-
植物叶片发育过程一般可分为原基形成、初级形态和次生形态等3个阶段[1]。在原基形成阶段,叶原基的顶端分生组织有较高浓度的生长素和细胞分裂素[2]。一般而言,新展开的叶子有较低的净光合速率与较高的暗呼吸速率和低传导的二氧化碳转移[3]。在初级形态阶段,叶片生长、细胞分裂、光合速率达到最高水平。在次生形态阶段,细胞停止增殖,叶片停止生长[4]。据报道,在番茄Solanum lycopersicum叶片成熟过程中,其总类胡萝素含量呈指数形式增加[5];在板栗Castanea millissima和紫丁香Syringa oblata叶片发育过程中,叶绿素含量也逐渐增加[6-7]。借助于光合膜的能量通量理论提出的“JIP分析法”[8],可以观察到植物叶片发育不同阶段的PSⅡ氧化还原状态和电子传递链末端电子受体间的PSⅠ受体侧的效率[9],植物叶片光合能量转换过程获得更清晰的认识。在早春期间,毛竹Phyllostachys edulis具有显著的爆发式生长特性已经获得深入研究[10-12],但是与毛竹竹秆爆发式生长相对应的叶片放叶至展叶过程中,有关光合色素、光化学效率及光合能力的变化特征尚未开展研究。为此,本研究以毛竹为研究对象,利用叶绿素荧光分析技术等,研究毛竹从叶片放叶至展叶过程中的光合色素质量分数、光合速率及光合激发能分配,试图阐明毛竹叶片发育过程中光合结构和功能的变化规律,为毛竹林的科学管理提供理论依据。
-
在浙江省临安市的毛竹林试验基地,选择生长健壮的2年生毛竹5株,1株为1个独立实验。从2015年4月20日幼叶展叶时开始取样测定,隔5 d取样1次,至2015年5月20日止共取样6次。
-
根据ARNON[13]的方法略做修改,称取毛竹叶片0.2 g,剪碎后置于带盖的试管中,加5.0 mL体积分数为80%的丙酮溶液在暗处浸提48 h,分别在470,645和663 nm处测定其光密度D(λ)值,参考LICHTENTHALER[14]的方法,计算叶绿素a,叶绿素b和类胡萝卜素的质量分数。取样1次·株-1,5次重复。
-
光响应曲线用Li-6400XT便携式光合仪(Li-COR,美国)中自动测定程序light-curve测定。测定时,设定的光合有效辐射强度为: 2 000,1 800,1 600,1 400,1 200,1 000,800,500,300,200,100,50,0 μmol·m-2·s-1,二氧化碳摩尔分数为370 μmol·mol-1,温度为25 ℃。根据直角双曲线修正模型拟合计算光饱和点(LSP)和光补偿点(LCP)等光合指标。选取5株,测定1次·株-1,5次重复。
-
快速叶绿素荧光动力学曲线采用非调制式叶绿素荧光仪(Yaxin-1161型)测定。测定前,叶片先暗适应20 min,然后用3 000 μmol·m-2·s-1饱和蓝闪光照射1 s,以10 μs(2 ms之前)和1 ms(2 ms之后)的间隔记录荧光信号,即可测得叶绿素荧光动力学参数。重复5次·处理-1。叶绿素荧光动力学参数的计算参考STRASSER等[15]的计算方法。暗适应下PSⅡ最大量子产率φPo=Fv /Fm=(Fm-Fo)/Fm;捕获的激子能电子传递的效率φO≡ETO /TRO=(1-VJ);用于热耗散的量子比率φDo≡1-φPo =(Fo /Fm);单位面积反应中心数目RC /CSO =(Fv /Fm)×(VJ /Mo)×Fo;单位反应中心耗散掉的能量DIO /RC=(ABS /RC)-(TRO /RC),其中:TRO /RC= Mo·(1/VJ)表示单位反应中心捕获的用于还原PSⅡ电子受体蛋白QA的能量;ABS /RC=Mo(Fv /Fm)/VJ表示单位反应中心复合体吸收的能量;ETO /RC =Mo×(1/VJ)×φo表示用于电子传递的能量;Mo=4×(F300μs-Fo)/(Fm-Fo)表示荧光曲线的相对初始斜率;VJ =(FJ-Fo)/(Fm-Fo)表示2 ms时的可变荧光。上述公式中,Fm表示暗适应后的最大荧光强度;Fo表示暗适应后的最小荧光强度;F300μs表示在暗适应后照光300 μs时的荧光强度;FJ表示在暗适应后照光2 ms时的荧光强度。
-
数据均为5次重复的平均值±标准误差,利用Origin 8软件进行统计分析和作图。采用One-Way ANOVA方法进行统计检验和最小显著差异法(LSD)多重比较(P<0.05)。
-
随着毛竹叶片展开天数的增加,毛竹叶片叶绿素a,叶绿素b和类胡萝卜素质量分数不断增加(表 1)。在展叶后第15天时,毛竹叶片中的叶绿素a,叶绿素b和类胡萝卜素质量分数比第5天时分别增加了233.8%,253.8%和142.1%。在第15天后,叶绿素a,叶绿素b和类胡萝卜素基本保持不变。由此说明在叶片发育进程中,第15天时,毛竹叶发育成熟,成熟叶片的叶绿素质量分数比幼叶多。此外,说明随着毛竹叶片发育进程推进,光合色素质量分数不断增加。
表 1 毛竹发育过程中光合色素质量分数变化
Table 1. Change of the pigment content in bamboo development processes
时间/d 叶绿素a/(mg·g-1) 叶绿素b/(mg·g-1) 类胡萝卜素/(mg·g-1) 叶绿素总和/(mg·g-1) 叶绿素a/叶绿素b 5 0.65 ± 0.11 e 0.26 ± 0.11 e 0.19 ± 0.03 d 0.91 ± 0.21 d 2.68 ± 0.65 b 10 1.25 ± 0.14 d 0.41 ± 0.02 d 0.33 ± 0.06 c 1.67 ± 0.12 c 3.04 ± 0.44 a 15 1.87 ± 0.24 c 0.71 ± 0.17 c 0.46 ± 0.03 b 2.58 ± 0.40 b 2.63 ± 0.21 b 20 2.27 ± 0.37 b 0.91 ± 0.26 b 0.54 ± 0.18 a 3.18 ± 0.38 a 2.49 ± 0.67 c 25 2.52 ± 0.03 a 1.03 ± 0.11 a 0.60 ± 0.02 a 3.55 ± 0.09 a 2.48 ± 0.31 c 30 2.55 ± 0.06 a 1.05 ± 0.05 a 0.59 ± 0.03 a 3.61 ± 0.10 a 2.43 ± 0.06 c 说明:每个值均为平均值±标准误 (n=5)。根据LSD测验 (P < 0.05),不同小写字母表示叶绿素a,叶绿素b和类胡萝卜素的 -
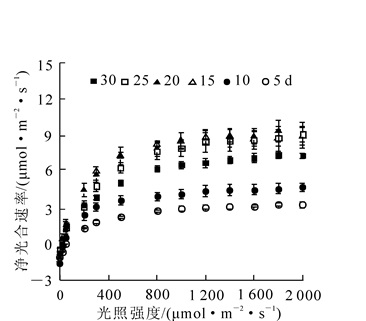
衡量叶片光合能力的重要指标是最大净光合速率。研究表明:2年生毛竹的叶片在6个不同的发育阶段,其净光合速率都有变化(图 1)。随着毛竹叶发育进程天数的增加,净光合速率呈缓慢上升趋势,在第15天到第20天时,净光合速率达到最大值,约为9 μmol·m-2·s-1;在20 d以后的一段时间里,净光合速率呈上下波动,趋于稳定状态。表明毛竹叶在第15天到第20天之间的光合能力是最强的。
-
叶片发育过程中各个时期的快速叶绿素荧光诱导动力学曲线(图 2)表明,叶片发育过程中各个时期的快速叶绿素荧光诱导动力学曲线均具有O,J,I,P相等的典型特征位点。随着叶片的发育,快速叶绿素荧光诱导动力学曲线的形状发生了变化,在初期的O相,叶片各个发育阶段的差异不明显,从J相开始,I相和P相荧光随叶片发育进程逐渐升高,叶片各个发育阶段的差异逐渐增大,在I相和P相表现出明显的差异。
-
毛竹叶片发育过程中PSⅡ反应中心数和活性反应中心数变化(图 3)表明,叶片发育过程中叶片单位面积含有的PSⅡ反应中心的数量(RC/CSo)和活性反应中心数(RC/ABS)逐渐增加,发育到第15天,RC/CSo升高了14.2%;RC/ABS升高了54.4%。由此表明在叶片发育过程中,有活性的反应中心增加更快。
-
毛竹叶片发育过程PSⅡ反应中心能量分配的变化(图 4)表明,在叶片发育过程中,PSⅡ单位反应中心吸收的光能(ABS/RC)和用于还原QA的能量(TRo/RC)逐渐降低,在5~15 d下降速度较快,在第15天后趋于稳定。ETo/RC数值保持平稳,表明在叶片发育过程中用于电子传递的能量变化不大。DIO/RC在5~15 d迅速减少,15 d后稳定在较低的水平,表明在叶片发育过程中单位反应中心的能量耗散越来越少,在15 d后叶片发育完成,维持较低的能量耗散。
-
图 5表示毛竹叶片发育过程PSⅡ受体侧的变化。随着叶片的发育成熟,Mo不断减少说明在叶片发育过程中QA被还原的速率迅速升高,QA完全被还原所需要的能量下降。最大光化学效率(φPo)上升最少,基本稳定;φEo和ψO的变化规律一致,在5~15 d间上升较快,而在15~30 d趋于稳定,表明叶片发育过程中PSⅡ受体侧QA下游的电子传递接收的能量无论绝对值还是占总能量的比例都是不断升高的,φEo的上升表明在叶片发育过程中用于QA下游电子传递的量子不断增加,ψO的上升表明叶片发育过程中PSⅡ反应中心捕获的激子中用于QA下游电子传递的激子占捕获激子总数的比例不断增加。
-
叶绿素主要作用是植物吸收和传递光能,类胡萝卜素的作用是吸收光能传递给中心色素叶绿素a,保护叶绿素免遭光氧化伤害[16]。一般情况下,植物叶片从幼叶伸出到完全扩展成熟的发育过程中,首先需要建立光能吸收与转换的机构,然后再逐渐完善气孔功能和羧化功能[17]。毛竹叶片叶绿素a,叶绿素b和类胡萝卜素质量分数均随叶片发育呈增加趋势。毛竹叶片在生长初期,各种色素质量分数都较低,随着叶片的发育,各种色素质量分数都在不断形成而增加,但由于叶绿素a比叶绿素b增加的幅度小,导致叶片中叶绿素a/叶绿素b值的降低。叶绿素b具有吸收和传递光能的作用,有利于叶片吸收更多的光能。可见毛竹在叶片发育过程中,通过叶绿素循环调节自身叶绿素a /叶绿素b比值的对策,适应外界环境中光的变化[18]。
快速叶绿素荧光诱导动力学曲线包含大量有关光合原初光化学反应的信息,能更好地反映植物叶片光合机构的状况[19]。在毛竹叶片发育过程中,PSⅡ反应中心吸收的光量子将电子传递到电子传递链中QA下游的电子受体的概率(φEo),以及捕获的激子将电子传递到电子传递链中QA-下游的电子受体的概率(ψO)逐渐上升。该结果表明:毛竹叶片发育过程中,PSⅡ受体侧PQ库被还原的能力急剧上升,库容增大,QA自身氧化还原越来越容易,导致受体侧电子传递畅通,毛竹叶片的PSⅡ反应中心功能逐渐完善,光合能力逐渐增强。
在毛竹叶片发育过程中,叶片单位面积上有活性的反应中心(RC /CSo)的数量明显增加,PSⅡ的光能转化效率升高,单位反应中心吸收(ABS /RC)和捕获(TRo /RC)的光能在叶片发育过程明显降低。ABS/RC和TRo /RC降低,减少了单位反应中心的激发压,而单位PSⅡ反应中心的光化学反应活性(ETo /RC)几乎没有变化。表明毛竹在叶片发育过程中,PSⅡ中有活性的反应中心数目逐渐增加,活性反应中心捕获的光能优先保证推动PSⅡ电子传递(ETo/RC)[20]。φPo是PS Ⅱ最大光化学量子产量,其大小反映了PS Ⅱ反应中心内原初光能的转换效率(最大PS Ⅱ的光能转换效率),φPo在非胁迫条件下变化极小,在受到环境胁迫时,随胁迫的增大而降低[21]。本研究中,毛竹叶片发育过程φPo无显著变化,表明毛竹在叶片发育过程中,环境胁迫不明显。
综上所述,毛竹在叶片发育过程中,叶绿素质量分数和净光合速率逐渐增加,15 d叶片发育完成,PSⅡ反应中心的功能逐渐完善,能够充分利用毛竹群落生境的光能资源。
Photosynthetic characteristics in the development process of Phyllostachys edulis
-
摘要: 运用植物气体交换测定和叶绿素荧光测定等分析技术,测定分析毛竹Phyllostachys edulis叶片发育过程中的色素质量分数、光响应进程和叶绿素荧光参数的变化特征。结果表明:① 毛竹叶片从幼叶生长到叶片成熟的各发育阶段,叶绿素质量分数和净光合速率不断增加而趋于稳定,叶片对光能的捕获和利用能力也随之增强。② 叶片从伸出到成熟,叶片的最大光化学效率(φPo)的变化不明显。③ 毛竹叶片经放叶、展叶至第15天,PSⅡ反应中心数不断增加,其数量约升高54.4%。④ PSⅡ电子受体蛋白QA被还原的速率和还原需要的能量不断减少,用于推动QA下游电子传递的能量不断增加。因此,毛竹叶片光合功能从幼叶形成至第15天完全展叶,叶片发育趋于完善,表明毛竹叶片具备正常的光合生理功能,可以充分利用毛竹群落生境的光能资源。Abstract: Using gas exchange and chlorophyll fluorescence technologies, changes in the net photosynthetic rate, pigment content, and chlorophyll fluorescence parameters of bamboo (Phyllostachys edulis) leaves were tested. Results showed that 1) as young bamboo leaves grew to maturity, in different developmental stages, the chlorophyll content and net photosynthetic rate increased, as did the light energy capture and utilization ability. 2) From leaf out to maturity, the maximum photochemical efficiency of leaves (φPo) did not change. 3) Also, for bamboo leaves, from leaf out to 15 d, the PS Ⅱ reaction center number increased about 54.4%. 4) The rate at which the PS Ⅱ receptor side primary quinone electron acceptor (QA) decreased and the energy required for reduction continuously decreased with the energy used to drive the QA downstream electron transfer increasing. Therefore, bamboo leaf photosynthetic functions from the time the young leaves formed to after 15 d of fully expanded leaves tended to be optimal, and leaves of Ph. edulis with normal photosynthetic physiological functions could make full use of the habitat community's solar energy resources.
-
表 1 毛竹发育过程中光合色素质量分数变化
Table 1. Change of the pigment content in bamboo development processes
时间/d 叶绿素a/(mg·g-1) 叶绿素b/(mg·g-1) 类胡萝卜素/(mg·g-1) 叶绿素总和/(mg·g-1) 叶绿素a/叶绿素b 5 0.65 ± 0.11 e 0.26 ± 0.11 e 0.19 ± 0.03 d 0.91 ± 0.21 d 2.68 ± 0.65 b 10 1.25 ± 0.14 d 0.41 ± 0.02 d 0.33 ± 0.06 c 1.67 ± 0.12 c 3.04 ± 0.44 a 15 1.87 ± 0.24 c 0.71 ± 0.17 c 0.46 ± 0.03 b 2.58 ± 0.40 b 2.63 ± 0.21 b 20 2.27 ± 0.37 b 0.91 ± 0.26 b 0.54 ± 0.18 a 3.18 ± 0.38 a 2.49 ± 0.67 c 25 2.52 ± 0.03 a 1.03 ± 0.11 a 0.60 ± 0.02 a 3.55 ± 0.09 a 2.48 ± 0.31 c 30 2.55 ± 0.06 a 1.05 ± 0.05 a 0.59 ± 0.03 a 3.61 ± 0.10 a 2.43 ± 0.06 c 说明:每个值均为平均值±标准误 (n=5)。根据LSD测验 (P < 0.05),不同小写字母表示叶绿素a,叶绿素b和类胡萝卜素的 -
[1] DONNELLY P M, BONETTA D, TSUKAYA H, et al. Cell cycling and cell enlargement in developing leaves of Arabidopsis[J]. Dev Biol, 1999, 215(2):407-419. [2] TRAAS J, MONÉGER F. Systems biology of organ initiation at the shoot apex[J]. Plant Physiol, 2010, 152(2):420-427. [3] CAI Z Q, SLOT M, FAN Z X. Leaf development and photosynthetic properties of three tropical tree species with delayed greening[J]. Photosynthetica, 2005, 43(1):91-98. [4] COSGROVE D J. Growth of the plant cell wall[J]. Nat Rev Mol Cell Biol, 2005, 6(11):850-861. [5] 李京, 惠伯棣, 裴凌鹏.番茄果实在成熟过程中类胡萝卜素含量的变化[J].中国食品学报, 2006, 6(2):122-125. LI Jing, HUI Bodi, PEI Lingpeng. Variation in the carotenoid amount of tomato fruit during ripening[J]. J Chin Inst Food Sci Technol, 2006, 6(2):122-125. [6] 孙山, 张立涛, 杨兴华, 等.板栗幼叶展叶过程的反射光谱和叶绿素荧光动力学[J].林业科学, 2009, 45(4):162-166. SUN Shan, ZHANG Litao, YANG Xinghua, et al. Spectral reflectance and chlorophyll fluorescemce kinetics of young leaves at the various stages of leaf expansion in field-grown chestnut plants[J]. Sci Silv Sin, 2009, 45(4):162-166. [7] 田野, 张会慧, 张秀丽, 等.紫丁香叶片发育过程中花色素苷含量与叶绿素荧光和激发能分配的关系[J].南京林业大学学报 (自然科学版), 2014, 38(1):59-64. TIAN Ye, ZHANG Huihui, ZHANG Xiuli, et al. The relationship between leaf anthocyanin content and chlorophyll fluorescence, as well as excited energy distribution during leaf expansion of Syringa oblata Lindl.[J]. J Nanjing For Univ Nat Sci Ed, 2014, 38(1):59-64. [8] STRASSER B J, STRASSER R J. Measuring fast fluorescence transients to address environmental questions:The JIP-test[M]//MATHIS P. Photosynthesis:from Light to Biosphere. Dordrecht:Kluwer Academic Publishers, 1995:977-980. [9] POLLASTRINI M, HOLLAND V, BRÜGGEMANN W, et al. Interactions and competition processes among tree species in young experimental mixed forests, assessed with chlorophyll fluorescence and leaf morphology[J]. Plant Biol, 2014, 16(2):323-331. [10] 李洪吉, 蔡先锋, 袁佳丽, 等.毛竹快速生长期光合固碳特征及其与影响因素的关系[J].浙江农林大学学报, 2016, 33(1):11-16. LI Hongji, CAI Xianfeng, YUAN Jiali, et al. Photosynthetic carbon fixation in Phyllostachys edulis during its fast growth period[J]. J Zhejiang A & F Univ, 2016, 33(1):11-16. [11] 曾莹莹, 王玉魁, 蔡先锋, 等.毛竹林爆发式生长期立竹器官营养成分的动态变化[J].浙江农林大学学报, 2015, 32(2):372-377. ZENG Yingying, WANG Yukui, CAI Xianfeng, et al. Nutritional elements in organs of a Phyllostachys edulis stand during its fast growth period[J]. J Zhejiang A & F Univ, 2015, 32(2):372-377. [12] 袁佳丽, 温国胜, 张明如, 等.毛竹快速生长期的水势变化特征[J].浙江农林大学学报, 2015, 32(5):722-728. YUAN Jiali, WEN Guosheng, ZHANG Mingru, et al. Water potential with Phyllostachys edulis in its fast-growth periods[J]. J Zhejiang A & F Univ, 2015, 32(5):722-728. [13] ARNON D I. Copper enzymes in isolated chloroplasts[J]. Polyphenol Beta Vulgaris Plant Physiol, 1949, 24(1):1-15. [14] LICHTENTHALER H K. Chlorophylls and carotenoids:pigments of photosynthetic biomembranes[J]. Methods Enzymol, 1987, 148(1):350-382. [15] STRASSER R J, TSIMILL-MICHAEL M, SRIVASTAVA A. Analysis of the chlorophyll a fluorescence transient[G]//PAPAGEORGIOU G, GOVINDJEE. Advances in Photosynthesis and Respiration. Berlin:Springer, 2004:321-362. [16] TANAKA R, TANAKA A. Chlorophyll cycle regulates the construction and destruction of the light-harvesting complexes[J]. Biochim Biophys Acta, 2011, 1807(8):968-976. [17] OLSON J M. Photosynthesis in the Archean era[J]. Photosynth Res, 2006, 88(2):109-117. [18] ZHOU Yanhong, LAM H M, ZHANG Jianhua. Inhibition of photosynthesis and energy dissipation induced by water and high light stresses in rice[J]. J Exp Bot, 2007, 58(5):1207-1217. [19] HOLLAND V, KOLLER S, BRÜGGEMANN W. Insight into the photosynthetic apparatus in evergreen and deciduous European oaks during autumn senescence using OJIP fluorescence transient analysis[J]. Plant Biol, 2014, 16(4):801-808. [20] KALAJI H M, OUKARROUM A, ALEXANDROV V. et al. Identification of nutrient deficiency in maize and tomato plants by in vivo chlorophyll a fluorescence measurements[J]. Plant Physiol Biochem, 2014, 81(1):16-25. [21] DEMMIG-ADAMS B, LII W W A. Xanthophyll cycle and light stress in nature:uniform response to excess direct sunlight among higher plant species[J]. Planta, 1996, 198(3):460-470. -

-
链接本文:
https://zlxb.zafu.edu.cn/article/doi/10.11833/j.issn.2095-0756.2017.03.008







 下载:
下载: