-
糖基转移酶(glycosyltransfereases, GTs)能把不同活化糖基转移到糖链、核酸、蛋白质、脂质以及各种有机复合物等特异受体分子上,形成多糖分子和糖基化衍生物[1-2],在生物代谢中作用巨大。糖基转移酶广泛参与植物代谢反应。首先,细胞的各种重要代谢产物如糖蛋白、糖脂、核酸、淀粉和蔗糖等的糖基都必须在糖基转移酶作用下添加上去[4-5];其次,组成细胞壁的纤维素、半纤维素和果胶等细胞组分的形成都需要糖基转移酶参与[2];另外,它还可以通过调节各种植物生长调节剂的糖基化,改变生长调节剂活性,参与植物生长发育以及对逆境因子的响应过程[6-7]。植物中糖基转移酶及其相应基因功能研究对了解植物代谢效应,以及植物细胞对胞内、胞外环境的响应都有重要意义。编码该类酶的基因在基因组中成员众多,如拟南芥Arabidopsis thanliana中有450多个,水稻Oryza sativa中有600多个,杨树Populus trichocarpa中有800多个[1, 3]。该类基因通常以基因家族形式存在,目前已知的糖基转移酶家族超过90个,其中植物中有40多个[3]。植物中GT8糖基转移酶基因家族组成复杂,该基因家族成员数量众多,仅拟南芥中就有41个属于GT8家族的基因[1]。根据编码蛋白序列特征和功能,可将GT8基因家族分成2大类。一类为半乳糖醛酸转移酶(gAlac uronosyl transferase, GAUT),包括GAUT和GATL(GAUT-Like)2个子类。目前研究认为:该类基因主要参与果胶和木聚糖的生物合成,如拟南芥中GAUT1编码的蛋白为半乳糖醛酸聚糖糖基转移酶,参与果胶合成[8]。AtGAUT12的突变能大幅度降低植株中木聚糖含量[9],GUX1,GUX2和GUX5也都参与植物木聚糖合成[10],而果胶和木聚糖是细胞壁合成所必需的,表明GT8家族的基因在植物细胞壁合成中发挥重要作用[11-12]。另一类包括植物类糖原淀粉合成起始蛋白(plant glycogenin-lilke starch intiation proteins, PGSIPs)和乳糖苷合成酶(galactinol synthses, GolSs)。其中PGSIPs参与淀粉生物合成的启动[13];GolSs则是棉子糖类多糖合成的关键酶,后者能够作为渗透调节剂在植物逆境响应中发挥重要作用[14-15]。糖基转移酶家族成员众多,糖基转移酶功能特异性很强,即使添加同一个活化糖基,也可能因为受体不同而需要不同糖基转移酶来完成[5];而且,不同家族成员在功能上也存在交叉现象[16]。这些问题给糖基转移酶基因功能研究带来了很大困难。植物中糖基转移酶相关基因功能研究仍处在起步阶段,林木中此类基因的具体功能更是知之甚少。桉树Eucalyptus是世界上三大用材树种之一,其生产和地域分布极易受到低温、干旱、高温和高盐等非生物逆境因子的影响。EgrGATL1(Eucgr. I01882)是巨桉Eucalyptus grandis GT8家族中一个基因成员,前期研究中发现该基因表达受低温胁迫诱导,表明该基因可能与巨桉逆境响应有一定关系。目前一般认为植物GATL子类中的基因也主要与果胶和木聚糖生物合成有关[9, 17-18],但在水稻中也发现多个OsGATL基因受低温、干旱和ABA处理的诱导,暗示植物中GATL基因可能在非生物逆境胁迫响应中发挥了一定功能[19]。本研究从EgrGATL的序列特征和低温、干旱和高盐胁迫以及脱落酸(ABA),茉莉酸甲酯(MeJA)处理下基因表达角度上,分析了EgrGATL1与巨桉抗逆的关系,为其进一步功能的研究提供依据。
-
本研究所用材料为巨桉G5无性系,来源于中国林业科学研究院热带林业研究所曾炳山研究员实验室。无性系幼苗种植于浙江农林大学苗圃地。选取苗龄3个月,长势一致的巨桉无性系幼苗作为实验材料。在植物生长箱(Snijders MC1000,荷兰)中正常培养,日温为25 ℃,夜温为22 ℃;光照/黑暗为14 h/10 h;光强为150 μmol·m-2·s-1,相对湿度为70%。
-
根据基因注释编号Eucgr. I01882,从https://phytozome.jgi.doe.gov/pz/portal.html#!info?alias=Org_ Egrandis下载该基因核酸序列和蛋白序列。设计全长引物(表 1)进行聚合酶链式反应(PCR)扩增,测序验证。其编码蛋白氨基酸序列用Protparam()软件进行分子量、等电点分析。结构域搜寻用保守域数据库(CDD. https://www.ncbi.nlm.nih.gov/cdd)结合EgrGATL1不同植物中同源蛋白多序列比对(Clustalx)以及GT8基因家族相关基因分析文献进行[1, 20]。
表 1 实时荧光定量所用引物序列
Table 1. List of primer sequences used in RT-PCR
引物用途 引物名称 引物序列(5′→3′) 全长扩增 EgrGATL1-F CTTCTTCTTCCCATATCGCAGC EgrGATL1-F TTGATTCTCGAGCCGAACATAG qRT-PCR分析 Egr18SrRNA-qRT-F CGCGCTACACTGATGTATTC Egr18SrRNA-qRT-R GTACAAAGGGCAGGGACGTA EgrGATL1-qRT-F ATCTGCTTCCTCCTGCTCC EgrGATL1-qRT-R CCCTCAGGTACTCGAAATCC EgrGATL1进化分类按照ULVSKOV对GATL基因的分类标准[21],分别选取不同子组中相应拟南芥GATL蛋白序列,与EgrGATL1一起,用MEGA软件构建1 000个自举重复无根邻接进化树,对其作进化分类。
-
3个月苗龄巨桉幼苗于4 ℃低温生长箱中,为避免由于取样时间点不同对基因表达造成影响,实验采用先后间隔0,24,36,42,46 h依次放入生长箱处理,以25 ℃生长条件下幼苗作为对照(ck)。处理完毕后一起收获叶片(摘取枝条顶芽下面第3~5片完全展开的叶片),迅速投入液氮中,提取RNA进行数字表达谱(DGE)测序(诺禾致源)。结果中差异表达基因(>2倍)用加权基因共表达网络分析(WGCNA)计算与EgrNAC1具有共表达关系基因的Pearson系数(rco),根据rco大小进行基因排序。选取rco或rco绝对值>0.9的基因,将这些基因的编码蛋白序列在UniProtKB蛋白数据库中进行Blastp(https://blast.ncbi.nlm.nih.gov/Blast.cgi)比对,在域值E值<e-5范围内筛选匹配度最好的一项提取蛋白序列编码。然后将所有注释蛋白的序列编码提交到可视化整合蛋白注释数据库(DAVID)中,对比京都基因与基因组百科全书(KEGG)进行分析(),对能够在KEGG中找到相应代谢途径的共表达基因进行统计、分析。
-
参考魏晓玲等[22]的方法,在生长箱中以25 ℃生长条件下幼苗作为对照(ck),-8,-4,0,4,8和42 ℃分别处理2 h;4 ℃不同时间处理实验同1.3。处理后植株叶片迅速放入液氮,用于RNA提取。高盐处理则将植株置于不同塑料容器(60 L)内,处理组塑料容器内保持植株栽培盆1/3高度的200 mmol·L-1氯化钠溶液,对照组则用清水保持同样液面高度。同样采用间隔0,24,48,60,66 h的方法依次放入处理苗;干旱处理用5,3,2,1 d不浇水植株作为处理组,正常浇水的作为对照。100 μmol·L-1 ABA和100 μmol·L-1 MeJA处理采用植株叶面喷施的方法,ABA以喷施激素溶液后6,12,24,48 h植株作为处理组;MeJA以喷施激素溶液后2,12,24 h植株作为处理组,未喷施植株作为对照组(ck)。实验结束后,统一收获叶片,提取RNA。实验设9株·处理-1,3株·重复-1,共3个重复。
-
根据王亚红等[23]的方法提取样品RNA。组织特异性表达分析以正常生长条件下苗龄3个月的巨桉根、木质部、韧皮部和叶为RNA提取材料。RNA反转录利用PrimeScrip®RT reagent Kit(TaKaRa,大连,中国)试剂盒完成。定量荧光染料SYBR-Green(Takara,大连,中国)和BIO-RAD CFX96实时PCR系统(Bio-Rad,美国)用于EgrGATL1基因的定量RT-PCR。以Egr18SrRNA作为内参基因,实验设3个重复。结果用Bio-Rad CFX Manager(Version 1.5.5.34)软件进行分析。并用GraphPad(ver 4.0)进行作图。引物序列见表 1。
-
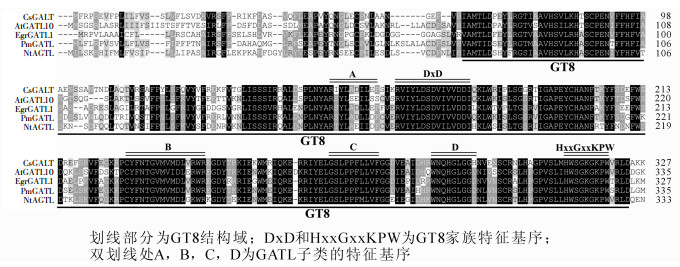
EgrGATL1基因全长cDNA为1 319 bp,开放阅读框长1 062 bp,没有内含子,编码一个353个氨基酸残基组成的蛋白。Protparam软件预测其分子量为39.5 kD,等电点为6.83。将其编码蛋白质序列与其他物种中同源蛋白质进行多序列比对。结果表明:其与橙Citrus sinensis(CsGATL),拟南芥(AtGATL10),梅Prunus mume(PmGATL)和烟草Nicotiana tomentosiformis(NtGATL)等的半乳糖醛酸转移酶类似蛋白(GATL)氨基酸序列相似程度很高,蛋白质序列中含DxD和HxxGxxKPW 2个GT8家族的典型特征基序(motif),表明该类蛋白质属于糖基转移酶GT8家族的蛋白质;同时,蛋白质序列中还有A(RxYLxxIL),B(CxFN),C(LPPF)和D(WxxxGL)基序,它们则是GT8家族中GATL子类成员编码蛋白质序列的重要特征[20]。这些结果说明EgrGATL1是半乳糖醛酸转移酶类似基因(图 1)。

图 1 EgrGATLl蛋白与其他植物同源蛋白序列的比对
Figure 1. Mutiple alignment of EgrGATLl and its homologus proteins from other plants
根据YIN等[1]对GT8基因家族中GATL子类的分类,将拟南芥中不同类别的GATL基因编码蛋白质序列与EgrGATL1一起进行进化树构建,结果中可以看出EgrGATL1属于GATL-a子组,与拟南芥中的AtGATL10(At3G28340)基因编码蛋白质亲缘关系最近(图 2)。
-
在4 ℃不同时间(0,2,6,12,24,48 h)处理下,EgrGATL1基因被强烈诱导,各处理时间下EgrGATL1的RPKM值分别为0.77,75.29,82.44,113.23,74.67和182.95。各处理时间点叶片中EgrGATL1的表达水平均为对照(0 h)的97倍以上。分析4 ℃不同处理时间下EgrGATL1共表达基因,选取其正向共表达基因90个(rco>0.90),负向共表达基因106个(rco>0.90),共196个基因进行KEGG分析。发现能与现有KEGG途径相匹配的基因有25个,其中参与基础代谢的基因18个,参与次生代谢物生物合成的基因13个(部分基因参与了1个以上的代谢途径)。这些KEGG途径包括各种糖相关代谢及合成途径,如糖酵解代谢,果糖和甘露糖代谢,半乳糖代谢,磷酸戊糖途径,氨基糖和核糖代谢,N-糖苷生物合成,淀粉和蔗糖代谢等;这些途径中包含11个基因。氨基酸代谢和生物合成途径如甘氨酸、丝氨酸和酸酸代谢,半胱氨酸和甲硫氨酸代谢,酪氨酸代谢,精氨酸和脯氨酸代谢,谷胱甘肽代谢以及氨基酸的生物合成等,包含8个基因。其他还包括嘌呤代谢,烟酸和烟酰胺代谢,卟啉与叶绿素代谢,碳固定与代谢以及脂肪酸代谢等(图 3)。尽管EgrGATL1高相关性共表达基因中,可用于KEGG分析的基因比例不高(12.8%),但仍能在一定程度上说明这些基因可能在代谢层面上主要参与糖代谢和氨基酸代谢,暗示这些代谢途径与EgrGATL1作用发挥具有一定协同性。
-
EgrGATL1在不同组织、器官中的表达有一定差异,木质部和韧皮部中表达水平较高,叶片中次之,根中表达量较低(图 4)。作为GATL家族成员,可能在细胞壁生物合成过程中会发挥一定作用。
-
定量RT-PCR结果表明:在4 ℃不同时间处理下,处理2 h后EgrGATL1表达即出现上调,为对照(25 ℃)的6.8倍,处理12 h后表达量达最大,是对照的42.8倍;随着时间延长,表达量有所下降,但仍处于被诱导状态(图 5A)。不同低温(-8,-4,0,4,8 ℃)下EgrGATL1表达都被诱导,但零上水平低温对基因诱导作用更强,在8 ℃时诱导表达量最高,相对表达量是对照的143倍;高温胁迫(42 ℃)对EgrGATL1表达并没有明显改变(图 5B)。

图 5 4 ℃不同时间(A)和不同温度(B)处理下巨桉中EgrGATL1基因的相对表达差异
Figure 5. Relative expression of EgrGATL1 under time course treatment at 4 ℃ (A) and different temperatures (B)
在200 mmol·L-1氯化钠处理下,EgrGATL1受高盐抑制,巨桉叶片中基因表达随处理时间延长不断下降,处理48 h后,基因表达水平下降为对照处理的0.02倍(图 6A)。干旱处理前2 d基因表达被诱导,随后表达水平出现了一定波动,先是下降,而后又有所上升(图 6B)。在100 μmol·L-1脱落酸处理下,EgrGATL1表达同样随时间延长受到明显抑制。有意思的是,脱落酸处理与盐胁迫处理,基因受抑制的规律几乎完全同步(图 6A,图 6C)。EgrGATL1表达对茉莉酸甲酯也有响应,茉莉酸甲酯处理2 h,即可强烈诱导基因表达,使EgrGATL1表达水平达到对照的211.3倍,之后诱导作用减弱,24 h后表达水平仅为对照的2.1倍,显示茉莉酸甲酯对EgrGATL1产生的是瞬时诱导作用(图 6D)。
-
从蛋白质序列分析可以知道EgrGATL1是一个典型半乳糖醛酸转移酶(GATL)类似基因,在子类分类中属于GATL-a子组[20]。一般认为GATL子类成员可能参与细胞壁中果胶或者木聚糖生物合成[24]。如AtGATL1,AtGATL13和AtGATL16参与细胞次生壁形成中木聚糖生物合成[9-10, 17];AtGATL9参与果胶生物合成[10]。这些基因的功能改变都会在一定程度上影响细胞壁的结构。也有GATL成员参与种皮黏液形成,AtGATL5被认为调控构成种皮黏液主要成分黏性鼠李半乳糖醛酸聚糖链的大小[25]。EgrGATL1在木质部和韧皮部中相对根和叶片的高表达现象,也在一定程度上说明它也有可能参与植物细胞壁组分的生物合成。
不同低温(-8,-4,0,4,8 ℃),低温(4 ℃)不同处理时间(0,2,6,12,24,48 h)以及干旱都能诱导EgrGATL1表达,说明该基因在植物非生物逆境响应中可能也发挥一定作用。水稻中7个GATL基因中有6个受干旱诱导,5个受低温诱导[19],表明GATL基因参与非生物逆境胁迫响应不是孤立的现象。植物受到冷胁迫时,细胞中混合多糖含量大幅度提高,半乳糖醛酸和糖醛酸含量增加[26];细胞壁成分中果胶和非纤维素成分往往发生积累,其中果胶含量的提高能增强植物细胞抗压强度和细胞壁厚度,降低细胞遭受低温危害的程度[27-28]。干旱条件下,植物也可能通过果胶修饰,产生细胞壁重构作用,提高抗旱能力[26]。抗干旱的小麦品种Triticum aestivum ‘Capeiti’在干旱条件下,细胞中果胶鼠李半乳糖醛酸聚糖Ⅰ(RGⅠ)和Ⅱ(RGⅡ)侧链含量大幅度增加,而干旱敏感型品种却没有类似情况发生。果胶中RGⅠ和RGⅡ侧链的增加能够提高其水合能力,增加植物的保水性能,进而提高其抗寒性[29]。因此,低温、干旱条件下巨桉EgrGATL1的诱导表达极有可能是因为该基因通过参与细胞壁组分生物合成,进行细胞壁重构来影响植物非生物逆境抗性的。细胞壁重构在植物非生物逆境响应中发挥作用的研究是目前植物抗逆研究的一个热点[26]。在细胞壁重构过程中,涉及大量糖代谢途径[30-31],低温(4 ℃)不同时间处理下EgrGATL1共表达基因KEGG途径分析中,各种糖代谢途径的相关共表达基因可能与EgrGATL1基因功能发挥有一定协同作用。这为EgrGATL1进一步功能研究提供了线索。
糖基转移酶还可能对植物生长调节剂的作用产生影响,进而改变植物的生长、发育及逆境响应过程。生长素(IAA),脱落酸,细胞分裂素(CK),油菜素内酯(BRs)和水杨酸(SA)等都可以被糖基转移酶糖基化而改变激素活性[32]。细胞分裂素以核糖化的形式在木质部运输[33];而拟南芥中糖基转移酶UGT71B6则可以通过形成脱落酸糖酯的形式使脱落酸失活,进而调控干旱、高盐等逆境下植物的响应[34]。本研究中脱落酸和盐胁迫对EgrGATL1基因表达都有抑制作用,而且随处理时间变化,基因受抑制的规律几乎完全同步。这暗示EgrGATL1有可能参与巨桉盐胁迫下脱落酸的调控,对这一推测的证实及其相应具体调控机制的研究,将是下一步开展的重点内容。茉莉酸甲酯处理下EgrGATL1的表达也存在瞬时诱导作用,尽管茉莉酸甲酯的信号转导作用参与了植物干旱和高盐胁迫逆境响应过程[35],但它与糖基转移酶之间的关系目前仍不清楚,同样需要进一步研究加以揭示。
Glycosyltransferases gene EgrGATL1 in Eucalyptus grandis
-
摘要: EgrGATL1(Eucgr.I01882)是巨桉Eucalyptus grandis糖基转移酶GT8家族中GATL子类GATL-a子组的成员,多参与细胞壁组分如果胶、木聚糖等的生物合成。实时定量聚合酶链式反应(qRT-PCR)分析表明:EgrGATL1在木质部和韧皮部的相对表达量较高;不同低温(-8,-4,0,4,8℃)和4℃不同时间(0,2,6,12,24,48 h)处理对EgrGATL1都有强烈诱导作用。4℃不同时间处理下,EgrGATL1基因共表达产物主要参与代谢途径分析数据库(KEGG)中的糖代谢和氨基酸代谢途径。干旱胁迫对EgrGATL1有诱导作用;100 μmol·L-1茉莉酸甲酯(MeJA)处理对EgrGATL1表达呈现瞬时诱导效应;200 mmol·L-1氯化钠(NaCl)和100 μmol·L-1脱落酸(ABA)对其表达有抑制作用,而且随处理时间延长,2种处理对EgrGATL1的抑制规律有很强同步性。这些结果说明:EgrGATL1有可能通过参与细胞壁组分生物合成和ABA等植物生长调节剂活性调控,在巨桉低温、干旱和高盐等非生物逆境响应过程中发挥一定作用。Abstract: In Eucalyptus grandis, EgrGATL1 (Eucgr. I01882), a member of the glycosyltransferases 8 family belongs to the GATL subfamily, which contributes to the biosynthesis of cell wall components such as pectin and xylan, is classified as the GATL-a subgroup. In this study, the protein sequence of EgrGATL1 was analyzed and cis-elements were searched in the promoter sequence of this gene with MathInspector software. Real time fluorescence quantitative Polymerase Chain Reaction(qRT-PCR) method was used to evaluate expression pattern of EgrGATL1 under treatments of low temperatures (-8, -4, 0, 4, 8℃), time course at 4℃ (0, 2, 6, 12, 24, 48 h), drought, 100 μmol·L-1 MeJA, 200 mmol·L-1 NaCl and 100 μmol·L-1 ABA. In addition, a KEGG analysis was used to test co-expression genes of EgrGATL1 under treatment of time course at 4℃. The protein sequence analysis showed EgrGATL1 contains a typical GT8 domain. Results of expression in different tissues showed that expression of EgrGATL1 was higher in xylem and phloem than in roots and leaves. Low temperature (-8, -4, 0, 4, 8℃) and time course treatment at 4℃ both can promote the expression of EgrGATL1. With KEGG analysis, 25 genes co-expressed with EgrGATL1 can match to KEGG pathways, 11 genes belong to the sugar metabolism pathways and 8 genes were distributed to amino acid metabolism pathways. EgrGATL1 was also induced by drought and showed transient induction with the 100 μmol·L-1 MeJA treatment. And, there is no significant difference between the expression patterns of EgrGATL1 with treatments of 200 mmol·L-1 NaCl and 100 μmol·L-1 ABA. Thus, EgrGATL1 was possibly involved in cell wall remodeling and with activity of hormones such as ABA, thereby implying a possible role in low temperature, drought, and salinity stress responses in E. grandis.
-
Key words:
- botany /
- Eucalyptus grandis /
- EgrGATL1 /
- abiotic stress /
- gene expression
-
表 1 实时荧光定量所用引物序列
Table 1. List of primer sequences used in RT-PCR
引物用途 引物名称 引物序列(5′→3′) 全长扩增 EgrGATL1-F CTTCTTCTTCCCATATCGCAGC EgrGATL1-F TTGATTCTCGAGCCGAACATAG qRT-PCR分析 Egr18SrRNA-qRT-F CGCGCTACACTGATGTATTC Egr18SrRNA-qRT-R GTACAAAGGGCAGGGACGTA EgrGATL1-qRT-F ATCTGCTTCCTCCTGCTCC EgrGATL1-qRT-R CCCTCAGGTACTCGAAATCC -
[1] YIN Yanbin, MOHNEN D, GELINEO-ALBERSHEIM, et al. Glycosyltransferases of the GT8 Family[J]. Annu Plant Rev, 2011, 41:167-172. [2] LAIRSON LL, HENRISSAT B, DAVIES GJ, et al. Glycosyltransferases:structures, functions, and mechanisms[J]. Annu Rev Biochem, 2008, 77:521-555. [3] LAO J, OIKAWA A, BROMLEY J R, et al. The plant glycosyltransferase clone collection for functional genomics[J]. Plant J, 2014, 79(3):517-529. [4] VOGT T, JONES P. Glycosyltransferases in plant natural product synthesis:characterization of a supergene family[J]. Trends Plant Sci, 2000, 5(9):380-386. [5] CAMPBELL J A, DAVIES G J, BULONE V, et al. A classification of nucleotide-diphospho-sugar glycosyltransferases based on amino acid sequence similarities[J]. Biochem J, 1997, 326(3):929-939. [6] TIWARI P, SANGWAN R S, SANGWAN N S. Plant secondary metabolism linked glycosyltransfrases:an update on expanding knowledge and scopes[J]. Biotechnol Adv, 2016, 34(5):714-739. [7] PALANIYANDI S A, CHUNG G, KIM S H, et al. Molecular cloning and characterization of the ABA-specific glucosyltransferase gene from bean (Phaseolus vulgaris L.)[J]. J Plant Physiol, 2015, 178(2):1-9. [8] STERLING J D, ATMODJO M A, INWOOD S E, et al. Functional identification of an Arabidopsis pectin biosynthetic homogalacturonan galacturonosyltransferase[J]. Proc Nat Acad Sci USA, 2006, 103(13):5236-5241. [9] BROWN D M, GOUBET F, WONG V W, et al. Comparison of five xylan synthesis mutants reveals new insight into the mechanisms of xylan synthesis[J]. Plant J, 2007, 52(6):1154-1168. [10] RENNIE E A, HANSEN S F, BAIDOO E E, et al. Three members of the Arabidopsis glycosyltransferase family 8 are xylan glucuronosyltransferases[J]. Plant Physiol, 2012, 159(4):1408-1417. [11] COSGROVE D J. Growth of the plant cell wall[J]. Nat Rev Mol Cell Biol, 2005, 6(11):850-861. [12] PEAUCELLE A, BRAYBROOK S, HÖFTE H. Cell wall mechanics and growth control in plants:the role of pectins revisited[J]. Front Plant Sci, 2012, 3:121. doi:10.338g/fpls. 2012. 00121. [13] CHATTERJEE M, BERBEZY P, VYAS D, et al. Reduced expression of a protein homologous to glycogenin leads to reduction of starch content in Arabidopsis leaves[J]. Plant Sci, 2005, 168(2):501-509. [14] NISHIZAWA-YOKOI A, YABUTA Y, SHIGEOKA S. Galactinol and raffinose constitute a novel function to protect plants from oxidative damage[J]. Plant Physiol, 2008, 147(3):1251-1263. [15] LAO N T, LONG D, KIANG S, et al. Mutation of a family 8 glycosyltransferase gene alters cell wall carbohydrate composition and causes a humidity-sensitive semi-sterile dwarf phenotype in Arabidopsis[J]. Plant Mol Biol, 2003, 53(5):687-701. [16] COUTINHO P M, HENRISSAT B. Annotating Carbohydrateactive Enzymes in Plant Genomes:Present Challenges[M]. Oxford:Wiley-Blackwell Publishing Ltd., 2010:93-107. [17] LEE C H, ZHONG R Q, RICHARDSON E A, et al. The PARVUS gene is expressed in cells undergoing secondary wall thickening and is essential for glucuronoxylan biosynthesis[J]. Plant Cell Physiol, 2007, 48(12):1659-1672. [18] KONG Yingzhen, ZHOU Gongke, AVCI U, et al. Two poplar glycosyltransferase genes, PdGATL1.1 and PdGATL1.2, are functional orthologs to PARVUS/AtGATL1 in Arabidopsis[J]. Mol Plant, 2009, 2(5):1040-1050. [19] LIU Jinlong, LUO Mansi, YAN Xin, et al. Characterization of genes coding for galacturonosyltransferase-like (GATL) proteins in rice[J]. Genes Genom, 2016, 38(10):917-929. [20] YIN Yanbin, CHEN Huiling, HAHN M G, et al. Evolution and function of the plant cell wall synthesis-related glycosyltransferase family 8[J]. Plant Physiol, 2010, 153(4):1729-1746. [21] ULVSKOV P. Annual Plant Reviews, Plant Polysaccharides:Biosynthesis and Bioengineering[M]. New Jersey:John Wiley & Sons, 2011. DOI:10.1002/9781444391015 [22] 魏晓玲, 程龙军, 窦锦青, 等.巨桉EgrDREB2A基因结构及表达特性分析[J].林业科学, 2015, 51(2):80-89. WEI Xiaoling, CHENG Longjun, DOU Jinqing, et al. The structure and expression characteristics of EgrDREB2A gene in Eucalyputs grandis[J]. Sci Silv Sin, 2015, 51(2):80-89. [23] 王亚红, 刘缙, 王玉国.高质量提取银杏种仁RNA的改良方法[J].中国农学通报, 2010, 26(15):48-52. WANG Yahong, LIU Jin, WANG Yuguo. An improved method for RNA isolation from seeds of Ginkgo biloba L.[J]. Chin Agric Sci Bull, 2010, 26(15):48-52. [24] KONG Yingzhen, ZHOU Gongke, YIN Yanbin, et al. Molecular analysis of a family of Arabidopsis genes related to galacturonosyl transferases[J]. Plant Physiol, 2011, 155(4):1791-1805. [25] KONG Yingzhen, ZHOU Gongke, ABDEEN A A, et al. GALACTURONOSYLTRANSFERASE-LIKE5 is involved in the production of Arabidopsis seed coat mucilage[J]. Plant Physiol, 2013, 163(3):1203-1217. [26] TENHAKEN R. Cell wall remodeling under abiotic stress[J]. Front Plant Sci, 2015, 5:771. doi:10.338g/fpls. 2014. 00771. [27] QU Tangdong, LIU Rugao, WANG Weilin, et al. Brassinosteroids regulate pectin methylesterase activity and AtPME41 expression in Arabidopsis under chilling stress[J]. Cryobiology, 2011, 63(2):111-117. [28] DOMON J M, BALDWIN L, ACKET S, et al. Cell wall compositional modifications of Miscanthus ecotypes in response to cold acclimation[J]. Phytochemistry, 2013, 85(2):51-61. [29] LEUCCI M R, LENUCCI M S, PIRO G, et al. Water stress and cell wall polysaccharides in the apical root zone of wheat cultivars varying in drought tolerance[J]. J Plant Physiol, 2008, 165(11):1168-1180. [30] PAULY M, KEEGSTRA K. Cell-wall carbohydrates and their modification as a resource for biofuels[J]. Plant J, 2008, 54(4):559-568. [31] le GALL H, PHILIPPE F, DOMON J M, et al. Cell wall metabolism in response to abiotic stress[J]. Plants, 2015, 4(1):112-166. [32] GACHON C M M, LANGLOIS-MEURINNE M, SAINDRENAN P. Plant secondary metabolism glycosyltransferases:the emerging functional analysis[J]. Trends Plant Sci, 2005, 10(11):542-549. [33] BEVERIDGE C A, MURFET I C, KERHOAS L, et al. The shoot controls zeatin riboside export from pea roots:evidence from the branching mutant rms4[J]. Plant J, 1997, 11(2):339-345. [34] DONG Ting, XU Zhengyi, PARK Y M, et al. Abscisic acid uridine diphosphate glucosyltransferases play a crucial role in abscisic acid homeostasis in Arabidopsis[J]. Plant Physiol, 2014, 165(1):277-289. [35] RIEMANN M, DHAKAREY R, HAZMAN M, et al. Exploring jasmonates in the hormonal network of drought and salinity responses[J]. Front Plant Sci, 2015, 6:1077. doi:10.3389/fpls.2015.01077. -

-
链接本文:
https://zlxb.zafu.edu.cn/article/doi/10.11833/j.issn.2095-0756.2018.04.004






 下载:
下载: