-
在昆虫与植物的关系中,植物化学成分、形态特征和各种环境因子等影响着昆虫对寄主选择,其中寄主植物释放的挥发性物质起着关键的作用[1]。植物的特异性化学物质诱导昆虫产生多种行为,如寄主定向、产卵行为、取食行为等[2]。研究植食性昆虫对寄主植物气味的趋性有助于了解植食性昆虫寄主选择的机制[3],探索新的害虫防控措施,在利用天然活性物质、抗虫育种和生物防治中具有积极意义[4]。大量文献报道:昆虫利用植物所释放的化学信号物质进行寄主定向。如马铃薯甲虫Leptinotarsa decemlineata依靠马铃薯Solanum tuberosum叶片气味产生定向选择[5],十字花科Brassicaceae所释放的芥子油对小菜蛾Plutella xylostella具有吸引作用[6],寄主白菜Brassica pekinensis气味对桃蚜Myzus persicae有明显的引诱作用[7],烟草甲Lasioderma serricorne对不同辣椒Capsicum annuum挥发物表现出极显著的趋向作用[8]。根据缨翅目Thysanoptera蓟马科Thripidae昆虫借助植物气味寻找寄主的特性,将烟碱乙酸酯和苯甲醛混合在一起制成诱芯在田间使用,能够准确预测花蓟马的发生及为害时期,并且能大量诱杀蓟马成虫[9]。天牛科Cerambycidae、郭公甲科Cleroidea、象甲科Curculionidae和小蠹虫科Scolytidae的昆虫能被松针叶的挥发物α-蒎烯、β-蒎烯、月桂烯和莰烯所引诱[10]。但有关寄主植物气味是否对绿带妒尺蛾Phthonoloba viridifasciata存在引诱作用还未见报道。
绿带妒尺蛾隶属于鳞翅目Lepidoptera尺蛾科Geometridae,是桫椤Alsophila spinulosa和小黑桫椤Alsophila metteniana的食叶昆虫之一[11]。在贵州赤水桫椤国家级自然保护区内1 a发生4~5代,幼虫以取食嫩叶为主,虫害暴发期,可将嫩叶和成叶取食殆尽,严重影响了桫椤的正常发育和繁殖[12]。桫椤和小黑桫椤隶属于桫椤科Cyatheaceae桫椤属Alsophila蕨类植物,其中桫椤是世界濒危植物[13-14]、中国二级珍惜保护植物[15]。目前桫椤和小黑桫椤对绿带妒尺蛾有效防治研究较少。避免使用化学农药带来的问题,寻找绿色的防控措施对促进有害生物的可持续控制意义重大[16]。研究绿带妒尺蛾幼虫对桫椤和小黑桫椤的趋向行为和寄主植物叶片的化学成分,能为桫椤和小黑桫椤的虫害防控筛选合适的引诱剂提供依据。
-
绿带妒尺蛾幼虫采自贵州赤水桫椤国家级自然保护区。将野外在桫椤上采集到的初孵幼虫放置于SPX型智能生化培养箱(SPX-280, 宁波江南仪器厂)内饲喂成2龄幼虫,饲养条件:温度(26±1) ℃,相对湿度为(70±10)%,光周期为14 h 光照/10 h黑暗(7:00—20:00为光照期,20:00—7:00为黑暗期)。预实验表明:初孵幼虫活动能力弱,而幼虫虫龄越大,由于取食经历会形成记忆对饲养植物偏爱,故选择2龄幼虫进行饥饿处理(24 h)作为待测幼虫。
-
直径12.0 cm的培养皿、直径1.5 cm的打孔器、绿色滤纸、镊子、圆形滤纸、养虫盒(长14.3 cm×宽8.5 cm×高4.0 cm)、黑色塑料薄膜、正己烷有机溶剂(天津市科密欧化学试剂有限公司)、HP6890/5975C气质联用仪GC/MS(美国安捷伦公司)、150 mL锥形瓶、胶头滴管等。
-
采样点位于贵州赤水桫椤国家级自然保护区内大水沟(28°25′10.69″N,106°01′14.85″E),海拔为614 m。供试植物为桫椤、小黑桫椤、中华复叶耳蕨Arachniodes chinensis和肾蕨Nephrolepis auriculata。将野外采集到的当年生新鲜叶片带回室内低温保存,测定前将4种蕨类植物嫩叶用蒸馏水冲洗干净后,打孔器制作成1.5 cm大小的植物叶碟。对照为非寄主植物中华复叶耳蕨和肾蕨,以及绿色叶碟(外形与桫椤和小黑桫椤叶碟大小相同的绿色滤纸)。
-
光照和黑暗条件下,桫椤和小黑桫椤叶碟分别与绿色叶碟组合,各取2片叶碟共4个处理,1次1头,每处理重复测定60头。
-
黑暗条件下,桫椤和小黑桫椤分别与肾蕨和中华复叶耳蕨组合,共4个处理,1次1头,每处理重复测定50头;桫椤和小黑桫椤配对,各取2片叶碟,1次1头,重复测定65头。
-
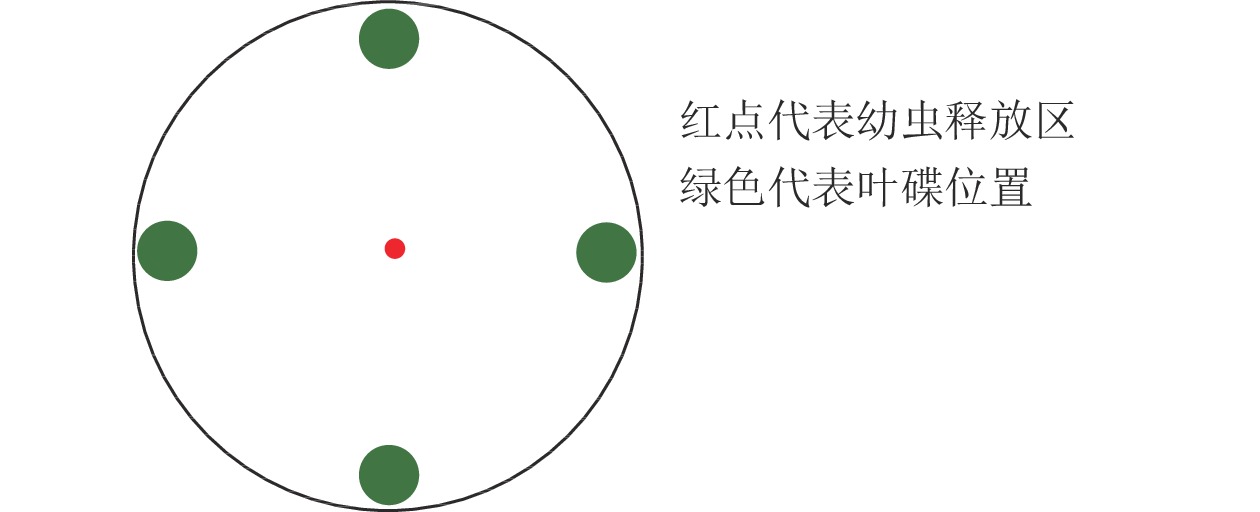
黑暗条件下,4种蕨类植物共存时,各取2片叶碟,“十字”交叉等距离放置于培养皿底部边缘,共1个处理,1次1头,重复测定120头。
-
利用培养皿[17]测定绿带妒尺蛾幼虫对不同叶碟的选择频次。选用直径为12.0 cm的培养皿,在底部铺垫相同直径的圆形滤纸,测定前在滤纸上均匀喷洒少量蒸馏水保湿。将制作好的叶碟(图1)放置,以“十”字交叉排列等距离放在培养皿的底部边缘。放置完成后,在培养皿中央接入1头已饥饿的绿带妒尺蛾幼虫,以幼虫接触叶碟并停留(3 min)作为选择的标准(一直停留在待选择区域的幼虫则记为无效选择,不计数),每头观察15 min,记录观察幼虫对不同叶碟的趋向选择情况,每次测定结束后更换新的试虫,并清理培养皿中的实验材料,蒸馏水清洗干燥后用于下次测定[18]。
整个实验在室内进行,测定时间为13:00—17:00。黑暗条件将培养皿用黑色塑料薄膜遮盖。
-
称取5 g桫椤和小黑桫椤新鲜嫩叶,分别放置于150 mL锥形瓶中。用胶头滴管提取10 mL的正己烷加入瓶中浸湿叶片,同时每间隔5 min振荡1次,30 min后加入2 mL的正己烷(分3次润洗)经无水硫酸钠过滤,获得粗提物。重复3次。放入−20 ℃的冰箱内密封低温保存。进样前,用99.99%的高纯氮气浓缩至1 mL。
-
使用HP6890/5975C气质联用仪 GC/MS(美国安捷伦公司)对2种桫椤植物嫩叶粗提物样品进行化学成分分析。色谱柱为Agilent HP-5MS(60 m×0.25 mm×0.25 μm)弹性石英毛细管柱,初始温度70 ℃(保留2 min),以5 ℃·min−1升温至110 ℃,再以10 ℃·min−1升温至310 ℃,运行时间为45 min;汽化室温度为250 ℃;载气为高纯氦气(99.999%);柱前压为0.127 5 MPa,载气流量为1.0 mL·min−1,不分流,溶剂延迟时间为6 min。离子源为电子轰击离子源(EI),离子源温度为230 ℃;四极杆温度为150 ℃;电子能量为70 eV;发射电流为34.6 μA;倍增器电压为1 729V;接口温度为280 ℃;质量范围为29~500。
-
用Excel统计数据,用R语言进行数据分析,使用卡方检验比较绿带妒尺蛾幼虫对不同处理的趋性选择,用Origin 2021作图。对总离子流图中的各峰经质谱计算机数据系统检索及核对Nist17和Wiley275标准质谱数据,用峰面积归一化法测定了各化学成分的相对含量。选择率=(选择虫数/测试虫总数)×100%[10]
-
如表1所示:无论在光照或黑暗条件下,绿带妒尺蛾幼虫对2种蕨类植物叶碟的选择频次均极显著大于对绿色叶碟的选择频次(P<0.01),说明寄主植物气味可对绿带妒尺蛾幼虫产生引诱作用。光照环境下对桫椤和小黑桫椤的选择率略低于黑暗环境下的选择率,可能是在视觉作用下,绿带妒尺蛾幼虫被外形相似的绿色叶碟所“蒙骗”,导致光照条件下选择绿色叶碟的频次略大于在黑暗下的选择频次,视觉可能在寄主选择中也发挥着一定得作用。
表 1 绿带妒尺蛾幼虫对桫椤和小黑桫椤叶碟的趋性选择
Table 1. Selection frequencies of P. viridifasciata larvae towards A. spinulosa and A. metteniana leaf discs respectively
组合 条件 选择
频次选择
率/%χ2 P 显著性 桫椤/绿色叶碟 光照 41 68.7 8.07 0.004 5 ** 黑暗 49 81.7 24.07 9.306E−07 ** 小黑桫椤/绿色叶碟 光照 46 78.3 17.07 3.609E−05 ** 黑暗 48 80.0 21.60 3.359E−06 ** 说明:组合为实验组/对照组,绿色叶碟为对照,n=60; **表示差异极显著(P<0.01) -
由表2显示:黑暗条件下,绿带妒尺蛾幼虫对桫椤分别与中华复叶耳蕨、肾蕨组合,小黑桫椤分别与中华复叶耳蕨、肾蕨组合的趋性选择均差异极显著(P<0.01),说明绿带妒尺蛾幼虫能依靠寄主植物气味信息准确识别寄主。绿带妒尺蛾幼虫对桫椤和小黑桫椤的选择频次无显著差异(P>0.05),说明2种植物的气味都可以对绿带妒尺蛾幼虫产生一定的吸引作用。
表 2 绿带妒尺蛾幼虫在不同蕨类植物比较时的选择频次
Table 2. Selection frequencies of P. viridifasciata larvae towards combinations of different ferns
组合 选择频次 χ2 P 显著性 实验组 对照组 桫椤/中华复叶耳蕨 41 9 20.48 6.026E−06 ** 桫椤/肾蕨 37 13 11.52 0.000 6 ** 小黑桫/中华复叶耳蕨 39 11 15.68 7.501E−05 ** 小黑桫椤/肾蕨 37 13 11.52 0.000 6 ** 桫椤/小黑桫椤 36 29 0.75 0.385 3 ns 说明:组合为实验组/对照组,n=50;**表示差异极显著 (P<0.01);ns表示差异不显著(P>0.05) -
如表3所示:黑暗条件下,在4种蕨类叶碟共存时,绿带妒尺蛾幼虫对不同蕨类叶碟的选择频次差异显著(P<0.05),其中对桫椤的选择频次最高,占总频次的38.3%。这说明在多植物环境下,绿带妒尺蛾幼虫可以准确判断最适宜的寄主植物,桫椤叶片所释放的挥发物对绿带妒尺蛾幼虫的定向选择具有更强烈的引诱作用。
表 3 绿带妒尺蛾幼虫在4种蕨类植物共存条件下的选择反应
Table 3. Selection responses of P. viridifasciata larvae towards different ferns presented together
植物 选择频次 选择率/% χ2 P 显著性 桫椤 46 38.3 15.8 0.001 2 ** 小黑桫椤 34 28.3 中华复叶耳蕨 21 17.6 肾蕨 19 15.8 说明:n=120;*表示差异极显著(P<0.01) -
通过GC-MS分析得到桫椤和小黑桫椤嫩叶浸提物的总离子流图TIC(图2)。初步确定2种桫椤的化学组分主要是烃类、醇类、醛类等,共检测出34种化合物。其中桫椤的化合物成分有23种,小黑桫椤的化合物成分有19种,同时2种桫椤共有物质有8种。由表4可知:2种桫椤的主要成分为烃类,桫椤中相对含量最高的是二十五烷(7.75%),小黑桫椤相对含量最高的是正二十七烷(8.84%)。其次是醇类,桫椤有5种,小黑桫椤2种,桫椤和小黑桫椤共有 γ-谷甾醇、豆甾烷醇,γ-谷甾醇(5.25%)和豆甾烷醇(8.21%)在小黑桫椤的挥发性成分中所占比例要高于桫椤,而叶醇(3.04%)仅在桫椤中存在。醛类、有机酸类、脂类、胺类在桫椤和小黑桫椤中的相对含量均相对较低,对甲氧基肉桂酸辛酯在桫椤中的相对含量仅有0.35%,庚醛在小黑桫椤中的相对含量仅为0.1%。说明在化合物组成上,桫椤和小黑桫椤既存在相似性,同时也具有一定的差异性。
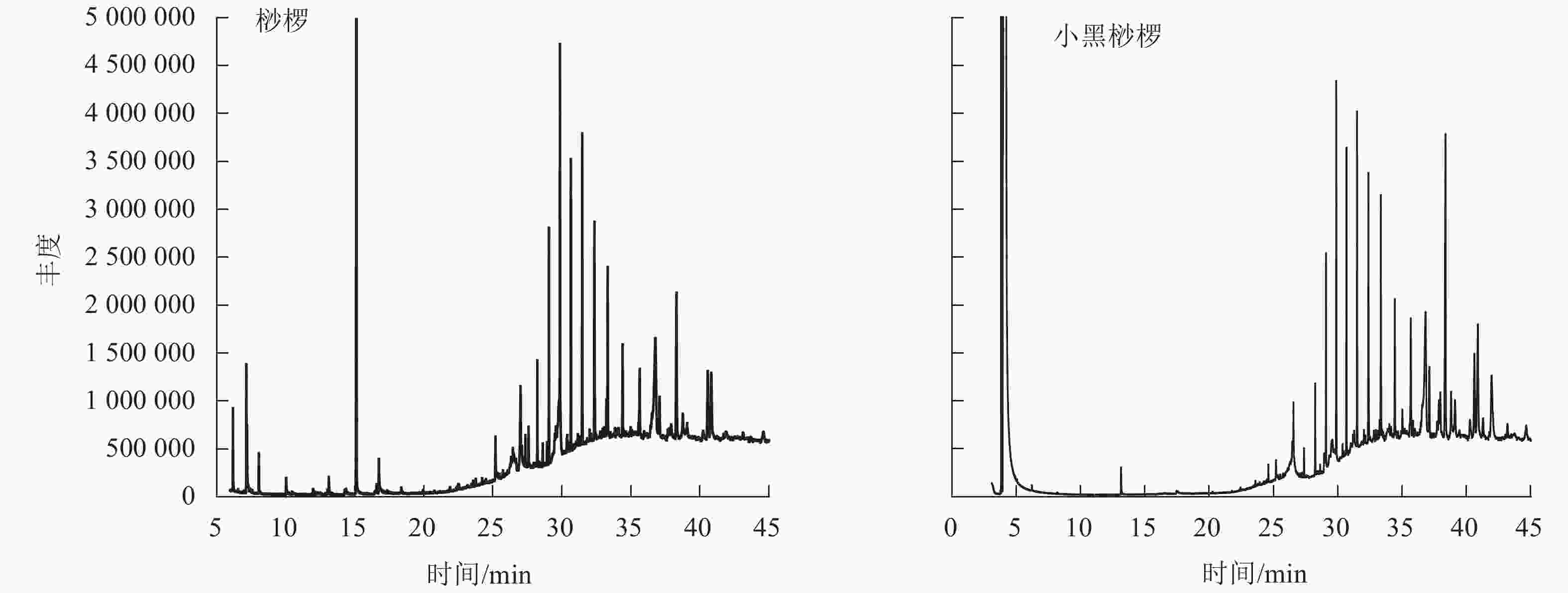
图 2 桫椤和小黑桫椤嫩叶浸提物总离子流图
Figure 2. Total ion current diagram of the young leaves of A. spinulosa and A. metteniana
表 4 寄主植物叶片浸提物成分分析
Table 4. Analysis of chemical compounds of leaf extractive substances from host plants
化合物 CAS号 相对含量/% 化合物 CAS号 相对含量/% 桫椤 小黑桫椤 桫椤 小黑桫椤 十二烷 dodecane 112-40-3 0.95±0.14 正三十二烷 dotriacontane 544-85-4 1.58±0.18 2.74±0.05 二十二烷 docosane 629-97-0 0.63±0.08 叶醇 3-hexen-1-ol, (Z)- 928-96-1 3.04±1.52 二十四烷 tetracosane 646-31-1 3.90±0.01 1-辛烯-3-醇 1-octen-3-ol 3391-86-4 0.43±0.13 二十五烷 pentacosane 629-99-2 7.75±1.10 8.71±1.72 麦角甾烷醇 ergostanol 1694036 0.78±0.38 二十六烷 hexacosane 630-01-3 5.76±0.35 γ-谷甾醇 gamma.-sitosterol 83-47-6 4.40±0.99 5.25±0.56 二十七烷 heptacosane 593-49-7 6.08±0.35 豆甾烷醇 stigmastanol 19466-47-8 4.39±0.70 8.21±0.69 二十八烷 octacosane 630-02-4 6.66±1.14 7.32±0.41 反式-2-己烯醛 2-hexenal, (E)- 6728-26-3 3.93±1.60 二十九烷 nonacosane 630-03-5 4.21±0.25 壬醛 nonanal 124-19-6 0.70±0.25 1.80±1.00 三十烷 triacontane 638-68-6 2.50±0.18 庚醛 heptanal 111-71-7 0.10±0.06 三十一烷 hentriacontane 630-04-6 2.31±0.23 棕榈酸 n-hexadecanoic acid 21096 0.79±0.45 正二十三烷 tricosane 638-67-5 1.53±0.06 2.15±0.54 α-亚麻酸 9,12,15-octadecatrienoic
acid, (Z,Z,Z)-463-40-1 2.12±1.72 正二十四烷 tetracosane 646-31-1 5.52±1.22 对甲氧基肉桂酸辛酯 2-propenoic acid,
3-(4-methoxyphenyl)-, 2-ethylhexyl ester5466-77-3 0.35±0.17 0.40±0.29 正二十六烷 hexacosane 630-01-3 8.06±1.43 对甲氧基肉桂酸异辛酯 2-ethylhexyl
trans-4-methoxycinnamate83834-59-7 0.31±0.14 正二十七烷 heptacosane 593-49-7 8.84±1.60 油酸酰胺 9-octadecenamide, (Z)- 301-02-0 0.58±0.17 正二十九烷 nonacosane 630-03-5 6.73±0.39 十六烷基二甲基叔胺 dimethyl palmitamine 112-69-6 0.31±0.01 正三十烷 triacontane 638-68-6 4.18±0.14 十八烷基二甲基叔胺 dimantine 124-28-7 0.93±0.11 正三十一烷 hentriacontane 630-04-6 4.15±0.22 4-乙烯基-2-甲氧基苯酚 2-methoxy-
4-vinylphenol7786-61-0 0.34±0.02 说明:表中数据是平均值±标准差(n=3);若为空白,则说明未检测到或者相对含量很低 -
植物挥发性物质是一类组成复杂、分子量为100~200的混合物质,主要包括烷烃类、醇类、醛类、酸类等,并以一定的比例组成植物独有的化学指纹图谱对植食性昆虫产生嗅觉刺激[19]。本研究发现:桫椤和小黑桫椤叶片具有相似的化学指纹图谱,化合物成分组成都以烃类为主,其次是醇类、醛类、酸类、脂类等。植物挥发物在植食性昆虫寄主选择中发挥着重要作用[20]。植食性昆虫依据植物挥发物对特定植物进行定向选择,最终识别寄主植物进行取食或产卵[21]。本研究发现:在光照和黑暗环境下,桫椤和小黑桫椤分别与绿色叶碟组合时,绿带妒尺蛾幼虫对桫椤和小黑桫椤有明显的趋向选择,可见桫椤和小黑桫椤释放的气味对绿带妒尺蛾幼虫有引诱作用。植食性昆虫在寄主选择时,视觉和嗅觉发挥着重要作用[22]。如苹果实蝇Rhagoletis pomonella寻找寄主植物时,嗅觉会与视觉相互作用[23]。在光照条件下,寄主植物和绿色叶碟共存时,尽管外形相似,绿带妒尺蛾幼虫依然可以准确选择寄主植物。
植物挥发物作为引导植食性昆虫的一种利他素,为植食性昆虫—植物二级营养关系建立过程中的信息化学物[24]。植食性昆虫凭借植物的化学图谱识别寄主植物和非寄主植物[25]。在一定距离内,昆虫通过嗅觉感官感知浓度达到一定阈值水平的化合物组成所代表的植物信息[26]。本次研究表明:当寄主植物和非寄主植物对比时,绿带妒尺蛾幼虫可以准确识别寄主植物,说明幼虫可被寄主植物所吸引[27]。如曹凤勤等[28]研究B型粉虱Bemisia tabaci对不同植物挥发物的行为反应结果表明:寄主植物挥发物对其有明显引诱作用。黄胸蓟马Thrips hawaiiensis对寄主植物挥发物有特殊偏好[29]。荔枝Litchi chinensis叶片气味对粗胫翠尺蛾Thalassodes immissaria具有导向作用[30]。当2种寄主植物对比时,绿带妒尺蛾幼虫对两者的选择并无差异,可能同为桫椤属蕨类植物,两者在化合物组成和含量上存在相似性。本研究通过GC-MS分析桫椤和小黑桫椤叶片的化合物成分证实,2种桫椤共有8种化合物。
从进化的角度来看,昆虫面临着强大的选择压力,如在寻找寄主时,往往要面对复杂的气味环境[31]。昆虫的感觉系统和搜索机制已经进化到处理复杂的化学物质,从而有效地定位合适的宿主来最大化自身的适应性[32]。如在6种植物共存时,烟叶蛾初孵幼虫可准确识别最适宜寄主植物烟草Nicotiana tabacum[33]。本研究中,在4种蕨类都存在时,绿带妒尺蛾幼虫可准确识别最适宜寄主植物桫椤,说明对寄主气味存在高度的特异性和敏感性[34]。在桫椤中存在的叶醇具有一定的青草气味,同时也作为昆虫警报、集合利用的信息素,并且还可作为防御、忌避、通信和环境安全的重要工具[35-36],这可能是绿带妒尺蛾幼虫更趋向桫椤的原因之一。同时,在鳞翅目昆虫中,由于幼虫活动能力受限,主要依赖于成虫对寄主植物的产卵选择[37],如产卵假说认为雌性成虫更容易将卵产在有利于后代发育的寄主植物上[38]。田间采集情况发现,幼虫主要集中分布在桫椤叶上,但成虫具体如何产卵选择,还需进一步研究。
-
绿带妒尺蛾幼虫鉴别寄主植物和非寄主的能力较强,能被桫椤和小黑桫椤所吸引,同时桫椤释放的挥发物对其具有更强烈的引诱作用。GC-MS结果显示:在桫椤中存在的叶醇和壬醛有望作为绿带妒尺蛾的引诱剂成分。
Host selection of Phthonoloba viridifasciata and analysis of chemical compounds in leaves of host plants
-
摘要:
目的 通过研究绿带妒尺蛾Phthonoloba viridifasciata幼虫对寄主植物的趋向反应和对寄主植物叶片的化学成分分析,为后续研发绿带妒尺蛾引诱剂提供科学依据。 方法 在室内用培养皿测定了在不同叶碟组合下,绿带妒尺蛾2龄幼虫对不同叶碟的趋性选择。使用正己烷制作寄主植物样品粗提物,气相色谱-质谱联用仪(GC-MS)分析寄主植物嫩叶浸提物的化学成分。使用卡方检验比较绿带妒尺蛾幼虫对不同处理的趋性选择。 结果 ①光照和黑暗条件下,桫椤Alsophila spinulosa和小黑桫椤Alsophila metteniana分别与绿色叶碟配对时,绿带妒尺蛾幼虫对桫椤和小黑桫椤的选择频次极显著高于对绿色叶碟的选择频次(P<0.01)。光照条件下的选择率(桫椤68.7%,小黑桫椤78.3%)略低于黑暗条件下的选择率(桫椤81.7%,小黑桫椤80.0%)。②黑暗条件下,绿带妒尺蛾幼虫对寄主植物桫椤和小黑桫椤的选择频次极显著高于对非寄主植中华复叶耳蕨Arachniodes chinensis和肾蕨Nephrolepis auriculata的选择频次(P<0.01)。③黑暗条件下,4种蕨类植物叶碟共存时,绿带妒尺蛾幼虫对桫椤的选择频次显著高于对其他3种蕨类植物的选择频次(P<0.05),其中对桫椤的选择频次最高,达46次,占总频次的38.3%。④桫椤和小黑桫椤的化学组分主要是烃类,其次是醇类、醛类等,GC-MS共检测出30余种化合物,其中桫椤23种,小黑桫椤19种,2种植物相同组分有γ-谷甾醇、豆甾烷醇、对甲氧基肉桂酸辛酯、壬醛和其他烷烃等8种化合物。 结论 绿带妒尺蛾幼虫鉴别寄主植物和非寄主植物能力较强,能被寄主桫椤和小黑桫椤所吸引。在桫椤中存在的叶醇和壬醛有望作为绿带妒尺蛾的引诱剂成分。图2表4参38 Abstract:Objective This study aims to investigate the tropism response of Phthonoloba viridifasciata larvae to host plants and chemical compounds in leaves of host plants, so as to provide scientific reference for the development of attractants for P. viridifasciata. Method The selection of the 2nd instar larvae of P. viridifasciata to different leaf disc combinations was determined by petri dishes in the laboratory. The crude extracts of host plants were prepared with hexane and the chemical compounds were analyzed by gas chromatography-mass spectrometry (GC-MS). Chi square test was used to compare the taxis selection of the larvae of P. viridifasciata to different treatments. Result (1) Under light and dark conditions, the selection frequency of P. viridifasciata towards Alsophina spinulosa and A. metteniana were significantly higher than that towards green leaf discs (P<0.01) when A. spinulosa and A. metteniana were paired with green leaf discs. Under light conditions, the selection ratio under light (A. spinulosa 68.7%, A. metteniana 78.3%) was slightly lower than that under darkness (A. spinulosa 81.7%, A. metteniana 80.0%). (2) Under dark conditions, the selection frequency of the larvae of P. viridifasciata to the host plants A. spinulosa and A. metteniana were significantly higher than that to the non-host plants Arachniodes chinensis and Nephrolepis auriculata (P<0.01). (3) Under dark conditions, when the leaves and discs of 4 ferns coexisted, the selection frequency of A. spinulosa by the larvae of P. viridifasciata was significantly higher than that of the other 3 ferns (P<0.05), in which the selection frequency of A. spinulosa was the highest, up to 46 times, accounting for 38.3% of the total frequency. (4) At least 30 compounds were detected by GC-MS, including 23 from A. spinulosa, 19 from A. metteniana, and 8 shared compounds such as γ-sitosterol, stigmastanol, 2-propenoic acid, 3-(4-methoxyphenyl)-, 2-ethylhexyl ester, nonanal, and other alkanes. The chemical compounds in leaves of A. spinulosa and A. metteniana were mainly hydrocarbons, followed by alcohols and aldehydes. Conclusion The larvae of P. viridifasciata have a strong ability to distinguish host plants from non-host plants, and can be attracted by host plants A. spinulosa and A. metteniana. Mixture of 3-Hexen-1-ol, (Z)- and nonanal can be used as attractants for P. viridifasciata. [Ch, 2 fig. 4 tab. 38 ref.] -
表 1 绿带妒尺蛾幼虫对桫椤和小黑桫椤叶碟的趋性选择
Table 1. Selection frequencies of P. viridifasciata larvae towards A. spinulosa and A. metteniana leaf discs respectively
组合 条件 选择
频次选择
率/%χ2 P 显著性 桫椤/绿色叶碟 光照 41 68.7 8.07 0.004 5 ** 黑暗 49 81.7 24.07 9.306E−07 ** 小黑桫椤/绿色叶碟 光照 46 78.3 17.07 3.609E−05 ** 黑暗 48 80.0 21.60 3.359E−06 ** 说明:组合为实验组/对照组,绿色叶碟为对照,n=60; **表示差异极显著(P<0.01) 表 2 绿带妒尺蛾幼虫在不同蕨类植物比较时的选择频次
Table 2. Selection frequencies of P. viridifasciata larvae towards combinations of different ferns
组合 选择频次 χ2 P 显著性 实验组 对照组 桫椤/中华复叶耳蕨 41 9 20.48 6.026E−06 ** 桫椤/肾蕨 37 13 11.52 0.000 6 ** 小黑桫/中华复叶耳蕨 39 11 15.68 7.501E−05 ** 小黑桫椤/肾蕨 37 13 11.52 0.000 6 ** 桫椤/小黑桫椤 36 29 0.75 0.385 3 ns 说明:组合为实验组/对照组,n=50;**表示差异极显著 (P<0.01);ns表示差异不显著(P>0.05) 表 3 绿带妒尺蛾幼虫在4种蕨类植物共存条件下的选择反应
Table 3. Selection responses of P. viridifasciata larvae towards different ferns presented together
植物 选择频次 选择率/% χ2 P 显著性 桫椤 46 38.3 15.8 0.001 2 ** 小黑桫椤 34 28.3 中华复叶耳蕨 21 17.6 肾蕨 19 15.8 说明:n=120;*表示差异极显著(P<0.01) 表 4 寄主植物叶片浸提物成分分析
Table 4. Analysis of chemical compounds of leaf extractive substances from host plants
化合物 CAS号 相对含量/% 化合物 CAS号 相对含量/% 桫椤 小黑桫椤 桫椤 小黑桫椤 十二烷 dodecane 112-40-3 0.95±0.14 正三十二烷 dotriacontane 544-85-4 1.58±0.18 2.74±0.05 二十二烷 docosane 629-97-0 0.63±0.08 叶醇 3-hexen-1-ol, (Z)- 928-96-1 3.04±1.52 二十四烷 tetracosane 646-31-1 3.90±0.01 1-辛烯-3-醇 1-octen-3-ol 3391-86-4 0.43±0.13 二十五烷 pentacosane 629-99-2 7.75±1.10 8.71±1.72 麦角甾烷醇 ergostanol 1694036 0.78±0.38 二十六烷 hexacosane 630-01-3 5.76±0.35 γ-谷甾醇 gamma.-sitosterol 83-47-6 4.40±0.99 5.25±0.56 二十七烷 heptacosane 593-49-7 6.08±0.35 豆甾烷醇 stigmastanol 19466-47-8 4.39±0.70 8.21±0.69 二十八烷 octacosane 630-02-4 6.66±1.14 7.32±0.41 反式-2-己烯醛 2-hexenal, (E)- 6728-26-3 3.93±1.60 二十九烷 nonacosane 630-03-5 4.21±0.25 壬醛 nonanal 124-19-6 0.70±0.25 1.80±1.00 三十烷 triacontane 638-68-6 2.50±0.18 庚醛 heptanal 111-71-7 0.10±0.06 三十一烷 hentriacontane 630-04-6 2.31±0.23 棕榈酸 n-hexadecanoic acid 21096 0.79±0.45 正二十三烷 tricosane 638-67-5 1.53±0.06 2.15±0.54 α-亚麻酸 9,12,15-octadecatrienoic
acid, (Z,Z,Z)-463-40-1 2.12±1.72 正二十四烷 tetracosane 646-31-1 5.52±1.22 对甲氧基肉桂酸辛酯 2-propenoic acid,
3-(4-methoxyphenyl)-, 2-ethylhexyl ester5466-77-3 0.35±0.17 0.40±0.29 正二十六烷 hexacosane 630-01-3 8.06±1.43 对甲氧基肉桂酸异辛酯 2-ethylhexyl
trans-4-methoxycinnamate83834-59-7 0.31±0.14 正二十七烷 heptacosane 593-49-7 8.84±1.60 油酸酰胺 9-octadecenamide, (Z)- 301-02-0 0.58±0.17 正二十九烷 nonacosane 630-03-5 6.73±0.39 十六烷基二甲基叔胺 dimethyl palmitamine 112-69-6 0.31±0.01 正三十烷 triacontane 638-68-6 4.18±0.14 十八烷基二甲基叔胺 dimantine 124-28-7 0.93±0.11 正三十一烷 hentriacontane 630-04-6 4.15±0.22 4-乙烯基-2-甲氧基苯酚 2-methoxy-
4-vinylphenol7786-61-0 0.34±0.02 说明:表中数据是平均值±标准差(n=3);若为空白,则说明未检测到或者相对含量很低 -
[1] 钦俊德. 昆虫与植物的关系[J]. 生物学通报, 1985, 20(10): 16 − 18. QIN Junde. The relationship of insects and plants [J]. Bulletin of Biology, 1985, 20(10): 16 − 18. [2] 杜家纬. 植物-昆虫间的化学通讯及其行为控制[J]. 植物生理学报, 2001, 27(3): 193 − 200. DU Jiawei. Plant-insect chemical communication and its behavior control [J]. Acta Phytophysiologica Sinica, 2001, 27(3): 193 − 200. [3] 阎雄飞, 李善才. 枣食芽象甲对不同品种枣树挥发物气味行为反应[J]. 中国农学通报, 2012, 28(22): 197 − 200. YAN Xiongfei, LI Shancai. The behavior responses of Scythropus yasunatsui to volatiles emanating from different cultivars of jujube tree [J]. Chinese Agricultural Science Bulletin, 2012, 28(22): 197 − 200. [4] 杜永均, 严福顺. 植物挥发性次生物质在植食性昆虫、寄生植物和昆虫天敌关系中的作用机理[J]. 昆虫学报, 1994, 37(2): 233 − 250. DU Yongjun, YAN Fushun. The role of plant volatile in tritrophic interactions among phytophagous insects, their host plants and natural enemies [J]. Acta Entomologica Sinica, 1994, 37(2): 233 − 250. [5] 戴建青, 韩诗畴, 杜家纬. 植物挥发性信息化学物质在昆虫寄主选择行为中的作用[J]. 环境昆虫学报, 2010, 32(3): 407 − 414. DAI Jianqing, HAN Shichou, DU Jiawei. Progress in studies on behavioural effect of semiochemicals of host plant to insects [J]. Journal of Environmental Entomology, 2010, 32(3): 407 − 414. [6] VERKERK R H J, WRIGHT D J. Multitrophic interactions and management of the diamondback moth: a review [J]. Bulletin of Entomological Research, 1996, 86(3): 205 − 216. [7] 王雪娇, 查友贵, 肖春, 等. 桃蚜对不同植物挥发物的趋性选择研究[J]. 云南农业大学学报(自然科学), 2012, 27(2): 165 − 169. WANG Xuejiao, ZHA Yougui, XIAO Chun, et al. Study on the preference selectivity of Myzus persicae to different plant order sources [J]. Journal of Yunnan Agricultural University (Natural Science) , 2012, 27(2): 165 − 169. [8] MAHROOF R M, PHILLIPS T W. Orientation of the Cigarette Beetle, Lasioderma serricorne (F. ) (Coleoptera: Anobiidae) to plant-derived volatiles [J]. Journal of Insect Behavior, 2007, 20(1): 99 − 115. [9] 莫圣书, 赵冬香, 陈青. 植物挥发物与昆虫行为关系研究进展[J]. 热带农业科学, 2006, 26(6): 84 − 89, 93. MO Shengshu, ZHAO Dongxiang, CHEN Qing. Advances on relationships between volatiles and insect behavior [J]. Chinese Journal of Tropical Agriculture, 2006, 26(6): 84 − 89, 93. [10] 向玉勇, 刘同先, 张世泽. 植物挥发物在植食性昆虫寄主选择行为中的作用及应用[J]. 玉溪师范学院学报, 2015, 31(8): 1 − 7. XIANG Yuyong, LIU Tongxian, ZHANG Shize. Effect of plant volatiles on host selecting behavior of phytophagous insects and their application [J]. Journal of Yuxi Normal University, 2015, 31(8): 1 − 7. [11] 徐东山. 桫椤Alsophila spinulosa主要害虫的生物学及生态学研究[D]. 贵阳: 贵州师范大学, 2020. XU Dongshan. Study on Biology and Ecology of Alsophila spinulosa Main Pests[D]. Guiyang: Guizhou Normal University, 2020. [12] 何琴琴, 杨卫诚, 梁盛, 等. 赤水桫椤自然保护区桫椤尺蛾科害虫的初步研究[J]. 环保科技, 2019, 25(3): 29 − 32,40. HE Qinqin, YANG Weicheng, LIANG Sheng, et al. A preliminary study on the individual development of viridifasciata of the Alsophila spinulosa pests in Chishui [J]. Environmental Protection and Technology, 2019, 25(3): 29 − 32,40. [13] 吕朝燕, 张宝成, 王加真, 等. 桫椤研究进展的可视化分析[J]. 北方园艺, 2018(19): 172 − 179. LÜ Chaoyan, ZHANG Baocheng, WANG Jiazhen, et al. Visual analysis of the research progress of Alsophila spinulosa [J]. Northern Horticulture, 2018(19): 172 − 179. [14] MA Yiyong, JIAO Peng, QI Zhuo, et al. Characterization of the complete chloroplast genome of Alsophila spinulosa, an endangered species endemic to China [J]. Mitochondrial DNA Part B, 2020, 5(3): 2262 − 2263. [15] 国家林业局. 国家重点保护野生植物名录(第一批)[J]. 中国林业, 2003(22): 50 − 53. State Forestry Administration. List of national key protected wild plants (First batch) [J]. Forestry of China, 2003(22): 50 − 53. [16] 陆宴辉, 张永军, 吴孔明. 植食性昆虫的寄主选择机理及行为调控策略[J]. 生态学报, 2008, 28(10): 5113 − 5122. LU Yanhui, ZHANG Yongjun, WU Kongming. Host-plant selection mechanisms and behavioral manipulation strategies of phytophagous insects [J]. Acta Ecologica Sinica, 2008, 28(10): 5113 − 5122. [17] 吴明峰, 高尚, 杨耀, 等. 棉铃虫和烟青虫初孵幼虫对植物顶尖嫩叶的偏好性差异[J]. 植物保护, 2016, 42(3): 63 − 69. WU Mingfeng, GAO Shang, YANG Yao, et al. Differences in the neonates’host preferences between Helicoverpa armigera and Helicoverpa assulta [J]. Plant Protection, 2016, 42(3): 63 − 69. [18] 刘婷. 棉铃虫Helicoverpa armigera(Hübner)在多寄主植物气味环境中的寄主选择行为[D]. 郑州: 河南农业大学, 2013. LIU Ting. Host Selection Behavior of Helicoverpa armigera (Hübner) under the Odor Environment of Multiple Host Plants[D]. Zhengzhou: Henan Agricultural University, 2013. [19] 卢伟, 侯茂林, 文吉辉, 等. 植物挥发性次生物质对植食性昆虫的影响[J]. 植物保护, 2007, 33(3): 7 − 11. LU Wei, HOU Maolin, WEN Jihui, et al. Effects of plant volatiles on herbivorous insects [J]. Plant Protection, 2007, 33(3): 7 − 11. [20] PAN H S, XIU C L, WILLIAMS L, et al. Plant volatiles modulate seasonal dynamics between hosts of the Polyphagous mirid bug Apolygus lucorum [J]. Journal of Chemical Ecology, 2021, 47(1): 87 − 98. [21] VISSER J H. Host odor perception in phytophagous insects [J]. Annual Review of Entomology, 1986, 31(1): 121 − 144. [22] 钦俊德. 诠释植食性昆虫是怎样选择食料植物的[J]. 生物学通报, 2003, 38(6): 1 − 3. QIN Junde. Explain how phytophagous insects choose feeding plants [J]. Bulletin of Biology, 2003, 38(6): 1 − 3. [23] ALUJA M, PROKOPY R J. Host odor and visual stimulus interaction during intratree host finding behavior of Rhagoletis pomonella flies [J]. Journal of Chemical Ecology, 1993, 19(11): 2671 − 2696. [24] 赵冬香, 高景林, 陈宗懋. 植食性昆虫对寄主植物的定向行为研究进展[J]. 热带农业科学, 2004, 24(2): 63 − 68. ZHAO Dongxiang, GAO Jinglin, CHEN Zongmao. Research progress of behavioral orientation of phytophagous insects to their host plants [J]. Chinese Journal of Tropical Agriculture, 2004, 24(2): 63 − 68. [25] 林海清, 陈少波. 植食性害虫及其天敌的定向行为研究进展[J]. 福建农业学报, 2009, 24(2): 191 − 196. LIN Haiqing, CHEN Shaobo. Advance in research on the orientation mechanism of herbivorous insects and natural enemies [J]. Fujian Journal of Agricultural Sciences, 2009, 24(2): 191 − 196. [26] 王鹏, 张龙. 植食性昆虫的嗅觉选食过程及其机制研究进展[J]. 环境昆虫学报, 2021, 43(3): 633 − 641. WANG Peng, ZHANG Long. Olfaction in the process of herbivorous insect food selection [J]. Journal of Environmental Entomology, 2021, 43(3): 633 − 641. [27] ROSS K, ANDERSON M. Larval responses of three vegetable root fly pests of the genus Delia (Diptera: Anthomyiidae) to plant volatiles [J]. Bulletin of Entomological Research, 1992, 82(3): 393 − 398. [28] 曹凤勤, 刘万学, 范中南, 等. B型烟粉虱对3种寄主植物及其挥发物的行为反应[J]. 昆虫学报, 2008, 51(8): 830 − 838. CAO Fengqin, LIU Wanxue, FAN Zhongnan, et al. Behavioural responses of Bemisia tabaci B-biotype to three host plants and their volatiles [J]. Acta Entomologica Sinica, 2008, 51(8): 830 − 838. [29] CAO Yu, REITZ S R, GERMINARA G S, et al. Host preference of Thrips hawaiiensis for different ornamental plants [J]. Journal of Pest Science, 2022, 95: 761 − 770. [30] 李鹏燕, 成家宁, 陈炳旭, 等. 粗胫翠尺蛾对不同荔枝品种的选择性研究[J]. 果树学报, 2012, 29(3): 434 − 439. LI Pengyan, CHENG Jianing, CHEN Bingxu, et al. Study on the selectivity of Thalassodes immissaria (Lepidoptera: Ge-ometridae) to litchi varieties [J]. Journal of Fruit Science, 2012, 29(3): 434 − 439. [31] 盛子耀, 李为争, 原国辉. 植物气味多样性与昆虫关系的研究进展[J]. 应用昆虫学报, 2019, 56(4): 652 − 661. SHENG Ziyao, LI Weizheng, YUAN Guohui. Advances in studies on the relationship between plant odor diversity and insects [J]. Chinese Journal of Applied Entomology, 2019, 56(4): 652 − 661. [32] MEINERS T. Chemical ecology and evolution of plant-insect interactions: a multitrophic perspective [J]. Current Opinion in Insect, 2015, 8: 22 − 28. [33] 王琼. 植物背景气味对烟夜蛾寄主选择的影响[D]. 郑州: 河南农业大学, 2015. WANG Qiong. Effect of Plant Background Odor on Host Selection of Helicoverpa assulta (Guenée)[D]. Zhengzhou: Henan Agricultural University, 2015. [34] BRUCE T J A, PICKETT J A. Perception of plant volatile blends by herbivorous insects-finding the right mix [J]. Phytochemistry, 2011, 72(13): 1605 − 1611. [35] 杨钦, 王天星, 杨轶雄, 等. 8种竹叶夏季挥发性成分特征[J]. 四川农业大学学报, 2021, 39(2): 180 − 190. YANG Qin, WANG Tianxing, YANG Yixiong, et al. Characteristics of volatile components of eight bamboo leaves in summer [J]. Journal of Sichuan Agricultural University, 2021, 39(2): 180 − 190. [36] 李正名, 蒋益民. 叶醇及其衍生物的立体有择合成[J]. 化学通报, 1989, 12: 23 − 24. LI Zhengming, JIANG Yimin. Stereoselective synthesis of leaf alcohols and their derivatives [J]. Chemistry Bulletin, 1989, 12: 23 − 24. [37] ROY N. Synergism in host selection behavior of three generalist insects towards leaf cuticular wax of sesame cultivars [J]. Neotropical Entomology, 2021, 50(5): 812 − 827. [38] PÖYKKÖ H. Females and larvae of a geometrid moth, Cleorodes lichenaria, prefer a lichen host that assures shortest larval period [J]. Environmental Entomology, 2006, 35(6): 1669 − 1676. -

-
链接本文:
https://zlxb.zafu.edu.cn/article/doi/10.11833/j.issn.2095-0756.20220168






 下载:
下载: