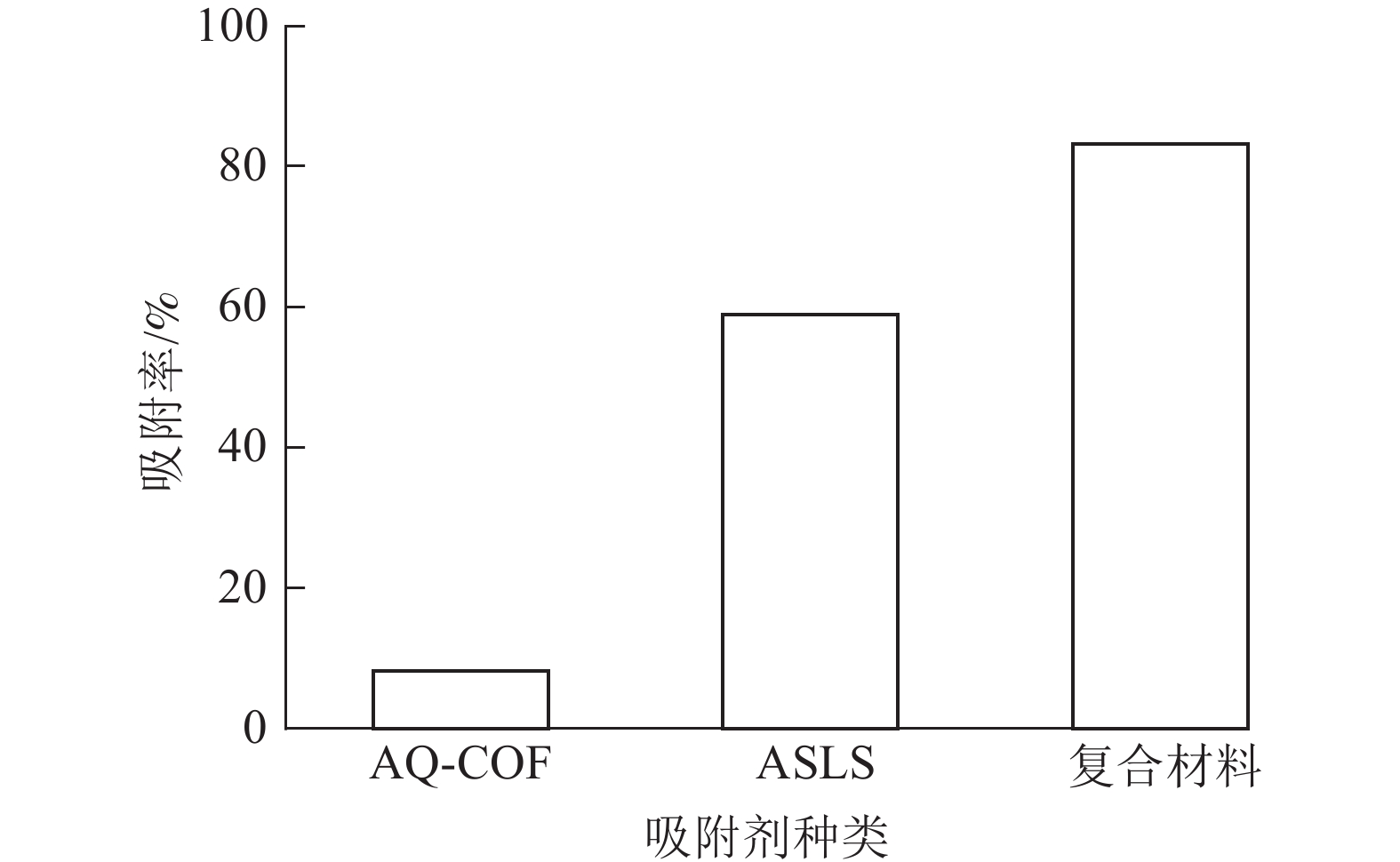
为了探究AQ-COF对ASLS吸附性能的影响,将2 mg AQ-COF与30 mg ASLS复合,制备AQ-COF/ASLS复合吸附剂。当温度为25 ℃,刚果红质量浓度为200 mg·L−1,体积为25 mL时,研究复合吸附剂对刚果红的吸附效果。如图9所示:复合前ASLS的吸附率为58.90%,AQ-COF对刚果红吸附率仅为8.20%,复合后AQ-COF/ASLS吸附剂对刚果红的吸附率达83.15%,吸附率明显提升,推测在AQ-COF和ASLS的相互作用下,ASLS被AQ-COF分散得更为均匀,因为AQ-COF中含有大量的醌结构,电负性较大的羰基结构与ASLS中带负电的磺酸基及电子云密度较高的胺基之间相互作用进行自组装[21],使ASLS中活性位点更多暴露出来,这与SEM的分析结果相一致。刚果红分子与吸附剂之间主要通过磺酸基、胺基、羟基等官能团之间的静电作用、氢键作用、π—π相互作用等进行吸附。并且胺化木质素磺酸钠、AQ-COF均为三维多孔结构,两者复合自组装之后增大了三维空间复杂性,为吸附剂提供更多的可吸附位点,从而有效提高吸附效率。
-
本研究制备的胺化木质素磺酸钠、AQ-COF/胺化木质素磺酸钠复合吸附剂对刚果红的最大平衡吸附量分别为153.0和216.7 mg·g−1,与其他生物质材料吸附剂相比,铁改性香蒲生物质炭为45.3 mg·g−1[22],Fe3O4@壳聚糖磁球为29.8 mg·g−1[23],葡萄糖酸钠派生多孔碳为102.0 mg·g−1[24],EL-PEI@Fe3O4-Mg为74.7 mg·g−1[25]。可见,本研究制备的胺基官能团化学改性的吸附剂吸附能力普遍高于没有经过官能团化学改性的吸附剂,说明通过化学改性引入活性胺基,在酸性环境中有利于提高质子化氨基的质量浓度,从而有效提升木质素磺酸钠对刚果红等阴离子染料的吸附性能。而任建鹏等[14]研究表明:木质素-聚苯胺复合材料的最大平衡吸附量为431.2 mg·g−1,吸附效果优于本研究制备的吸附剂,是因为其结构中含有大量的活性伯胺、仲胺官能团,可以通过静电作用、氢键作用、π—π作用等对染料分子进行吸附,因而吸附效果明显,进一步说明在木质素磺酸钠中引入活性胺基是提升其对刚果红吸附效果的有效方法。
此外,共价有机框架材料具有丰富的活性位点和高的比表面积,可与胺化木质素磺酸钠结合,利用两者之间的协同与自组装作用,可有效提高胺化木质素磺酸钠吸附剂的比表面积,为吸附剂提供更多的可吸附活性位点。本研究表明:复合前ASLS对刚果红的吸附率为58.90%,复合后AQ-COF/ASLS吸附剂对刚果红的吸附率达83.15%。可见,利用胺化木质素磺酸钠与COF复合可以明显提升材料的吸附效率,为木质素磺酸钠在刚果红吸附中的应用提供了新的思路。AQ-COF/胺化木质素磺酸钠复合吸附剂结构调控、吸附条件的优化,以及其对刚果红吸附性能的进一步提高可在后续进一步研究。
-
以木质素磺酸钠为原料,成功制备了ASLS、AQ-COF/ASLS 2种吸附剂,表明木质素磺酸钠成功胺化,且具有较强吸附效果。当ASLS用量为10 mg,染料初始质量浓度为200 mg·L−1,温度为25 ℃时,实际最大吸附量可达153.0 mg·g−1,并在80 min时达到吸附平衡,酸性环境可显著提高吸附效率;吸附等温线和吸附动力学分别符合Langmuir方程和准二级动力学方程,表明胺化改性的木质素磺酸钠对刚果红具有良好的吸附效果和循环使用性能。此外,AQ-COF/ASLS最大平衡吸附量为216.7 mg·g−1,因此,利用胺化木质素磺酸钠与COF复合是制备高效吸附剂的有效方法。
Preparation of sodium lignosulfonate adsorption materials and their adsorption properties for Congo red
doi: 10.11833/j.issn.2095-0756.20230585
- Received Date: 2023-12-06
- Accepted Date: 2024-04-08
- Rev Recd Date: 2024-03-21
- Available Online: 2024-07-12
- Publish Date: 2024-07-12
-
Key words:
- sodium lignosulfonate /
- amination modification /
- organic framework polymers /
- Congo red /
- adsorption
Abstract:
| Citation: | LI Junyang, HUO Lizhu, GONG Zhuxiang, et al. Preparation of sodium lignosulfonate adsorption materials and their adsorption properties for Congo red[J]. Journal of Zhejiang A&F University, 2024, 41(4): 870-878. DOI: 10.11833/j.issn.2095-0756.20230585 |









 DownLoad:
DownLoad: