-
芸香Ruta graveolens又名臭草、香草、百应草、小叶香,是芸香科Rutaceae芸香属Ruta多年生草本植物。植株高达1 m,叶羽状复叶,灰绿或带蓝绿色;花金黄色,花期3-6月;原产地中海沿岸,在中国均有栽培[1]。芸香植株各部分均有浓烈的特殊气味,具有杀虫抑菌效果[2-4];全株均可入药,有清热解毒、凉血散瘀、利尿、消肿、止咳平喘等功效[5-6]。目前,对芸香科的研究主要集中在生理特性[7-8]、分子生物学[9-10]、精油化学成分[3-4, 11-13]及抑菌效果[14-15]等方面,并未见有关芸香花瓣及叶片挥发性有机物成分随时间变化的相关报道。现有的研究探究芸香的有机物成分主要采用精油作为试验材料[16-18],精油的提取常常会受提取温度和方法的干扰,造成某些挥发性物质受到破坏,不能完全反映芸香释放的挥发性有机物的成分。因此,本研究采用顶空固相微萃取技术吸附采集不同时期芸香叶片释放的挥发性有机成分,结合气质联用仪检测并分析不同季节及1 d中不同时间释放的挥发性有机物成分及相对含量,揭示其叶片挥发性有机物的季节变化规律和日变化规律;并用相同的方法探究叶片和花瓣释放的挥发性有机物成分的差异,为科学利用芸香营造生态型、保健型、芳香园林景观提供理论依据。
-
芸香苗龄为5~6年生,株高70~75 cm,长势繁茂,无病虫害,栽植于深圳职业技术学院西校区芳香植物园内。
-
分别于3月、6月、9月、12月的16日上午9:00-10:00在同一位置的植物上摘取向阳侧上、中、下部位的健康无损伤的叶片10片,分别剪碎混匀,称取0.5 g放置5 mL萃取瓶中密封,静置30 min,环境温度为(22.0±3.0) ℃。将型号为DVB-CAR-PDMS 100 μm的SPME纤维头(美国Supelco公司)通过聚四氟乙烯瓶垫插入到萃取瓶中,置于样品正上方0.5 cm左右,顶空萃取40 min,然后将纤维头插入6890N/5975气相色谱-质谱联用仪(美国Agilent公司)的气相色谱进样口,解吸10 min。每次收集设置3次重复,吸附空萃取瓶中的气体作为空白对照。花瓣挥发性有机物的测定于3月16日进行,9:00-10:00采摘盛开的花5朵;挥发性有机物日动态变化的测定于6月16日进行,8:00-20:00隔2 h采样1次。色谱条件:HP-5MS弹性石英毛细管柱色谱柱,长为30 m,内径为0.25 mm,液膜厚为0.25 μm,载气为高纯氦气,不分流进样,恒流流速为1.0 mL ·min-1,进样口温度为230 ℃,接口温度为280 ℃。季节变化和花瓣挥发物测定初始温度为50 ℃,保持4 min,以6 ℃·min-1升至150 ℃,保持2 min,然后以7 ℃·min-1升至250 ℃,保持8 min。日动态变化规律测定初始温度为50 ℃,保持4 min,以4 ℃·min-1升至150 ℃,保持2 min,然后以8 ℃·min-1升至250 ℃,保持6 min。质谱条件:电子轰击(EI)离子源,电子能量为70 eV,离子阱温度为230 ℃,四级杆温度为150 ℃,原子质量扫描范围为30~500。
-
挥发性有机物成分经气相色谱分离,各色谱峰、质谱峰分别利用气相色谱-质谱仪中MSD Productivity ChemStation和NIST Mass Spectral Database 2008谱库进行分析鉴定。各成分在样品气体中的浓度采用峰面积归一法进行计算。相对含量=(该物质峰面积/样品所有气体峰面积之和)×100%。
-
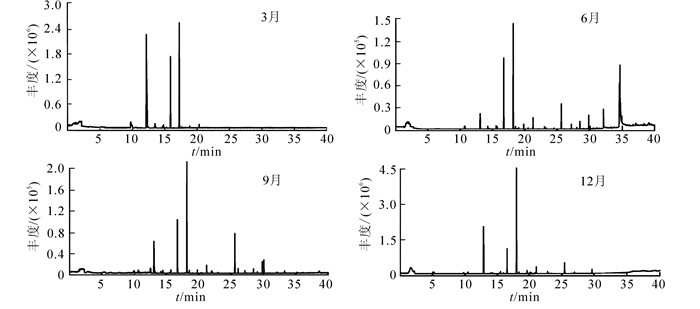
芸香叶片1 a中不同季节释放的挥发性有机物中,共鉴定出29种化合物(图 1,表 1),其中酯类11种,酮类7种,烷类4种,萜烯类,酸类和醇类各2种,以及少量的酚类和其他化合物;主要成分为乙酸壬酯、4-(3, 4-亚甲基二氧基苯基)-2-丁酮、2-壬酮和2-十一(烷)酮等。1 a中检测到叶片释放的挥发性有机物种类数量呈先增多后减少的趋势;6月最多,为18种,9月最少,仅为10种(图 2)。3月挥发性有机物成分检测到酮类、酯类和烷类3类化合物,以酮类最多,其次为酯类;其中2-壬酮的相对含量最高,为48.98%。6月检测到酯类、酮类、萜烯类、酸类、烷类、酚类和醇类7类化合物,以酯类最多,7种。9月检测到的挥发性有机物种类仅为6月的55.56%,酮类和酯类占50.00%,其中2-十一(烷)酮的相对含量最高,为34.13%。12月检测到的挥发物中以酮类为主,占当月总种类的54.55%。1 a中每个季节都可以检测到乙酸壬酯、2-壬酮和2-十一(烷)酮。

图 1 芸香叶片不同季节释放挥发性有机物的总离子流图
Figure 1. TIC of VOCs released from leaves of Ruta graveolens in different seasons
表 1 芸香叶片释放的挥发性有机物成分及其相对含量的季节变化
Table 1. Seasonal variations of components and relative contents of VOCs released from leaves of Ruta graveolens
挥发性有机物 t/min CAS号 分子式 相对含量/% 3月 6月 9月 12月 2-octanone 仲辛酮 9.37 000111-13-7 C8H16O 0.19 - - - 3-hexen-1-ol, acetate, (Z)- (Z)-乙酸-3-己烯-1-醇酯 10.99 003681-71-8 C8H16O2 5.96 2.41 - 2.15 2-nonanone 2-壬酮 13.86 000821-55-6 C9H18O 48.98 6.22 17.74 34.21 cyclohexene, 3, 4-diethenyl-3-methyl- 3, 4-二乙烯基-3-甲基环己烷 14.99 061141-77-3 C11H16 2.47 0.24 1.53 - cyclohexene 环己烯 15.45 000110-83-8 C6H10 - - 2.91 - cyclotrisiloxane, hexamethyl- 六甲基环三硅氮烷 16.02 000541-05-9 C6H18O3Si3 - - - 4.65 1-nonene 壬烯 16.40 000124-11-8 C9H18 - 14.25 - - butanoic acid, 3-hexenyl ester, (Z)- (Z)-丁酸-3-己烯酯 17.13 016491-36-4 C10H18O2 0.63 0.86 - - cyclopentasiloxane, decamethyl- 十甲基环五硅氧烷 17.54 000541-02-6 C10H30O5Si5 - - - 0.47 chloroacetic acid, octyl ester 氯乙酸辛酯 18.45 005451-98-9 C10H19ClO2 - - 5.08 - acetic acid, sec-octyl ester 乙酸仲辛酯 19.07 054515-77-4 C10H20O3 16.93 - - - 2-decanone 2-癸酮 20.05 000693-54-9 C10H20O 1.14 0.52 - 0.09 2-undecanone 2-十一(烷)酮 20.21 000112-12-9 C11H22O 21.89 20.02 34.13 37.88 tridecyl acetate 十三基醋酸 20.39 1000351-76-9 C15H30O2 - - 2.43 - 2-dodecanone 2-十二烷酮 20.83 006175-49-1 C12H24O 0.68 1.23 - 2.04 acetic acid, nonyl ester 乙酸壬酯 21.38 000143-13-5 C11H22O2 0.41 0.61 15.46 10.20 butylated hydroxytoluene 2, 6-二叔丁基对甲基苯酚 23.38 000128-37-0 C15H24O - 0.42 - - 2-acetoxy tridecane 2-乙酰氧基十三烷 25.26 1000245-61-2 C15H30O 0.72 - - - 2-tridecanone 2-十三烷酮 25.71 000593-08-8 C13H26O - 0.78 - 1.30 2-propenoic acid, 3-phenyl-, methyl ester 肉桂酸甲酯 25.78 000103-26-4 C10H10O2 - - - 0.96 7H-furo[3, 2-g][1]benzopyran-7- one 未命名 30.29 000066-97-7 C11H6O3 - - 4.72 - 4-(3, 4-methylenedioxyphenyl) -2-butanone 4-(3, 4-亚甲基二氧基苯基)-2-丁酮 30.64 055418-52-5 C11H12O3 - 5.64 12.89 6.06 1, 3-benzodioxole, 5-(2, 2-dimethylethyl)- 未命名 33.45 028140-80-9 C11H14O2 - - 3.11 - 2H-furo[2, 3-H]-1-benzopyran-2-one 二氢山芹醇 34.46 000523-50-2 C14H14O4 - 0.59 - - (Z, Z)-9, 13-octadecadienoic acid (Z,Z)-9,13-十八烷二烯酸乙酯 34.78 000060-33-4 C18H32O3 - 16.80 - - (Z, Z)-9, 14-octadecadienoic acid (Z,Z)-9,14-十八烷二烯酸乙酯 34.85 000060-33-5 C18H32O4 - 10.81 - - (Z, Z)-9, 15-octadecadienoic acid (Z, Z)-9, 15-十八烷二烯酸乙酯 35.08 000060-33-6 C18H32O5 - 3.29 - - n-Hexadecanoic acid 十六烷酸 38.06 000057-10-3 C16H32O2 - 5.31 - - (Z, Z)-9, 12-octadecadienoic acid (Z, Z)-9, 12-十八烷二烯酸乙酯 40.92 000060-33-3 C18H32O2 - 10.01 - - 说明:“-”为未检测到或不存在 
图 2 1 a中不同季节芸香叶片释放的各类挥发物数量和相对含量的变化
Figure 2. Component numbers and relative contents of classified VOCs from leaves in different seasons of a year
1 a中不同季节,芸香叶片释放的酯类物质的相对含量呈先上升后下降的趋势,在6月最高,为44.78%,12月最低(图 2)。烷类和酮类化合物均呈先下降后上升的趋势,6月其相对含量最低,12月达最高。萜烯类、酸类和醇类化合物只在6月和9月检测到,且6月检测到的萜烯类和酸类化合物相对含量均高于9月;酚类物质仅在6月检测到。叶片释放的主要成分季节变化趋势如下:2-壬酮和2-十一(烷)酮的相对含量随季节呈高-低-高的趋势,而乙酸壬酯则呈现低—高—低的变化趋势。
-
芸香叶片1 d中不同时段释放的挥发性有机物共鉴定出41种(表 2)。其中酯类化合物10种,酮类7种,酸类和醛类各6种,烷类和醇类各4种,以及少量的萜烯类、炔类等化合物;主要成分为乙酸仲辛酯,2-壬酮,2-十一(烷)酮,十六烷酸,油酸等。8:00-20:00叶片释放的挥发性有机物种类呈先增多后减少的趋势(图 3),12:00最多,为22种,20:00最少,为14种。8:00检测到酮类、酯类、酸类和醛类等4类化合物,以酮类最多;但挥发物的相对含量以酸类最高,其中十六烷酸和油酸,分别为39.90%和19.31%。10:00挥发物种类较8:00增加了6种,出现了烷类、醇类和炔类;酯类数量最多,其次为酮类,2类物质的相对含量较8:00大幅上升,酮类占此时挥发物含量的58.11%,其中2-壬酮占35.10%;而酸类化合物的相对含量急剧下降,仅为3.31%。12:00-14:00挥发物种类较10:00变化不大,14:00出现了萜烯类,但相对含量较低。16:00-20:00种类逐渐减少,主要成分和相对含量均以酮类和酯类化合物为主;18:00酸类相对含量在1 d中最低,而此时萜烯类和醛类含量达最高。1 d中,每个时段均可检测到(Z)-乙酸-3-己烯-1-醇酯,乙酸壬酯,2-壬酮,2-癸酮,2-十一(烷)酮,2-十三烷酮和十六烷酸7种物质。
表 2 芸香叶片1 d中不同时间释放的挥发性有机物成分及其相对含量
Table 2. Components and relative contents of VOCs released from leaves of Ruta graveolens in one day
挥发性有机物 t/min 化合物相对含量/% 8:00 10:00 12:00 14:00 16:00 18:00 20:00 3-hexen-1-ol, acetate, (Z)- (Z)-乙酸-3-己烯-1-醇酯 10.99 2.32 0.96 1.35 1.41 1.82 1.32 0.68 2-nonanone 2-壬酮 13.86 3.84 35.10 28.04 31.54 34.76 36.96 36.39 (R)-(-)-5-hexen-2-ol (R)-(-)-5-己烯-2-醇 14.60 - - - - - 0.94 - cyclohexene, 3, 4-diethenyl-3 -methyl- 3, 4-二乙烯基-3-甲基环己烷 14.99 - 1.58 - - 2.02 - 1.92 1, 3 -cycloheptadiene 1, 3-环庚二烯 16.07 - - - - - 1.77 - 3-cyclohexene-1-methanol 3-环己烯-1-甲醇 16.07 - - 1.78 1.83 - - - butanoic acid, 3-hexenyl ester, (Z)- (Z)-丁酸-3-己烯酯 17.13 - 0.38 0.49 0.49 0.44 0.41 0.31 acetic acid, octyl ester 乙酸辛酯 18.69 - 0.42 0.40 0.38 0.40 0.40 - acetic acid, decyl ester 乙酸癸酯 18.69 - - - - - - 0.48 acetic acid, sec-octyl ester 乙酸仲辛酯 19.07 - 21.81 22.65 21.49 23.25 22.88 23.48 2-decanone 2-癸酮 20.05 0.37 1.24 1.11 1.22 1.31 1.56 1.34 2-undecanone 2-十一(烷)酮 20.21 17.24 20.86 22.31 21.46 24.25 23.75 26.73 pentafluoropropionic acid, nonyl ester 未命名 20.63 5.69 - - - - - - 2-dodecanone 2-十二烷酮 20.83 0.36 - - - - - - acetic acid, nonyl ester 乙酸壬酯 21.38 0.52 0.48 0.71 0.42 0.47 0.51 0.63 2-acetoxy tridecane 2-乙酰氧基十三烷 25.26 - 1.01 1.34 1.12 1.22 0.18 1.47 2-tridecanone 2-十三烷酮 25.71 0.90 0.70 0.85 0.73 0.90 1.66 1.03 2-propenoic acid, 3-phenyl-, methyl 肉桂酸甲酯 25.78 0.39 - - - - - - ester 4- (3, 4-methylenedioxyphenyl) -2-bu tanone 4-(3, 4-亚甲基二氧基苯基)-2-丁酮 30.64 3.61 0.21 0.26 0.37 0.30 0.26 - 2H-furo [2, 3-H]-1-benzopyran-2-one 二氢山芹醇 34.46 - 0.11 - 0.12 - - - hexadecanal 棕榈醛 35.58 2.09 - - - - - - tetradecanoic acid 十四烷酸 35.65 0.58 0.32 0.37 0.28 - - - n-hexadecanoic acid 十六烷酸 38.06 39.90 2.46 5.78 2.89 2.69 0.28 1.35 cyclopentadecanone, 2-hydroxy- 2-羟基环十五烷酮 38.45 - - - - 0.33 - - (Z)-ll-hexadecenoic acid 反-11-十六烯酸 38.45 - - - 0.07 - - - 9-tetradecenal, (Z)- (Z)-9-十四烯醛 38.72 1.51 - - - - - - 9-oxabicyclo [6.1.0] nonane 9-双环氧辛烷[6.1.0]壬烷 39.95 - - 0.09 - - - - cis-9-hexadecenoic acid 顺-9-十六碳烯酸 40.29 - - 0.91 - - - - hexadecanoic acid, trimethylsilyl ester 棕榈酸三甲基硅脂 40.81 1.37 - - - - - - (Z, Z)-9, 12-octadecadienoic acid (Z, Z)-9, 12-十八烷二烯酸乙酯 40.92 - 7.23 0.76 1.67 - - - cis, cis-7, 10, -hexadecadienal 顺, 顺-7, 10-十六碳二烯醛 41.27 - - 0.22 - - 5.35 - oleic acid 油酸 41.84 19.31 0.36 7.20 9.23 4.21 - 3.66 (Z) -9, 17-octadecadienal (Z)-9, 17-十八烷烯醛 41.84 - 1.95 0.71 - - - - cis-13-octadecenoic acid 顺-13-十八碳烯酸 42.19 - - 1.08 - - 0.02 - 2-octyl-cyclopropaneoctanal 2-辛基-环丙基辛醛 42.47 - 1.87 1.58 2.35 0.37 - 0.54 cis-9-oxabicyclo [6.1.0] nonane 顺-9-双环氧辛烷[6.1.0]壬烷 42.72 - - - - 0.51 1.00 - Z, Z-10, 12-hexadecadien-1-ol acetate 1-乙酯基-Z, Z-10,12-十六二烯 42.91 - - - 0.24 - - - 1, 2-benzisothiazole, 3-(hexahydro-1H-azepin-1-yl)-, 1, 1-dioxide 未命名 43.02 - 0.23 - - - - - Z, Z-3, 13-octadecadienol-2-methyl 未命名 43.15 - 0.05 - - - - - 7-pentadecyne 7-十五炔 43.29 - 0.69 - - - - - (Z)-9-octadecenal (Z)-9-十八烷烯醛 43.47 - - - 0.71 0.74 0.75 - 说明:“-”为未检测到或不存在 
图 3 1 d中不同时段芸香叶片释放的各类挥发物数量的变化
Figure 3. Component numbers of classified VOCs from leaves in different times of a day
1 d不同时段,芸香叶片释放的酯类化合物的相对含量呈先上升后下降的趋势,10:00达最大;酮类呈先上升后略微下降再上升的趋势,20:00达最大,为65.49%;酸类呈先下降后上升再下降的趋势,8:00最大,为59.80%。烷类和醛类相对含量变化不明显;萜烯类、炔类和醇类的相对含量较少,仅在少数几个时间段出现(表 3)。其中,主要成分日变化趋势如下:乙酸仲辛酯和2-十一(烷)酮的相对含量呈现低-高的变化趋势,十六烷酸则呈现高—低的变化趋势,2-壬酮的相对含量呈现低—高—低—高的变化趋势,油酸呈现出高—低—高—低的变化趋势。
表 3 芸香叶片1 d中不同时段挥发性有机物成分及其相对含量分类统计
Table 3. Classified statistic about components and relative contents of VOCs released from leaves in different time of a day
时刻 相对含量/% 酯 烯 烷 酮 酸 炔 醛 醇 其他 8:00 10.29 - - 26.31 59.80 - 3.60 - - 10:00 31.28 - 2.59 58.11 3.13 0.69 3.82 0.16 0.23 12:00 26.36 - 1.43 52.57 15.34 - 2.52 1.78 - 14:00 25.85 0.24 1.12 55.33 12.46 - 3.05 1.94 - 16:00 26.38 - 3.75 61.86 6.90 - 1.11 - - 18:00 25.51 1.77 1.18 64.20 0.30 - 6.10 0.94 - 20:00 25.57 - 3.39 65.49 5.01 - 0.54 - - 说明:“-”为未检测到或不存在 -
通过对芸香花瓣的SPME/GC/MS总离子流图分析(图 4),扣除本底空气中的杂质,3月花期时,花瓣释放的挥发性有机物中检测出8种挥发性成分,其中酮类化合物最多,有5种,其相对含量占花瓣总挥发物的82.24%;其次为萜烯类化合物,其相对含量为11.20%(表 4)。花瓣中检测到的相对含量最多的挥发性成分是2-十一(烷)酮,为66.10%,其次为2-壬酮和壬烯。
表 4 芸香花瓣释放的挥发性有机物成分及其相对含量
Table 4. Components and relative contents of VOCs released from flowers of Ruta graveolens
挥发性有机物 t/min CAS号 相对含量/% 2-nonanone 2-壬酮 13.86 000821-55-6 13.18 cyclohexene, 3, 4-diethenyl-3-methyl- 3, 4-乙烯基-3-甲基环己烷 14.99 061141-77-3 1.82 1-nonene 壬烯 16.40 000124-11-8 11.20 2-decanone 2-癸酮 20.05 000693-54-9 2.09 2-undecanone 2-十一(烷)酮 20.21 000112-12-9 66.10 tridecyl acetate 乙酸十三酯 20.39 1000351-76-9 4.74 2-dodecanone 2-十二烷酮 20.83 006175-49-1 0.34 2-tridecanone 2-十三烷酮 25.71 000593-08-8 0.53 说明:“-”为未检测到或不存在 -
目前,学者对芸香或芸香科其他植物挥发性有机物的研究多采用水蒸气蒸馏法和溶剂提取法[3-5, 11]。蒸馏法简单易行,适于难溶或不溶于水的成分的提取;但属于非活体提取,长时间高温提取会使某些挥发性物质受到破坏;溶剂提取法是根据相似相溶原理,选择适合的溶剂非常关键,但溶剂中的微量杂质沉淀在提取物中,影响提取物的纯度[19]。通过对比各种提取方法,本研究采用了顶空-固相微萃取采集法,此方法所需样品量少,无需溶剂,操作简化,集采样、萃取、浓集、进样于一体,非常适合芳香植物挥发性成分的研究,已在刺山柑Capparis spinosa,茶Camellia sinensis,黄兰Michelia chmpaka,麦秆菊Helichrysum bracteatum等多种植物挥发物的研究中得到应用[20-23]。
植物释放挥发性有机物的成分和相对含量在同种之间存在差异,与植物的品种、自身结构特点、生理状态和株龄等有密切关系[24-25]。如HADDOUCHI等[4]研究得出阿尔及利亚生长的芸香含有10种挥发性有机物,唐祖年等[15]从芸香挥发油中鉴定出21种成分,徐汉虹等[2]从芸香精油中鉴定出39种成分。本研究在芸香叶片中共鉴定出41种,花瓣中检测到8种,虽然与前人鉴定的结果不尽相同,但通过对比发现,芸香挥发性有机物的主要成分均为2-十一(烷)酮和2-壬酮。主要原因是植物的自身特性决定了不同植物所释放的有机物成分、含量和释放速率不同。
前人[26-31]对侧柏Platycladus orientalis,薄荷Mentha×piperita,红松Pinus koraiensis,桂花Osmanthus fragrans等植物挥发性有机物释放规律的研究表明,挥发物释放的种类和相对含量与采样时温度、湿度、气孔导度、光照以及蒸腾速率等关系密切。一般而言,随着温度、湿度、光照等的提高,植物释放的有机物也会增多。本研究表明,不同季节和1 d中不同时段芸香释放的挥发性有机物的种类均呈先上升后下降的趋势;其中,6月最多,为18种;1 d中12:00最多,为22种。在测试的4个月中,6月光照时间较长,温度较高,达30 ℃左右,植物进入生长旺盛阶段,植物自身旺盛的光合作用为有机物合成提供充足的碳源和还原力,体内有机物合成、代谢加快,强烈的蒸腾作用也为有机物的扩散创造了条件,使挥发性有机物的种类增多[32-34]。说明随着温度、光照等的提高,芸香叶片释放的挥发性有机物的种类也随之增多。另外,研究发现芸香花瓣释放的挥发性有机物种类少于叶片,叶片和花瓣挥发性有机物的主要成分均为酮类化合物,叶片释放的物质中以2-壬酮为主,花瓣中以2-十一(烷)酮为主。芸香释放的挥发性有机物种类多少、相对含量的高低与外界环境以及植物自身条件的密切相关,两者之间关系有待进一步深入研究。
植物释放的挥发性有机物具有一定的药用保健作用。前人[35-36]研究已证实,针叶树释放的萜烯类化合物使人情绪趋于放松状态,使人感觉清新、舒爽和愉悦;柠檬油、雪松醇、龙脑和1, 8-桉叶油能够降低心率和血压;萜烯类和生物碱类物质具有一定的抗菌和消炎作用[3-6, 16]。芸香叶片和花瓣释放的挥发性有机物的主要成分均具有一定的药用价值,可以起到杀虫抑菌效果,在某种程度上对调节人体情绪具有一定的作用。如:2-壬酮具有果香、甜香、皂香及椰子、奶油的气味,2-十一(烷)酮是芸香特有的香气,低浓度时有类似桃子的香气;这些有机物的气味或许可以使人趋于放松状态,从而间接起到调节人体情绪的作用。因此,在植物景观设计中,可以适当栽植芸香,营造保健型的生态植物景观。
Components and variations of volatile organic compounds released from leaves and flowers of Ruta graveolens
-
摘要: 为探究芸香Ruta graveolens叶片和花瓣释放的挥发性有机物的成分及其变化规律,采用顶空-固相微萃取与气相色谱-质谱联用技术,对芸香叶片和花瓣中释放的挥发性有机物成分进行采集,分析叶片和花瓣中挥发性有机物的主要成分、相对含量及叶片在不同时期挥发性有机物成分的差异。结果表明:1 a中不同季节芸香释放的挥发性有机物共鉴定出29种,其中酯类化合物11种,酮类化合物7种,为芸香挥发物的主要成分。1 a中,芸香叶片释放的挥发性有机物的种类呈先增加后减少的趋势;其中,6月检测到的挥发性有机物种类最多,为18种;9月最少,仅为10种。主要成分酯类物质的相对含量呈先上升后下降趋势,酮类化合物呈先下降后上升趋势。在6月的日变化检测中,挥发性有机物的种类呈先增多后减少趋势,依次为12:00 > 10:00 > 14:00 > 16:00,18:00 > 8:00 > 20:00,在12:00达到高峰,以酯类和酮类化合物为主。芸香花瓣释放的挥发性有机物种类少于叶片,主要成分为酮类化合物,以2-十一(烷)酮为主。芸香叶片和花瓣释放的挥发性有机物的主要成分均具有一定的药用保健作用,因此,芸香是营造保健型植物景观的理想材料。Abstract: In order to reveal dynamic changes of volatile organic compounds (VOCs) released in different stages of Ruta graveolens, leaves and flowers were used as materials to determine the components, relative content, and releasing dynamics of VOCs using the methods of headspace solid-phase microextraction (HS-SPME) and gas chromatography-mass spectrometry (GC-MS). Results showed 29 VOCs that were identified in different seasons of the year. The major VOCs released from leaves of R. graveolens were esters (11 components) and ketones (7 components). Components released from leaves over one year were the most in June (18 components) and the least in September (10 components). Relative content for esters was the most in June and the least in December, but for ketones was the most in December and the least in June. In the course of one day, VOC components were the order at the following daytime hours:12:00 > 10:00 > 14:00 > 16:00 > 18:00 > 8:00 > 20:00; values at 12:00 were maximum, mainly esters and ketones. Major components released from flowers (esters having 2-undecanone as the primary VOC) were much less than from leaves. The major components released from leaves and flowers all had medical and health care benefits. Thus, R. graveolens, an admirable ornamental plant, could be a healthy and aromatic plant for improving a landscape.
-
Key words:
- batany /
- Ruta graveolens /
- volatile organic compounds /
- seasonal variation /
- diurnal variation
-
表 1 芸香叶片释放的挥发性有机物成分及其相对含量的季节变化
Table 1. Seasonal variations of components and relative contents of VOCs released from leaves of Ruta graveolens
挥发性有机物 t/min CAS号 分子式 相对含量/% 3月 6月 9月 12月 2-octanone 仲辛酮 9.37 000111-13-7 C8H16O 0.19 - - - 3-hexen-1-ol, acetate, (Z)- (Z)-乙酸-3-己烯-1-醇酯 10.99 003681-71-8 C8H16O2 5.96 2.41 - 2.15 2-nonanone 2-壬酮 13.86 000821-55-6 C9H18O 48.98 6.22 17.74 34.21 cyclohexene, 3, 4-diethenyl-3-methyl- 3, 4-二乙烯基-3-甲基环己烷 14.99 061141-77-3 C11H16 2.47 0.24 1.53 - cyclohexene 环己烯 15.45 000110-83-8 C6H10 - - 2.91 - cyclotrisiloxane, hexamethyl- 六甲基环三硅氮烷 16.02 000541-05-9 C6H18O3Si3 - - - 4.65 1-nonene 壬烯 16.40 000124-11-8 C9H18 - 14.25 - - butanoic acid, 3-hexenyl ester, (Z)- (Z)-丁酸-3-己烯酯 17.13 016491-36-4 C10H18O2 0.63 0.86 - - cyclopentasiloxane, decamethyl- 十甲基环五硅氧烷 17.54 000541-02-6 C10H30O5Si5 - - - 0.47 chloroacetic acid, octyl ester 氯乙酸辛酯 18.45 005451-98-9 C10H19ClO2 - - 5.08 - acetic acid, sec-octyl ester 乙酸仲辛酯 19.07 054515-77-4 C10H20O3 16.93 - - - 2-decanone 2-癸酮 20.05 000693-54-9 C10H20O 1.14 0.52 - 0.09 2-undecanone 2-十一(烷)酮 20.21 000112-12-9 C11H22O 21.89 20.02 34.13 37.88 tridecyl acetate 十三基醋酸 20.39 1000351-76-9 C15H30O2 - - 2.43 - 2-dodecanone 2-十二烷酮 20.83 006175-49-1 C12H24O 0.68 1.23 - 2.04 acetic acid, nonyl ester 乙酸壬酯 21.38 000143-13-5 C11H22O2 0.41 0.61 15.46 10.20 butylated hydroxytoluene 2, 6-二叔丁基对甲基苯酚 23.38 000128-37-0 C15H24O - 0.42 - - 2-acetoxy tridecane 2-乙酰氧基十三烷 25.26 1000245-61-2 C15H30O 0.72 - - - 2-tridecanone 2-十三烷酮 25.71 000593-08-8 C13H26O - 0.78 - 1.30 2-propenoic acid, 3-phenyl-, methyl ester 肉桂酸甲酯 25.78 000103-26-4 C10H10O2 - - - 0.96 7H-furo[3, 2-g][1]benzopyran-7- one 未命名 30.29 000066-97-7 C11H6O3 - - 4.72 - 4-(3, 4-methylenedioxyphenyl) -2-butanone 4-(3, 4-亚甲基二氧基苯基)-2-丁酮 30.64 055418-52-5 C11H12O3 - 5.64 12.89 6.06 1, 3-benzodioxole, 5-(2, 2-dimethylethyl)- 未命名 33.45 028140-80-9 C11H14O2 - - 3.11 - 2H-furo[2, 3-H]-1-benzopyran-2-one 二氢山芹醇 34.46 000523-50-2 C14H14O4 - 0.59 - - (Z, Z)-9, 13-octadecadienoic acid (Z,Z)-9,13-十八烷二烯酸乙酯 34.78 000060-33-4 C18H32O3 - 16.80 - - (Z, Z)-9, 14-octadecadienoic acid (Z,Z)-9,14-十八烷二烯酸乙酯 34.85 000060-33-5 C18H32O4 - 10.81 - - (Z, Z)-9, 15-octadecadienoic acid (Z, Z)-9, 15-十八烷二烯酸乙酯 35.08 000060-33-6 C18H32O5 - 3.29 - - n-Hexadecanoic acid 十六烷酸 38.06 000057-10-3 C16H32O2 - 5.31 - - (Z, Z)-9, 12-octadecadienoic acid (Z, Z)-9, 12-十八烷二烯酸乙酯 40.92 000060-33-3 C18H32O2 - 10.01 - - 说明:“-”为未检测到或不存在 表 2 芸香叶片1 d中不同时间释放的挥发性有机物成分及其相对含量
Table 2. Components and relative contents of VOCs released from leaves of Ruta graveolens in one day
挥发性有机物 t/min 化合物相对含量/% 8:00 10:00 12:00 14:00 16:00 18:00 20:00 3-hexen-1-ol, acetate, (Z)- (Z)-乙酸-3-己烯-1-醇酯 10.99 2.32 0.96 1.35 1.41 1.82 1.32 0.68 2-nonanone 2-壬酮 13.86 3.84 35.10 28.04 31.54 34.76 36.96 36.39 (R)-(-)-5-hexen-2-ol (R)-(-)-5-己烯-2-醇 14.60 - - - - - 0.94 - cyclohexene, 3, 4-diethenyl-3 -methyl- 3, 4-二乙烯基-3-甲基环己烷 14.99 - 1.58 - - 2.02 - 1.92 1, 3 -cycloheptadiene 1, 3-环庚二烯 16.07 - - - - - 1.77 - 3-cyclohexene-1-methanol 3-环己烯-1-甲醇 16.07 - - 1.78 1.83 - - - butanoic acid, 3-hexenyl ester, (Z)- (Z)-丁酸-3-己烯酯 17.13 - 0.38 0.49 0.49 0.44 0.41 0.31 acetic acid, octyl ester 乙酸辛酯 18.69 - 0.42 0.40 0.38 0.40 0.40 - acetic acid, decyl ester 乙酸癸酯 18.69 - - - - - - 0.48 acetic acid, sec-octyl ester 乙酸仲辛酯 19.07 - 21.81 22.65 21.49 23.25 22.88 23.48 2-decanone 2-癸酮 20.05 0.37 1.24 1.11 1.22 1.31 1.56 1.34 2-undecanone 2-十一(烷)酮 20.21 17.24 20.86 22.31 21.46 24.25 23.75 26.73 pentafluoropropionic acid, nonyl ester 未命名 20.63 5.69 - - - - - - 2-dodecanone 2-十二烷酮 20.83 0.36 - - - - - - acetic acid, nonyl ester 乙酸壬酯 21.38 0.52 0.48 0.71 0.42 0.47 0.51 0.63 2-acetoxy tridecane 2-乙酰氧基十三烷 25.26 - 1.01 1.34 1.12 1.22 0.18 1.47 2-tridecanone 2-十三烷酮 25.71 0.90 0.70 0.85 0.73 0.90 1.66 1.03 2-propenoic acid, 3-phenyl-, methyl 肉桂酸甲酯 25.78 0.39 - - - - - - ester 4- (3, 4-methylenedioxyphenyl) -2-bu tanone 4-(3, 4-亚甲基二氧基苯基)-2-丁酮 30.64 3.61 0.21 0.26 0.37 0.30 0.26 - 2H-furo [2, 3-H]-1-benzopyran-2-one 二氢山芹醇 34.46 - 0.11 - 0.12 - - - hexadecanal 棕榈醛 35.58 2.09 - - - - - - tetradecanoic acid 十四烷酸 35.65 0.58 0.32 0.37 0.28 - - - n-hexadecanoic acid 十六烷酸 38.06 39.90 2.46 5.78 2.89 2.69 0.28 1.35 cyclopentadecanone, 2-hydroxy- 2-羟基环十五烷酮 38.45 - - - - 0.33 - - (Z)-ll-hexadecenoic acid 反-11-十六烯酸 38.45 - - - 0.07 - - - 9-tetradecenal, (Z)- (Z)-9-十四烯醛 38.72 1.51 - - - - - - 9-oxabicyclo [6.1.0] nonane 9-双环氧辛烷[6.1.0]壬烷 39.95 - - 0.09 - - - - cis-9-hexadecenoic acid 顺-9-十六碳烯酸 40.29 - - 0.91 - - - - hexadecanoic acid, trimethylsilyl ester 棕榈酸三甲基硅脂 40.81 1.37 - - - - - - (Z, Z)-9, 12-octadecadienoic acid (Z, Z)-9, 12-十八烷二烯酸乙酯 40.92 - 7.23 0.76 1.67 - - - cis, cis-7, 10, -hexadecadienal 顺, 顺-7, 10-十六碳二烯醛 41.27 - - 0.22 - - 5.35 - oleic acid 油酸 41.84 19.31 0.36 7.20 9.23 4.21 - 3.66 (Z) -9, 17-octadecadienal (Z)-9, 17-十八烷烯醛 41.84 - 1.95 0.71 - - - - cis-13-octadecenoic acid 顺-13-十八碳烯酸 42.19 - - 1.08 - - 0.02 - 2-octyl-cyclopropaneoctanal 2-辛基-环丙基辛醛 42.47 - 1.87 1.58 2.35 0.37 - 0.54 cis-9-oxabicyclo [6.1.0] nonane 顺-9-双环氧辛烷[6.1.0]壬烷 42.72 - - - - 0.51 1.00 - Z, Z-10, 12-hexadecadien-1-ol acetate 1-乙酯基-Z, Z-10,12-十六二烯 42.91 - - - 0.24 - - - 1, 2-benzisothiazole, 3-(hexahydro-1H-azepin-1-yl)-, 1, 1-dioxide 未命名 43.02 - 0.23 - - - - - Z, Z-3, 13-octadecadienol-2-methyl 未命名 43.15 - 0.05 - - - - - 7-pentadecyne 7-十五炔 43.29 - 0.69 - - - - - (Z)-9-octadecenal (Z)-9-十八烷烯醛 43.47 - - - 0.71 0.74 0.75 - 说明:“-”为未检测到或不存在 表 3 芸香叶片1 d中不同时段挥发性有机物成分及其相对含量分类统计
Table 3. Classified statistic about components and relative contents of VOCs released from leaves in different time of a day
时刻 相对含量/% 酯 烯 烷 酮 酸 炔 醛 醇 其他 8:00 10.29 - - 26.31 59.80 - 3.60 - - 10:00 31.28 - 2.59 58.11 3.13 0.69 3.82 0.16 0.23 12:00 26.36 - 1.43 52.57 15.34 - 2.52 1.78 - 14:00 25.85 0.24 1.12 55.33 12.46 - 3.05 1.94 - 16:00 26.38 - 3.75 61.86 6.90 - 1.11 - - 18:00 25.51 1.77 1.18 64.20 0.30 - 6.10 0.94 - 20:00 25.57 - 3.39 65.49 5.01 - 0.54 - - 说明:“-”为未检测到或不存在 表 4 芸香花瓣释放的挥发性有机物成分及其相对含量
Table 4. Components and relative contents of VOCs released from flowers of Ruta graveolens
挥发性有机物 t/min CAS号 相对含量/% 2-nonanone 2-壬酮 13.86 000821-55-6 13.18 cyclohexene, 3, 4-diethenyl-3-methyl- 3, 4-乙烯基-3-甲基环己烷 14.99 061141-77-3 1.82 1-nonene 壬烯 16.40 000124-11-8 11.20 2-decanone 2-癸酮 20.05 000693-54-9 2.09 2-undecanone 2-十一(烷)酮 20.21 000112-12-9 66.10 tridecyl acetate 乙酸十三酯 20.39 1000351-76-9 4.74 2-dodecanone 2-十二烷酮 20.83 006175-49-1 0.34 2-tridecanone 2-十三烷酮 25.71 000593-08-8 0.53 说明:“-”为未检测到或不存在 -
[1] 曾建飞.中国植物志:第43卷第2分册[M].北京:科学出版社, 1997. [2] 徐汉虹, 赵善欢, 周俊, 等.芸香精油的化学成分和杀虫活性初探[J].天然产物研究与开发, 1994, 6(4):56-61. XU Hanhong, ZHAO Shanhuan, ZHOU Jun, et al. Preliminary studies on insecticidal activity of the rue oil and analysis of its components[J]. Nat Product Res Dev, 1994, 6(4):56-61. [3] REDDY D N, ALRAJAB A J. Chemical composition, antibacterial and antifungal activities of Ruta graveolens L. volatile oils[J]. Cog Chem, 2016, 2(1):1-11. [4] HADDOUCHI F, CHAOUCHE T M, ZAOUALI Y, et al. Chemical composition and antimicrobial activity of the essential oils from four Ruta species growing in Algeria[J]. Food Chem, 2013, 141(1):253-258. [5] 顾关云, 蒋昱.芸香的化学成分与药理活性[J].国外医药:植物药分册, 2003, 18(2):47-50. GU Guanyun, JIANG Yu. Rue chemical constituents and pharmacological activities[J]. World Phytom, 2003, 18(2):47-50. [6] 袁海梅, 邱露, 谢贞建, 等.花椒属植物生物碱类成分及其药理活性研究进展[J].中国中药杂志, 2015, 40(23):4573-4584. YUAN Haimei, QIU Lu, XIE Zhenjian, et al. Research progress on alkaloids constituents from Zanthoxylum and their pharmacological activities[J]. China J Chin Mater Med, 2015, 40(23):4573-4584. [7] 刘文哲, 胡正海.中国芸香科植物叶分泌囊比较解剖学研究[J].植物分类学报, 1998, 36(2):119-127. LIU Wenzhe, HU Zhenghai. Comparative anatomy of secretory cavities in leaves of the Rutaceae in China[J]. Acta Phytotaxon Sin, 1998, 36(2):119-127. [8] 刘文哲, 胡正海.芸香科植物茎分泌囊的研究[J].西北植物学报, 1999, 19(3):456-460. LIU Wenzhe, HU Zhenghai. Studies on the secretory cavity of stems in Rutaceae[J]. Acta Bot Boreal-Occident Sin, 1999, 19(3):456-460. [9] 罗焜, 陈士林, 陈科力, 等.基于芸香科的植物通用DNA条形码研究[J].中国科学C辑:生命科学, 2010, 40(4):342-358. LUO Kun, CHEN Shilin, CHEN Keli, et al. Assessment of candidate plant DNA barcodes using the Rutaceae family[J]. Sci China Life Sci, 2010, 40(4):342-358. [10] 柴素芬, 陈兆贵, 洪彩霞.芸香科药用植物ISSR-PCR反应体系的建立及优化[J].安徽农业科学, 2008, 36(33):14433-14435. CHAI Sufen, CHEN Zhaogui, HONG Caixia. Construction and optimization of ISSR reaction system of medicinal Rutaceae plants[J]. J Anhui Agric Sci, 2008, 36(33):14433-14435. [11] 王兆玉, 郑家欢, 黎恩立, 等. 3种芸香科植物果皮挥发油成分GC-MS分析与比较[J].中药材, 2016, 39(5):1071-1074. WANG Zhaoyu, ZHENG Jiahuan, LI Enli, et al. GC-MS analysis and comparison of VOCs of 3 Rutaceae plants peel[J]. J Chin Med Mater, 2016, 39(5):1071-1074. [12] 刘向前, 魏圣淑, 白完淑, 等.中国产和韩国产芸香科植物的成分比较研究[J].中草药, 2003, 34(7):586-589. LIU Xiangqian, WEI Shengshu, BAI Wanshu, et al. Studies on constituents of Rutaceae plants growing in China comparied with those in Korea[J]. Chin Trad Herb Drug, 2003, 34(7):586-589. [13] 杨序成, 侯娜.顶空固相微萃取-气质联用法测定日本花椒挥发油成分分析[J].贵州科学, 2017, 35(1):94-96. YANG Xucheng, HOU Na. Composition analysis of volatile oil of Zanthoxylum japonica by SPMEGC-MS[J]. Guizhou Sci, 2017, 35(1):94-96. [14] 吴娇, 苗辉, 邝芷琪, 等.芸香科植物杀虫及抑菌活性研究进展[J].中国植保导刊, 2015, 35(10):18-26. WU Jiao, MIAO Hui, KUANG Zhiqi, et al. Research progress on insecticidal and antifungal activity of Rutaceae plants[J]. China Plant Prot, 2015, 35(10):18-26. [15] 唐祖年, 杨月, 杨扬, 等.芸香挥发油GC-MS分析及其生物活性研究[J].中国现代应用药学, 2011, 28(9):834-838. TANG Zunian, YANG Yue, YANG Yang, et al. Chemical composition and biological activity of the essential oil of Ruta graveolens[J]. Chin J Mod Appl Pharm, 2011, 28(9):834-838. [16] IVANOVA A, MIKHOVA B, NAJDENSKI H, et al. Antimicrobial and cytotoxic activity of Ruta graveolens[J]. Fitoterapia, 2005, 76(3/4):344-347. [17] de FEO V, de SIMONE F, SENATORE F. Potential allelochemicals from the essential oil of Ruta graveolens[J]. Phytochemistry, 2002, 61(5):573-578. [18] DZHURMANSKI A, ZHEKOVA G, ANGELOVA D. Accumulation dynamic of Ruta graveolens L. essential oil[J]. Agric Sci Technol, 2011, 3(4):343-345. [19] 蒋冬月, 李永红.植物挥发性有机物的研究进展[J].黑龙江农业科学, 2011(11):143-149. JIANG Dongyue, LI Yonghong. Review on volatile organic compounds of plant[J]. Heilongjiang Agric Sci, 2011(11):143-149. [20] ROMEO V, ZⅡNO M, GIUFFRIDA D, et al. Flavour profile of capers (Capparis spinosa L.) from the Eolian Archipelago by HS-SPME/GC-MS[J]. Food Chem, 2007, 101(3):1272-1278. [21] WU Yuanshuang, LÜ Shidong, LIAN Ming, et al. Study of characteristic aroma components of baked Wujiatai green tea by HS-SPME/GC-MS combined with principal component analysis[J]. CyTA J Food, 2016, 14(3):1-10. [22] 蒋冬月, 李永红, 夏兵, 等.黄兰叶片和花瓣挥发性成分及其抑菌效果[J].东北林业大学学报, 2012, 40(5):71-74. JIANG Dongyue, LI Yonghong, XIA Bing, et al. Components and antibacterial functions of volatile organic compounds from leaves and flowers of Michelia chmpaka[J]. J Northeast For Univ, 2012, 40(5):71-74. [23] GIULIANI C, LAZZARO L, CALAMASSI R, et al. A volatolomic approach for studying plant variability:the case of selected Helichrysum species (Asteraceae)[J]. Phytochemistry, 2016, 130:128. [24] BENJAMIN M T, WINER A M. Estimating the ozone-forming potential of urban trees and shrubs[J]. Atmos Environ, 1998, 32(1):53-68. [25] OWEN S M, HARLEY P, GUENTHER A, et al. Light dependency of VOC emissions from selected Mediterranean plant species[J]. Atmos Environ, 2002, 36(19):3147-3159. [26] 杨伟伟, 王成, 郄光发, 等.北京西山春季侧柏游憩林内挥发物成分及其日变化规律[J].林业科学研究, 2010, 23(3):462-466. YANG Weiwei, WANG Cheng, QIE Guangfa, et al. Compounds and diurnal variation of VOCs of Platycladus orientalis recreation forest in Beijing western hills in spring[J]. For Res, 2010, 23(3):462-466. [27] 李娟, 王成, 彭镇华, 等.侧柏春季挥发物浓度日变化规律及其影响因子研究[J].林业科学研究, 2011, 24(1):82-90. LI Juan, WANG Cheng, PENG Zhenhua, et al. The diurnal variation and influence factors of VOC of Platycladus orientalis in spring[J]. For Res, 2011, 24(1):82-90. [28] GERSHENZON J, McCONKEY M E, CROTEAU R B. Regulation of monoterpene accumulation in leaves of peppermint[J]. Plant Physiol, 2000, 122(1):205-213. [29] SON Y S, KIM K J, JUNG I H, et al. Seasonal variations and emission fluxes of monoterpene emitted from coniferous trees in East Asia:focused on Pinus rigida and Pinus koraiensis[J]. J Atmos Chem, 2015, 72(1):27-41. [30] SAATHOFF H, NAUMANN K H, HLER O M, et al. Temperature dependence of yields of secondary organic aerosols from the ozonolysis of α-pinene and limonene[J]. Atmos Chem Physics, 2009, 9(5):1551-1577. [31] 蔡宙霏, 陈雅奇, 许馨露, 等. 4个桂花品种开花进程释放VOCs动态变化分析[J].浙江农林大学学报, 2017, 34(4):608-619. CAI Zhoufei, CHEN Yaqi, XU Xinlu, et al. Changes of volatile organic compounds released during flowering in four Osmanthus fragrans cultivar groups[J]. J Zhejiang A & F Univ, 2017, 34(4):608-619. [32] STAUDT M, LHOUTELLIER L. Monoterpene and sesquiterpene emissions from Quercus coccifera exhibit interacting responses to light and temperature[J]. Biogeosci Discuss, 2011, 8(9):5691-5728. [33] SHARKEY T D, LORETO F. Water stress, temperature, and light effects on the capacity for isoprene emission and photosynthesis of Kudzu leaves[J]. Oecologia, 1993, 95(3):328-333. [34] HARLEY P, GUENTHER A, ZIMMERMAN P. Effects of light, temperature and canopy position on net photosynthesis and isoprene emission from sweetgum (Liquidambar styraciflua) leaves[J]. Tree Physiol, 1996, 16(1/2):25-32. [35] 高岩. 北京市绿化树木挥发性有机物释放动态及其对人体健康的影响[D]. 北京: 北京林业大学, 2005. GAO Yan. Releasing Variation and Effects on Human Health of Volatile Organic Compounds from Landscape Trees in Beijing[D]. Beijing: Beijing Forestry University, 2005. [36] 王艳英, 王成, 蒋继宏, 等.侧柏、香樟枝叶挥发物对人体生理的影响[J].城市环境与城市生态, 2010, 23(3):30-32, 37. WANG Yanying, WANG Cheng, JIANG Jihong, et al. Effect of VOCs from branch and leaf of Piatycladus orientalis and Cinnamomum camphora on human physiology[J]. Urban Environ Urban Ecol, 2010, 23(3):30-32, 37. -

-
链接本文:
https://zlxb.zafu.edu.cn/article/doi/10.11833/j.issn.2095-0756.2018.03.025






 下载:
下载: