-
猪圆环病毒2型(porcine circovirus type 2,PCV2)是引起猪Sus scrofa圆环病毒相关综合征(PCVAD)的主要病原体之一,主要引起断奶仔猪多系统衰竭综合征(PMWS)、母猪繁殖障碍、猪呼吸道疾病综合症(PRDC)、猪皮炎与猪肾病综合症(PDNS)、肠炎和先天性震颤等疾病[1-2]。PCV2对外界和一般消毒剂的抵抗力较强,在酸性环境及氯仿中可以存活较长时间甚至在高温环境(72 ℃)也能存活一段时间。PCV2易通过胎盘垂直传播,感染猪群的免疫功能下降后发病,常与猪繁殖与呼吸综合症病毒(PRRSV)或猪细小病毒(PPV)混合感染,也易继发猪副嗜血杆菌或猪链球菌2型感染[3]。PCV2主要感染哺乳期和育成期的仔猪,尤其5~12周龄仔猪特别易感,常并发或继发细菌感染而导致死亡率大大增加[4]。PCV2最早发现于加拿大,后蔓延至美国、西班牙、英国、丹麦、德国、荷兰、比利时、日本和韩国等,发病率为10%~80%,死亡率为15%~30%[5]。PCV2在中国猪群中早已存在,2002年后发病猪群逐渐增多,死亡率为20%~30%[6-7]。临床上防控该病主要采用疫苗接种方法,虽然控制了大规模流行的趋势,但是一些饲养管理不当的养殖场仍然存在较高的发病率。为及时诊断该疾病,及早接种疫苗和加强生物安全措施,亟需建立起一种高灵敏性、快速高效的检测方法,以减少疾病所导致的损失。实时荧光定量聚合酶链式反应(PCR)技术是一种简单、快速、灵敏度高的检测技术,应用到动物疫病诊断上,主要采用基于SYBR Green I和Taqman探针的2种荧光定量检测方法,相比而言,Taqman探针灵敏性更高。因此,本研究拟利用针对PCV2的ORF1基因特异引物和TaqMan探针,建立一种实时荧光定量PCR检测技术,为PCV2的实验室诊断和疫病预警预报提供检测工具。
-
猪圆环病毒(PCV2)、猪瘟病毒(CSFV)、猪繁殖与呼吸障碍综合征病毒(PRRSV)均由浙江农林大学动物预防医学与公共卫生实验室保存,猪流行性腹泻病毒(PEDV)-猪传染性胃肠炎病毒(TGEV)-轮状病毒(RV)三联疫苗购自哈尔滨维科生物技术有限公司。
-
从GenBank中下载PCV2特异性的ORF1基因(Genbank:MF616438.1)序列,利用Beacon Designer 7软件找出该基因上的高保守序列,设计1对特异引物和1条带有FAM(6-羧基荧光素)标记和碎灭为MGB(Minor Groove Binder,小沟结合物)的Taqman探针。上游引物(ORF1-F)序列:5′-TAGATCTCAAGGACAACGGAGTGA-3′;下游引物(ORF1-R)序列:5′-GTTACAGGGTGCTGCTCTGCA-3′;探针序列:5′-FAM-CAGACTCCCGCTCTC-MGB-NFQ-3′。所有序列均由上海金唯智生物科技有限公司合成。
-
应用特异引物(ORF1-F/R),以PCV2的DNA为模板进行PCR扩增。反应条件为94 ℃ 5 min;98 ℃ 10 s,60 ℃ 30 s,72℃ 30 s,40个循环;72 ℃ 10 min,15 ℃ 5 min。利用DNA凝胶回收试剂盒(上海生工生物有限公司)纯化回收PCR产物,连接至pMD18-T(大连宝生物工程有限公司)克隆载体后,转化大肠埃希菌Escherichia coli(DH5α)感受态细胞。经蓝白斑筛选得到阳性克隆,用菌落PCR和质粒PCR鉴定获得阳性重组质粒(命名为pSL1353),送生物公司(上海金唯智生物科技有限公司)测序验证。所获质粒经核酸蛋白浓度测定仪测定浓度和纯度,置于-20 ℃备用。
-
配制实时荧光定量PCR的反应体系,其中上、下游引物各0.4 μL,SYBR® Premix EX Taq 10.0 μL,模板(pSL1353) 2.0 μL,超纯水补足至20.0 μL。对反应条件进行优化,分别设置退火温度为54,56,58,60和62 ℃,以获得该方法的最佳退火温度。分别采用0.2,0.3,0.4和0.5 μmol·L-1的引物浓度,构建PCR反应体系,确定该方法的最佳引物浓度。采用0.1,0.2,0.3,0.4和0.5 μmol·L-1的探针浓度,上、下游引物各0.4 μL,rTaq酶和dNTP混合物10.0 μL,模板(pSL1353,超纯水为空白对照)2.0 μL,超纯水补足至20.0 μL,确定最佳Taqman探针浓度。
-
将1.2.2得到的pSL1353质粒以10倍梯度稀释成1013,1012,1011,1010,109,108,107和106拷贝·L-1;以1.2.3得到的优化条件进行荧光定量PCR扩增,重复3次·浓度-1;以阳性重组质粒拷贝数为横坐标,以各浓度下的循环数(Ct)值为纵坐标,建立Taqman探针实时荧光定量PCR标准曲线。其中拷贝数计算公式:拷贝数(拷贝·L-1)=质粒浓度(g·L-1)×阿弗加德罗常数/重组质粒分子量[8]。
-
将pSL1353质粒稀释成1 000×106,100×106,50×106,20×106,5×106和106拷贝· L-1,设置6重复·浓度-1,测定各浓度下的循环数(Ct),用PODLOD calculation program(version 8)[9]计算本检测方法的最低检测限值。
-
以空白模板对照,检测已知的PCV2 DNA样品,间隔1周做重复试验,重复3次·样品-1(组间);检测已知的3个PCV2 DNA样品(1013,1012,1011拷贝·L-1),重复6个·样品-1(组内)。计算组内和组间变异系数(CV),CV=SCt/Ct×100%,验证该方法的重复性。其中SCt为Ct的标准差,Ct为Ct的平均值。
-
以1.2.3建立的PCV2 Taqman探针实时荧光定量方法同时对CSFV,PRRSV,PEDV/TGEV/RV进行检测,以空白模板为对照,确定该方法的特异性。
-
以1.2.3建立的PCV2 Taqman探针实时荧光定量方法检测2016-2017年杭州市周边地区规模化生猪养殖场送检的50份疑似病料(腹股沟淋巴结)。
-
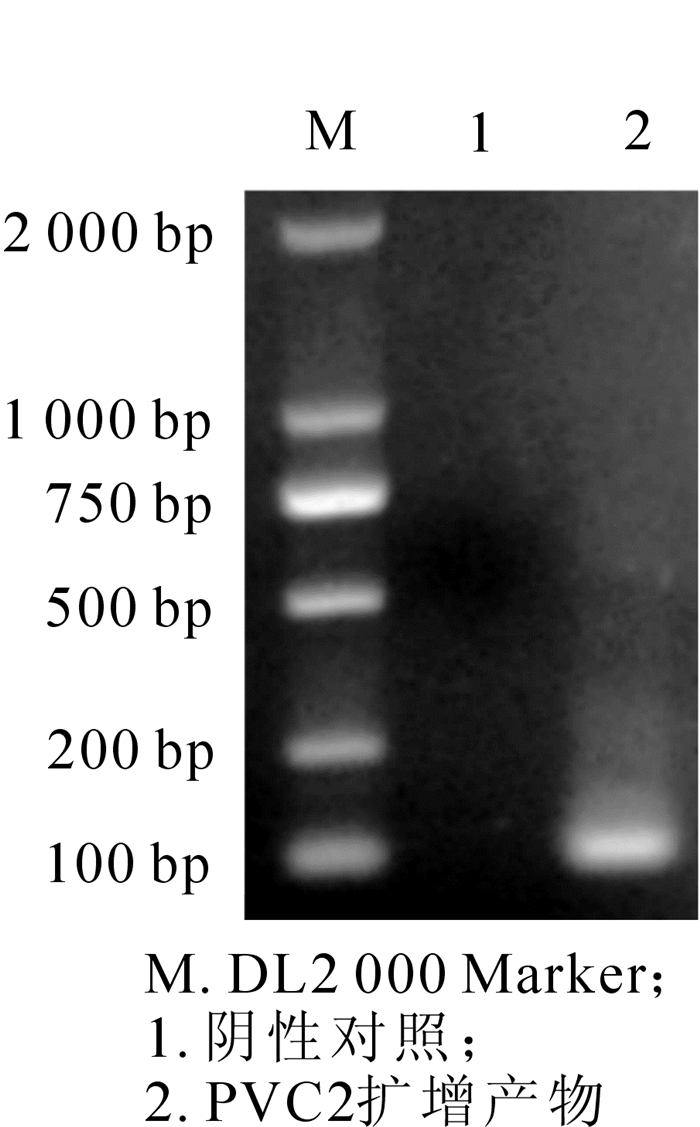
以PCV2疫苗毒株所提取的DNA为模板,利用特异性引物(ORF1-F/R)扩增出大小约100 bp的片段;扩增产物回收后借助T/A克隆技术,构建pMD18-T-PCV2-ORF1载体(pSL1353),经菌落PCR和质粒PCR鉴定为阳性克隆(图 1)。测序验证其序列,发现与NCBI上PCV2的序列(GenBank:KY940521.1)同源性达100%。提取pSL1353质粒作为本研究方法的阳性质控,可以作为判断试验成立与否的阳性模板。
-
依据1.2.3,发现退火温度为60 ℃时扩增效率最高。在PCV2质粒浓度1013拷贝·L-1时进行反应条件优化,根据有典型扩增曲线且Ct值最小的原则,发现上、下游引物浓度0.2 μmol·L-1(图 2A)和探针浓度0.2 μmol·L-1(图 2B)时扩增效果最佳。该结果可作为试剂盒开发及成本控制的理论依据。
-
将稀释(1013,1012,1011,1010,109,108,107,106拷贝·L-1)后的重组质粒作为模板,在2.2的Taqman探针实时荧光定量PCR优化条件下进行扩增(图 3A),得到标准曲线如图 3B所示,其线性回归方程为Ct=-0.671 4×lgA+38.16,其中A为模板浓度。相关系数(R2)为0.974 5,表明本研究所建立的方法在模板为2.1×1013~2.1×106拷贝·L-1时,检测到Ct值和模板之间具有较好的线性关系。
-
选取2.3中1013,1012,1011拷贝·L-1阳性质粒为模板,在2.2优化条件下进行重复试验。结果表明:组内样品扩增曲线重复性较好(图 4),组内与组间Ct值的变异系数较小(表 1), 表明该检测方法有较好的重复性。
表 1 实时荧光定量PCR的重复性验证
Table 1. Repetitive assay of the real-time quantitative PCR
质粒浓度/(拷贝·L-1) CV/% 组内 组间 1013 0.28 0.34 1012 0.14 0.32 1011 0.09 0.28 -
阳性标准质粒pSL353分别按照1 000×106,100×106,50×106,20×106,5×106和106拷贝·L-1,重复6次·浓度-1,进行实时荧光定量PCR扩增,有典型的扩增曲线且Ct值小于38时判断为阳性。结果表明:每个浓度均具有较好的重复(图 5)。PODLOD V8软件[9]公式计算,得到该方法的最低检测线为8.828×106拷贝·L-1(95%的检测限)(表 2)。
表 2 实时荧光定量PCR的灵敏度
Table 2. Sensitivity of the real-time quantitative PCR
质粒浓度/(×106拷贝·L-1) 样本数/个 阳性样本/个 1 000 6 6 100 6 6 50 6 6 20 6 6 5 6 5 0 6 0 -
分别以PCV2的基因组DNA,PEDV,TGEV,PEDV/TGEV/RV三联疫苗的cDNA为模板,利用1.2.3构建的检测方法进行实时荧光定量PCR扩增。结果表明:PCV2病毒组有特征性扩增曲线,其他各组则没有,说明该方法具有较好的特异性(图 6),为临床疾病混合感染情况下准确检测PCV2提供了工具。
-
以实验室收集到的2016-2017年杭州市周边地区规模化生猪养殖场的50份PCV2疑似病料(腹股沟淋巴结)为检测样本,提取样本中的DNA,利用本研究的PCV2实时荧光定量PCR检测方法进行检测。结果表明:送检50份样品中该方法检出40份阳性,待检样品的PCV2阳性率达80%(表 3),与普通PCR检测结果基本一致。
表 3 实时荧光定量PCR方法检测临床样品PCV2的结果
Table 3. Results of detecting PCV2 from clinical samples by fluorescent quantitative PCR
年份 阳性样本/个 阴性样本/个 样本总数/个 阳性率/% 95%置信区间下限/% 95%置信区间上限/% 2016 24 6 30 80 61.4 92.3 2017 16 4 20 80 56.3 94.3 总计 40 10 50 80 66.3 90.0 -
PCV2是目前规模化猪场疾病防控中最重要的病原体之一,通常伴随细菌感染[10],因此要检定出PCV2,需要建立高度灵敏的检测方法。YANG等[11]建立的基于SYBR Green I检测PCV2的方法检测范围是109~1017拷贝·L-1,郑兰兰等[12]建立的PCV2和PRV二重SYBR Green I荧光定量PCR方法对PCV2的检测灵敏度是215×106拷贝·L-1;本研究建立的方法能检测PCV2病毒基因组DNA最低浓度达到为8.825×106拷贝·L-1,比常规PCR和基于SYBR Green I的荧光定量PCR更加灵敏。影响实时荧光定量PCR方法的灵敏度涉及许多因素,如样品来源的复杂程度,引物和探针特异性,体系及扩增条件等,其中引物和探针的设计是最重要的关键点。SYBR Green I法是基于荧光染料与双链DNA结合后检测荧光值来判断产物的量,而Taqman探针法则是基于DNA酶的5′外切酶活性对探针的切割后,荧光信号的强弱判断扩增产物的量,因而探针的特异结合使得该方法具有更高的特异性。本研究针对PCV2的ORF1保守区域设计特异引物,有利于扩增PCV2所有基因型的毒株,其探针则是基于15碱基的特异序列,具有较高结合效率;普通Taqman探针一般为25~35 bp,而MGB探针由于在普通Taqman 3′端特殊的设计,长度更短,一般为10到15 bp,因而灵敏性更高,同时还具有淬灭无荧光、背景低等特点。所以本研究采用的淬灭基团为MGB的Taqman探针,提高了检测灵敏度,降低了背景值。
基于ORF2(Cap)基因分类方法,分子流行病学分析认为PCV2在临床上流行主要为PCV2a,PCV2b 2个基因型[13],且后者的流行明显超过前者;近期发现还存在PCV2c,2d,2e等基因型[14-15]。本研究在设计引物时针对相对保守的ORF1(Rep)基因,因此能够检测PCV2的所有基因型,为临床诊断提供了可靠的检测工具。
Development of Taqman real time fluorescent quantitative PCR for porcine circovirus type 2
-
摘要: 猪圆环病毒2型(PCV2)是一种引起仔猪Sus scrofa多系统衰竭综合征、免疫抑制、皮肤肾病综合征等疾病的病原体。根据GenBank中PCV2 ORF1基因序列设计合成特异性引物和探针,以PCV2基因组DNA为模板,通过聚合酶链式反应(PCR)扩增目的片段,并将目的片段克隆至pMD18-T载体,构建阳性标准品。以阳性标准品为模板,优化荧光定量PCR反应条件,建立标准曲线,进行灵敏性、重复性和特异性验证,并应用此方法对临床样品进行检测。结果表明:阳性质粒标准品构建成功,该检测方法的引物与探针的最佳浓度皆为0.2 μmol·L-1,线性检测范围为2.1×1013~2.1×106拷贝·L-1,最低检测限值为8.828×106拷贝·L-1,且与其他相关疾病无交叉反应;各批次检测变异系数低,检测方法稳定性好。本研究建立的敏感性高、特异性好的实时荧光定量PCR方法,为PCV2的快速检测提供了新工具。Abstract: Porcine circovirus type 2 (PCV2) is a pathogen causing postweaning multisystemic wasting syndrome (PMWS), immune suppression, and skin nephrotic syndrome of piglets. To construct specific method of detecting PCV2 nucleic acid basing on Taqman probes, PCV2 genomic DNA was used as a template for polymerase chain reaction (PCR). The specific primers of PCV2, open reading frame 1 (ORF1), and probes were designed and synthesized according to the gene sequence in GenBank. Then, the target fragment was amplified and cloned into a pMD18-T vector. The cyclic conditions of quantitative PCR were optimized by a template with a positive plasmid standard. The standard curve of real time fluorescent quantitative PCR for PCV2 was established and the sensitivity, repeatability, and specificity of the method were verified by three repeatability tests. Results showed that a positive plasmid was successfully constructed. The optimal concentration of primers and probe was 0.2 μmol·L-1, and the minimum detection threshold was 8.828×106 copies·L-1. The linear range of detection was 1013-106 copies·L-1. This method had no cross reaction of related diseases, a low coefficient of variation, and well repeatability between testing of every batch. From this study then, the high sensitivity and specific real-time quantitative PCR for PCV2 could be used as a tool for rapidly diagnosing PCV2 infectious diseases.
-
Key words:
- zoology /
- PCV2 /
- fluorescent quantitative RT-PCR /
- sensitivity /
- specificity /
- detection method
-
表 1 实时荧光定量PCR的重复性验证
Table 1. Repetitive assay of the real-time quantitative PCR
质粒浓度/(拷贝·L-1) CV/% 组内 组间 1013 0.28 0.34 1012 0.14 0.32 1011 0.09 0.28 表 2 实时荧光定量PCR的灵敏度
Table 2. Sensitivity of the real-time quantitative PCR
质粒浓度/(×106拷贝·L-1) 样本数/个 阳性样本/个 1 000 6 6 100 6 6 50 6 6 20 6 6 5 6 5 0 6 0 表 3 实时荧光定量PCR方法检测临床样品PCV2的结果
Table 3. Results of detecting PCV2 from clinical samples by fluorescent quantitative PCR
年份 阳性样本/个 阴性样本/个 样本总数/个 阳性率/% 95%置信区间下限/% 95%置信区间上限/% 2016 24 6 30 80 61.4 92.3 2017 16 4 20 80 56.3 94.3 总计 40 10 50 80 66.3 90.0 -
[1] AFGHAH Z, WEBB B, MENG Xiangjin, et al. Ten years of PCV2 vaccines and vaccination:is eradication a possibility?[J]. Vet Microbiol, 2016, 206:21. doi:10.1016/j.vetmic.2016.10.002. [2] CANELLI E, BORGHETTI P, FERRARI L, et al. Immune response to PCV2 vaccination in PRRSV viraemic piglets[J]. Vet Rec, 2016, 178(8):193. doi:10.1136/vr.103637. [3] OBER R A, THISSEN J B, JAING C J, et al. Increased microbiome diversity at the time of infection is associated with improved growth rates of pigs after co-infection with porcine reproductive and respiratory syndrome virus (PRRSV) and porcine circovirus type 2(PCV2)[J]. Vet Microbiol, 2017, 208:203-211. doi:10.1016/j.vetmic.2017.06.023. [4] FENG Hua, SEGALES J, FRAILE L, et al. Effect of high and low levels of maternally derived antibodies on porcine circovirus type 2(PCV2) infection dynamics and production parameters in PCV2 vaccinated pigs under field conditions[J]. Vaccine, 2016, 34(27):3044-3050. doi:10.1016/j.vaccine.2016.04.088. [5] XIAO Chaoting, HALBUR P G, OPRIESSNIG T. Global molecular genetic analysis of porcine circovirus type 2(PCV2) sequences confirms the presence of four main PCV2 genotypes and reveals a rapid increase of PCV2d[J]. J Gen Virol, 2015, 96(7):1830-41. doi:10.1099/vir.0.000100. [6] ZHANG Dan, HE Kongwang, WEN Libin, et al. Genetic and phylogenetic analysis of a new porcine circovirus type 2(PCV2) strain in China[J]. Arch Virol, 2015, 160(12):3149-3151. doi:10.1007/s00705-015-2615-8. [7] LIU Xin, WANG Fengxue, ZHU Hongwei, et al. Phylogenetic analysis of porcine circovirus type 2(PCV2) isolates from China with high homology to PCV2c[J]. Arch Virol, 2016, 161(6):1591-1599. doi:10.1007/s00705-016-2823-x. [8] 于静, 劳秀杰, 陈彦永, 等.猪圆环病毒2型实时荧光定量PCR检测方法的建立[J].浙江农林大学学报, 2016, 33(2):357-363. YU Jing, LAO Xiujie, CHEN Yanyong, et al. A real-time PCR method for detection of porcine circovirus 2[J]. J Zhejiang A & F Univ, 2016, 33(2):357-363. doi:10.11833/j.issn.2095-0756.2016.02.023. [9] WILRICH C, WILRICH P T. Estimation of the POD function and the LOD of a qualitative microbiological measurement method[J]. J AOAC Int, 2009, 92(6):1763-1772. [10] LIU Shuqing, LI Wentao, WANG Yang, et al. Coinfection with Haemophilus parasuis serovar 4 increases the virulence of porcine circovirus type 2 in piglets[J]. Virol J, 2017, 14(1):227. doi:10.1186/s12985-017-0890-6. [11] YANG Zongzhao, HABIB M, SHUAI Jiangbing, et al. Detection of PCV2 DNA by SYBR Green I-based quantitative PCR[J]. J Zhejiang Univ Sci B, 2007, 8(3):162-169. doi:10.1631/jzus.2007.B0162. [12] 郑兰兰, 张君涛, 李明凤, 等.猪伪狂犬病毒与猪圆环病毒2型二重SYBR Green Ⅰ实时荧光PCR检测方法的建立[J].中国兽医学报, 2014, 34(3):366-370, 378. ZHENG Lanlan, ZHANG Juntao, LI Mingfeng, et al. Simultaneous detection of porcine pseudorabies virus and porcine circovurs 2 by duplex real-time PCR[J]. Chin J Vet Sci, 2014, 34(3):366-370, 378. [13] NEIRA V, RAMOS N, TAPIA R, et al. Genetic analysis of porcine circovirus type 2 from pigs affected with PMWS in Chile reveals intergenotypic recombination[J]. Virol J, 2017, 14(10):191. doi:10.1186/s12985-017-0850-1. [14] LIU Jiankui, WEI Chuhua, DAI Ailing, et al. Detection of PCV2e strains in Southeast China[J]. Peer J, 2018, 6(14):e4476. doi:10.7717/peerj. 4476. [15] QU Tailong, LI Runcheng, YAN Meijun, et al. High prevalence of PCV2d in Hunan Province, China:a retrospective analysis of samples collected from 2006 to 2016[J]. Arch Virol, 2018, 163(7):1897-1906. doi:10.1007/s00705-018-3823-9. -

-
链接本文:
https://zlxb.zafu.edu.cn/article/doi/10.11833/j.issn.2095-0756.2018.06.018







 下载:
下载: