-
植物在生长发育过程中合成并释放低沸点、小分子量的次生代谢产物,统称为挥发性有机化合物(volatile organic compounds,VOCs)[1]。VOCs具有多种生理生态功能,是重要的植保素、引诱剂,能直接或间接参与植物的防御反应[2]。番茄Lycopersicon esculentum[3],黄花蒿Artemisia annua[4]的腺毛是其合成并存储代谢产物最主要场所,主要分布在花、茎、叶等地上组织,而花部位释放的萜类物质含量较高。植物鲜花释放的气味与植物种类、发育时期、生长环境和营养状态等有密切关系。目前,玫瑰Rosa rugosa,玉兰Magnolia denudata,蜡梅Chimonanthus praecox,桂花Osmanthus fragrans等多种具有重要观赏价值园林树木鲜花释放的VOCs已被鉴定[5-8]。不同桂花品种的香气在VOCs组分、数量、含量上存在差异[9],但金桂(Luteus group),银桂(Albus group)和丹桂(Aurantiacus group)的花香又具有一定的相似性[10],而且每种桂花释放的VOCs成分及含量均与环境因子紧密关联,生长环境、气温和光强是造成其差异的主要影响因素[11]。日本紫藤Wisteria floribunda在开花进程中,释放的VOCs以萜烯类为主,并随着花朵发育物质成分发生显著的变化[12];梅花Prunus mume在开放过程中,其香气成分也具有时空动态变化特征[13]。自然条件下有关4个桂花品种群开花进程中鲜花释放VOCs动态变化却鲜见报道。桂花是中国传统十大名花之一。其花香、花色、花朵数量及花期持续时间是育种的重要目标,因此,本研究以金桂、银桂、丹桂和四季桂(Asiaticus group)等4个桂花品种群的鲜花为研究对象,在自然环境中利用动态顶空气体循环采集法与热脱附-气相色谱-质谱(TDS-GC-MS)联用技术,以花蕾期、初花期、盛花期、衰败期为时间点,进行VOCs的采集与分析。综合分析相关参数在各时期的变化特征及种类与物质含量的差异。揭示4个桂花品种群在自然条件下鲜花开放过程释放VOCs的动态变化规律,为进一步研究桂花鲜花VOCs对人体健康的影响提出理论依据,从而为园林植物的合理配置提供科学的参考。
HTML
-
实验选取金桂、银桂、丹桂和四季桂各1个品种(未确定品种名),均来自于浙江农林大学东湖校区,在自然条件下选取长势良好、无病虫害、无机械损伤,树龄为15~20 a,树高为5 m,冠幅为3 m,胸径为20 cm且分枝点低,花蕾繁茂的树种。
-
于2015年9-10月,监测花期,并对花蕾期、初花期、盛花期、衰败期释放的VOCs进行采集。在晴朗无风的天气,用QC-2型大气采样仪采用动态顶空气体循环采集法[14]分别采集4个桂花品种不同开花阶段的VOCs。各个时期采样重复3次,气体循环流量为100 mL·min-1,采气时间20 min,采集后采摘鲜花称量。
-
VOCs用TDS-GC-MS技术分析,仪器及参数设置条件参考文献[15]。TDS(TD3型,德国GERSTEL公司)工作条件:系统载气压力20 kPa;进样口温度250 ℃;脱附温度250 ℃(10 min);冷阱温度-100 ℃(保持3 min);冷阱进样时温度骤然升温至260 ℃。GC(7890A型,Agilent公司)工作条件:色谱柱为30 m × 250.00 μm × 0.25 μm的HP-5MS柱;程序升温:初始温度40 ℃(保持4 min),以6 ℃·min-1的速率升至250 ℃(保持3 min),以10 ℃·min-1的速率升至270 ℃(保持5 min)。MS(5975C型,Agilent公司)工作条件:电离方式为EI,电子能量为70 eV,原子质量范围为28~450,接口温度为280 ℃,离子源温度为230 ℃,四级杆温度为150 ℃。
-
通过TDS-GC-MS技术分析,获得VOCs的总离子流量色谱图,对比气质联用仪计算机的NIST 2008谱库,以色谱峰的峰面积计算相对含量,并兼顾色谱保留时间,结合手工检索定性。再用Origin 8和SPSS 18.0软件进行制图和方差分析。
1.1. 材料
1.2. 方法
1.2.1. VOCs的采集
1.2.2. VOCs的分析
1.3. 数据分析
-
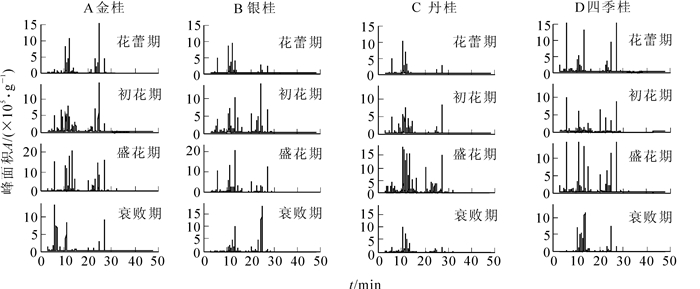
金桂鲜花VOCs成分的总离子流图见图 1A,其所释放的VOCs相对含量在开放进程中呈现先上升后下降的变化趋势,伴随着花朵发育,香味随之浓郁,所释放的VOCs成分和相对含量也随之显著增加。在开花进程中,共发现43种物质。从花蕾期到衰败期分别检测出23,32,38和18种物质,总释放量依次为302.2,353.3,556.8和171.2(峰面积A×105·g-1)。在盛花期VOCs种类和相对含量达到最大值,与花蕾期相比较,种类增加了16种,释放量增加了1.8倍。其中萜类增加到了23种,占盛花期总量的78.2%,醛类4种占盛花期总量的3.7%,烃类3种占盛花期总量的2.8%,醇类1种占盛花期总量的2.6%,酮类1种占盛花期总量的0.2%,酯类2种占盛花期总量的7.8%,4种芳香族类化合物占盛花期总量的3.9%及1种酸类化合物占盛花期总量的0.8%(表 1)。随着开花进程,β-紫罗兰酮、萜品醇和乙酸-3-己烯酯释放量呈现低—高—低的动态变化,而罗勒烯和β-蒎烯在花蕾期达到最大值之后呈逐步下降趋势。
序号 t/min 挥发性有机化合物 分子式 峰面积A/(×105·g-1) 花蕾期 初花期 盛花期 衰败期 1 3.50 2-甲基环己醇2-methyl cyclohexanol C7H14O 2.40 ± 0.34 1.45 ± 0.61 14.49 ± 2.13 4.96 ± 0.55 2 5.23 辛烯octene C8H16 5.39 ± 0.49 10.76 ± 0.48 7.05 ± 0.48 5.28 ± 0.21 3 5.36 己醛hexanal C6H12O — — 12.70 ± 1.92 — 4 6.35 糠醛furfural C5H4O2 5.41 ± 0.41 2.30 ± 0.30 2.29 ± 0.35 12.53 ± 2.08 5 6.82 2-己烯醛2-hexenal C6H10O — — — 2.88 ± 0.87 6 6.98 乙苯ethylbenzene C8H10 6.10 ± 0.11 5.22 ± 0.19 12.38 ± 2.35 1.73 ± 0.64 7 7.20 二甲苯xylene C8H10 — 5.45 ± 0.64 5.65 ± 0.78 — 8 7.77 环辛四稀cyclooctatetraene C8H8 3.50 ± 0.35 5.45 ± 0.41 3.78 ± 0.73 — 9 7.98 壬烷nonane C9H20 — — 4.96 ± 0.66 — 10 8.07 庚酵heptanal C7H14O — — 2.62 ± 0.53 — 11 8.92 α-蒎烯α-pinene C10H16 3.63 ± 0.37 21.98 ± 1.31 1.67 ± 0.60 — 12 9.32 莰稀camphene C10H16 — — 2.29 ± 0.41 — 13 10.46 7-辛烯-2-酮7-octen-2-one C8H14O 11.61 ± 1.54 4.51 ± 0.57 1.25 ± 0.34 0.38 ± 0.09 14 10.53 β-蒎烯β-pinene C10H16 41.71 ± 2.08 3.46 ± 0.43 18.55 ± 1.64 0.33 ± 0.05 15 10.86 水芹烯phellandrene C10H16 3.67 ± 0.34 7.33 ± 0.43 2.32 ± 0.40 — 16 10.99 乙酸-3-己烯酯3-hexenyl acetate C8H14O2 16.86 ± 3.37 10.70 ± 1.41 37.52 ± 3.00 17.60 ± 1.28 17 11.20 α-萜品烯α-terpinene C10H16 3.25 ± 0.33 — 1.58 ± 0.59 — 18 11.42 邻伞花烃o-cymene C10H14 — 9.08 ± 1.10 0.95 ± 0.06 — 19 11.53 柠檬烯limonene C10H16 18.54 ± 0.85 15.49 ± 2.11 11.47 ± 1.53 — 20 11.80 反式-罗勒烯trans-ocimene C10H16 15.90 ± 3.15 1.44 ± 0.42 5.18 ± 0.18 — 21 12.07 顺式-罗勒烯cis-ocimene C10H16 56.96 ± 9.09 8.80 ± 0.98 26.90 ± 3.61 0.34 ± 0.05 22 12.74 顺式-氧化芳樟醇cis-linalool oxide C10H18O2 1.55 ± 0.44 — 1.60 ± 0.22 — 23 13.43 芳樟醇linalool C10H18O 3.46 ± 0.31 6.27 ± 0.04 1.35 ± 0.47 — 24 13.48 萜品醇terpineol C10H18O 3.29 ± 0.35 1.58 ± 0.33 63.78 ± 0.75 18.82 ± 1.22 25 13.88 紫苏烯perillen C10H14O — 1.63 ± 0.39 3.37 ± 0.49 — 26 13.96 1,3, 8-薄荷三烯1,3, 8-menthatriene C10H14 2.59 ± 0.56 3.41 ± 0.73 1.30 ± 0.26 — 27 14.16 2-蒈烯2-carene C10H16 — — 1.54 ± 0.59 — 28 14.18 香芹醇carveol C10H16O 6.49 ± 0.34 6.20 ± 0.71 2.36 ± 0.48 5.85 ± 0.54 29 15.19 马鞭草烯醇verbenol C10H16O — — 0.95 ± 0.04 0.73 ± 0.03 30 15.32 环氧芳樟醇epoxylinalol C10H18O2 — — 1.34 ± 0.18 0.97 ± 0.03 31 15.59 3-己烯基丁酸3-hexenyl butyrate C10H18O2 — — 4.63 ± 0.70 — 32 16.14 癸醒decanal C10H20O — — 2.78 ± 0.71 — 33 17.78 香叶醇geraniol C10H18O — — — 8.87 ± 0.40 34 20.49 大马酮damascone C13H20O — 2.78 ± 0.65 — 7.60 ± 0.53 35 21.16 紫罗烯ionene C13H18 — 1.36 ± 0.51 1.87 ± 0.61 — 36 22.41 巨豆三烯酮megastigmatrienone C13H18O — 5.66 ± 0.38 2.65 ± 0.47 — 37 22.58 长叶烯longifolene C15H24 — 3.75 ± 0.32 8.35 ± 0.46 1.88 ± 0.87 38 22.96 石竹烯caryophyllene C15H24 26.57 ± 4.45 11.72 ± 1.93 15.35 ± 0.08 1.69 ± 0.83 39 23.17 α-紫罗兰酮α-ionone C13H20O — 3.60 ± 0.22 — — 40 23.45 二氢-紫罗兰酮dihydro-ionone C13H22O 11.85 ± 2.31 5.24 ± 0.76 2.76 ± 0.01 0.39 ± 0.08 41 24.20 癸内酯decalactone C10H18O2 24.00 ± 4.03 8.62 ± 0.39 5.87 ± 0.74 34.13 ± 3.10 42 24.42 衣兰油烯muurolene C15H24 — 2.25 ± 0.19 2.35 ± 0.41 — 43 24.64 β-紫罗兰酮β-ionone C13H20O 27.42 ± 3.48 175.75 ± 24.47 257.05 ± 28.95 44.28 ± 6.06 总峰面积 302.15 353.26 556.82 171.24 说明:“—”代表未检测到VOCs。 Table 1. The VOCs from the fresh flowers of Luteus cultivar in blooming process
-
银桂鲜花VOCs成分的总离子流图见图 1B。在整个花期中,银桂鲜花共检测出39种物质。与金桂相似,开花进程中所释放的物质种类和相对含量最高峰也出现在盛花期。据感官判断,其整体香味不似其他桂花浓烈,所散发出来的香味较为淡雅。从花蕾期到衰败期分别检测出20,29,36及18种成分,总释放量依次为106.3,287.9,1 100.1和180.9。经比较,盛花期较花蕾期多出了16种物质,相对含量也增加了10.3倍。其中萜类增加到了22种,占盛花期总量的93.8%,醛类4种占盛花期总量的1.4%,烃类1种占盛花期总量的0.2%,醇类4种占盛花期总量的0.9%,酯类1种占盛花期总量的3.0%,芳香族类4种占盛花期总量的0.6%(表 2)。随着花瓣的展开,β-紫罗兰酮、芳樟醇、石竹烯、二氢-紫罗兰酮和罗勒烯释放量也均呈现低—高—低的动态变化。
序号 t/min 挥发性有机化合物 分子式 峰面积A/(×105·g-1) 花蕾期 初花期 盛花期 衰败期 1 3.50 2-甲基环己醇2-methyl cyclohexanol C7H14O 0.57 ± 0.08 2.38 ± 0.36 2.43 ± 0.29 — 2 4.26 5-辛烯-1-醇5-octen-1-ol C8H16O — — 1.18 ± 0.15 — 3 4.72 芳庚tropilidene C7H8 — 5.23 ± 0.27 3.74 ± 0.35 8.04 ± 0.35 4 5.36 己醛hexanal C6H12O — 17.17 ± 2.29 6.59 ± 0.26 — 5 6.82 2-己烯醛2-hexenal C6H10O — — 3.67 ± 0.35 — 6 6.95 反式-3-己烯-1-醇trans-3-hexen-1-ol C6H12O — 3.72 ± 0.38 4.58 ± 0.44 — 7 7.20 二甲苯xylene C8H10 1.15 ± 0.18 2.26 ± 0.27 1.48 ± 0.08 — 8 7.78 壬烯nonene C9H18 — 3.49 ± 0.33 2.42 ± 0.24 — 9 8.07 庚醛heptanal C7H14O — — 1.09 ± 0.07 — 10 8.45 4-庚烯-1-醇4-hepten-1-ol C7H14O — — 1.51 ± 0.20 — 11 8.92 α-蒎烯α-pinene C10H16 0.13 ± 0.04 3.87 ± 0.59 1.52 ± 0.13 — 12 10.53 β-蒎烯β-pinene C10H16 16.81 ± 2.57 17.72 ± 2.33 23.35 ± 3.71 3.46 ± 0.33 13 10.86 水芹烯phellandrene C10H16 1.37 ± 0.09 2.26 ± 0.22 2.60 ± 0.40 — 14 10.99 乙酸-3-己烯酷3-hexenyl acetate C10H16O2 5.23 ± 0.27 17.48 ± 2.46 33.44 ± 4.40 26.33 ± 3.76 15 11.20 α-萜品烯α-terpinene C10H16 1.20 ± 0.17 0.64 ± 0.06 1.26 ± 0.13 — 16 11.42 邻伞花烃o-cymene C10H14 1.17 ± 0.15 0.48 ± 0.02 0.65 ± 0.05 — 17 11.53 柠檬烯limonene C10H16 7.67 ± 0.39 2.49 ± 0.61 4.58 ± 0.42 8.92 ± 0.21 18 11.61 桉树脑eucalyptol C10H18O — 6.56 ± 0.76 4.48 ± 0.67 — 19 11.80 反式罗勒烯trans-ocimene C10H16 6.45 ± 0.36 1.22 ± 0.27 2.61 ± 0.33 13.47 ± 3.96 20 12.07 顺式罗勒烯cis-ocimene C10H16 16.73 ± 2.50 2.69 ± 0.59 38.31 ± 1.94 5.72 ± 0.68 21 12.74 顺式氧化芳樟醇cis-linalool oxide C10H18O2 1.65 ± 0.24 2.31 ± 0.11 23.67 ± 3.70 4.82 ± 0.67 22 13.43 芳樟醇linalool C10H18O 25.39 ± 3.59 55.14 ± 3.87 224.43 ± 33.24 8.96 ± 0.50 23 13.61 脱氢芳樟醇hotrienol C10H16O 0.77 ± 0.03 4.35 ± 0.39 4.56 ± 0.59 — 24 13.88 紫苏烯perillene C10H14O — — 1.69 ± 0.21 — 25 14.18 香芹醇carveol C10H16O — — 0.72 ± 0.06 3.47 ± 0.44 26 15.19 马鞭草烯醇verbenol C10H16O 8.43 ± 0.44 4.42 ± 0.41 27.93 ± 1.60 3.77 ± 0.19 27 15.32 环氧芳樟醇epoxylinalol C10H18O2 — — 3.39 ± 0.43 1.76 ± 0.18 28 16.14 癸醛decanal C10H20O — 4.69 ± 0.69 4.48 ± 0.59 — 29 17, 78 香叶醇geraniol C10H18O — — — 13.14 ± 2.49 30 21.16 紫罗烯ionene C13H20O — 1.60 ± 0.37 1.62 ± 0.23 11.04 ± 0.70 31 22.41 巨豆三烯酉同me gasti gmatrienone C13H18O 0.56 ± 0.04 1.43 ± 0.23 — — 32 22.58 长叶烯longifolene C15H24O — 11.51 ± 1.70 8.45 ± 0.37 — 33 22.75 雪松烯cedrene C15H24 — — 0.74 ± 0.05 — 34 22.96 石竹烯caryophyllene C15H24O 1.38 ± 0.17 14.58 ± 1.94 58.46 ± 0.64 1.13 ± 0.01 35 23.17 α-紫罗兰酮α-ionone C13H20O — 3.74 ± 0.31 — 51.42 ± 0.44 36 23.45 二氢-紫罗兰酮dihydro-ionone C13H22O 0.56 ± 0.05 5.25 ± 0.34 38.42 ± 2.06 0.94 ± 0.05 37 24.48 兔耳草酵cyclamen aldehyde C13H22O 0.43 ± 0.08 3.43 ± 0.36 0.56 ± 0.08 7.66 ± 0.51 38 24.64 β-紫罗兰酮β-ionone C13H20O 8.67 ± 0.89 85.82 ± 2.54 555.05 ± 21.32 6.83 ± 0.31 39 25.27 甲基紫罗兰酮methyl ionone C14H22O — — 4.47 ± 0.58 — 总峰面积 106.32 287.93 1 100.13 180.88 说明:“—”代表未检测到VOCs。 Table 2. The VOCs from the fresh flowers of Albus cultivar in blooming process
-
丹桂鲜花VOCs成分的总离子流图见图 1C。随着丹桂鲜花的开放,所释放的鲜花VOCs成分逐渐增多,相对含量也随之升高,在盛花期达到最大值。而随着花的衰败,VOCs种类和相对含量也逐渐减少。在开花进程中,共检测到52种物质,从花蕾期到衰败期分别检测出18,33,52及21种成分,总释放量依次为76.6,284.5,478.2,223.4。从中可以发现,与花蕾期比较,盛花期物质种类多了34种,相对含量增加了6.2倍。其中萜类增加到了30种,占盛花期总量的65.7%,醛类7种占盛花期总量的13.1%,烃类2种占盛花期总量的1.2%,醇类2种占盛花期总量的3.3%,酮类2种占盛花期总量的1.6%,酯类3种占盛花期总量的13.0%,4种芳香族类化合物占盛花期总量的1.5%及2种酸类化合物占盛花期总量的0.6%(表 3)。开花进程中,芳樟醇、乙酸-3-己烯酯和罗勒烯释放量呈现低—高—低的动态变化,而β-蒎烯和柠檬烯含量则逐步上升,在衰败期达到最大值。
序号 t/min 挥发性有机化合物 分子式 峰面积A/(×105·g-1) 花蕾期 初花期 盛花期 衰败期 1 3.38 戍醛pentanal C5H10O 0.24 ± 0.05 8.80 ± 0.57 2.34 ± 0.58 — 2 3.50 2-甲基环己醇2-methyl cyclohexanol C7H14O — — 6.36 ± 0.50 0.65 ±0.05 3 4.21 羟丙酮hydroxyacetone C3H6O2 — — 6.41 ± 0.48 — 4 4.72 芳庚tropilidene C7H7 3.96 ± 0.23 0.49 ± 0.09 1.10 ± 0.25 9.50 ± 0.46 5 5.23 辛烯octene C8H16 — 3.51 ± 0.63 1.54 ± 0.64 2.45 ± 0.50 6 5.36 己醛hexanal C6H12O 2.28 ± 0.25 18.87 ± 1.96 24.19 ± 3.61 — 7 6.35 糠醛furfural C5H4O2 — 7.82 ± 0.81 3.91 ± 0.54 — 8 6.82 2-己烯醛2-hexenal C6H10O — 2.60 ± 0.48 5.41 ± 0.41 — 9 6.95 反式-3-己烯-1-醇trans-3-hexen-1-ol C6H12O — 25.69 ± 4.03 9.43 ± 0.58 — 10 7.20 二甲苯xylene C8H10O 2.72 ± 0.52 2.84 ± 0.50 2.56 ± 0.61 5C83 ± 0C80 11 7.78 壬烯nonene C9H18O 1.14 ± 0.08 2.64 ± 0.14 4.13 ± 0.40 3.69 ± 0.42 12 8.07 庚酵heptanal C7H14O — — 2.38 ± 0.42 — 13 8.92 α-蒎烯α-pinene C10H16 0.87 ± 0.05 0.77 ± 0.08 2.90 ± 0.52 4.51 ± 0.66 14 9.70 安息香醛benzaldehyde C7H6O — — 1.37 ± 0.02 — 15 10.19 月桂烯myrcene C10H16 0.92 ± 0.06 2.84 ± 0.72 4.37 ± 0.48 2C40 ± 0C54 16 10.53 β-蒎烯β-pinene C10H16 26.39 ± 3.39 6.48 ± 0.68 48.82 ± 6.96 56C91 ± 7C94 17 10.86 水芹烯phellandrene C10H16 2.41 ± 0.22 1.35 ± 0.04 10.16 ± 1.53 5C44 ± 0C34 18 10.99 乙酸-3-己烯酯3-hexenyl acetate C8H14O2 2.69 ± 0.45 29.37 ± 3.80 45.93 ± 6.79 5C58 ± 0C39 19 11.20 α-萜品烯α-terpinene C10H16 — 1.87 ± 0.49 6.23 ± 0.59 5C70 ± 0C29 20 11.42 邻伞花烃o-cymene C10H14 — 5.41 ± 0.31 2.13 ± 0.36 5C24 ± 0C32 21 11.53 柠檬烯limonene C10H16 14.03 ± 0.18 2.37 ± 0.39 27.69 ± 2.01 38C36 ± 5C18 22 11.80 反式-罗勒烯trans-ocimene C10H16 5.35 ± 0.34 1.67 ± 0.46 17.14 ± 2.77 16.67 ± 2.62 23 12.07 顺式-罗勒烯cis-ocimene C10H16 6.51 ± 0.15 14.68 ± 1.90 34.45 ± 4.41 21.45 ± 0.30 24 12.33 γ-萜品烯γ-terpinene C10H16 — 0.87 ± 0.05 3.13 ± 0.14 — 25 12.60 蔚烯fenchene C10H16 — — 1.30 ± 0.32 — 26 12.74 顺式-氧化芳樟醇cis-linalool oxide C10H18O2 0.87 ± 0.07 21.97 ± 2.75 11.75 ± 2.06 2C55 ± 0C49 27 13.11 萜品油烯terpinolene C10H16 4.19 ± 0.20 22.02 ± 2.53 23.02 ± 3.26 7C95 ± 0C36 28 13.43 芳樟醇linalool C10H18O — 15.44 ± 3.08 46.98 ± 7.17 16.48 ± 2.56 29 13.52 壬酵nonanal C9H18O 0.38 ± 0.09 7.75 ± 0.35 12.25 ± 1.75 1C48 ± 0C31 30 13.96 1,3, 8-薄荷三烯1,3, 8-menthatriene C10H14 — 0.66 ± 0.07 2.35 ± 0.13 — 31 14.18 香芹醇carveol C10H16O 1.24 ± 0.23 1.69 ± 0.34 5.36 ± 0.48 2C43 ± 0C30 32 15.17 2-癸烯醇2-decenol C10H20O — — 1.50 ± 0.39 — 33 15.32 环氧芳樟醇epoxylinalol C10H18O2 — 5.37 ± 0.49 8.44 ± 0.48 — 34 15.59 3-己烯基丁酸3-hexenyl butyrate C10H18O2 — — 1.29 ± 0.40 — 35 15.86 水杨酸甲酯methyl salicylate C8H8O3 — 6.6 ± 0.89 3.48 ± 0.54 — 36 16.14 癸醛decanal C11H20O — — 12.28 ± 2.12 — 37 17.09 3-己烯异戍酸3-hexenyl isovalerate C11H20O2 — — 1.68 ± 0.40 — 38 18.34 柠檬醛citral C10H16O — — 1.62 ± 0.05 — 39 21.16 紫罗烯ionene C13H18 — — 4.43 ± 0.39 — 40 22.08 绿叶烯patchoulene C15H24 — 0.61 ± 0.06 1.77 ± 0.32 — 41 22.41 巨豆三烯酮megastigmatrienone C13H18O — — 1.26 ± 0.39 — 42 22.58 长叶烯longifolene C15H24 — 3.94 ± 0.72 13.26 ± 2.06 — 43 22.75 雪松烯cedrene C15H24 — — 1.23 ± 0.31 — 44 22.96 石竹烯caryophyllene C15H24 — — 2.48 ± 0.58 — 45 23.17 α-紫罗兰酮α-ionone C13H20O — — 6.57 ± 0.52 — 46 23.45 二氢紫罗兰酮dihydro-ionone C13H22O — — 9.32 ± 0.39 — 47 24.20 癸内酯 C10H18O2 0.41 ± 0.07 44.31 ± 5.01 12.71 ± 2.17 8.13 ± 0.36 48 24.42 衣兰油烯muurolene C15H24 — 3.63 ± 0.55 4.69 ± 0.70 — 49 24.64 β-紫罗兰酮β-ionone C13H20O — 9.56 ± 0.60 4.21 ± 0.32 — 50 25.31 杜松烯cadinene C15H24 — — 1.25 ± 0.26 — 51 25.52 去氢白菖烯calamenene C15H22 — — 1.47 ± 0.50 — 52 27.22 甲基紫罗兰烯methyl-ionene C14H22O — — 6.20 ± 0.51 — 总峰面积 76.61 284.52 478.18 223.40 说明:“—”代表未检测到VOCs。 Table 3. The VOCs from the fresh flowers of Aurantiacus cultivar in blooming process
-
四季桂鲜花VOCs成分的总离子流图见图 1D。与其他品种的桂花相似,四季桂在整个花期中所释放的VOCs种类和含量也在盛花期达到最大值,在衰败期又有所下降。这与四季桂开花过程中所散发出来的香气浓度变化趋势相同。整个花期中,共发现46种物质,从花蕾期到衰败期分别检测出29,36,42及22种成分,总释放量依次为150.7,227.4,1 202.1和124.3。经分析,盛花期物质种类较花蕾期多13种,相对含量也大幅增加了8.0倍。其中萜类增加到了23种,占盛花期总量的94.4%,醛类7种占盛花期总量的2.0%,烃类1种占盛花期总量的0.1%,醇类3种占盛花期总量的0.9%,酮类1种占盛花期总量的0.2%,酯类2种占盛花期总量的1.5%,芳香族类5种占盛花期总量的0.9%(表 4)。随着开花的进程,萜品醇、β-紫罗兰酮、β-蒎烯、罗勒烯和柠檬烯释放量也均呈现低—高—低的动态变化。
序号 t/min 挥发性有机化合物 分子式 峰面积A/(×105·g-1) 花蕾期 初花期 盛花期 衰败期 1 3.50 2-甲基环己醇2-methyl cyclohexanol C7H14O — 6.42 ± 0.09 5.47 ± 0.65 — 2 4.26 5-辛烯-1-醇5-octen-1-ol C8H16O — — 1.37 ± 0.51 — 3 4.72 芳庚tropilidene C7H8 6.47 ± 0.16 12.21 ± 0.84 3.48 ± 0.64 4.53 ± 0.31 4 5.36 己醛hexanal C6H12O 3.80 ± 0.67 7.75 ± 0.78 6.69 ± 0.71 4.51 ± 0.45 5 6.35 糠醛furfural C5H4O2 2.49 ± 0.02 1.54 ± 0.46 5.56 ± 0.15 — 6 6.98 乙苯ethylbenzene C8H10 5.29 ± 0.28 3.64 ± 0.43 3.51 ± 0.55 — 7 7.20 二甲苯xylene C8H10 5.28 ± 0.20 4.73 ± 0.31 1.34 ± 0.01 — 8 7.77 环辛四烯cyclooctatetraene C8H8 4.60 ± 0.38 4.82 ± 0.52 1.71 ± 0.20 — 9 8.07 庚醛heptanal C7H14O 1.64 ± 0.35 1.47 ± 0.34 1.33 ± 0.39 — 10 8.38 山梨醛sorbaldehyde C6H6O — — 2.57 ± 0.45 — 11 8.92 α-蒎烯α-pinene C10H16 2.01 ± 0.01 4.58 ± 0.49 0.83 ± 0.04 — 12 9.72 间乙基甲苯m-ethyltoluene C9H12 2.35 ± 0.05 2.53 ± 0.54 1.22 ± 0.08 — 13 10.19 月桂烯myrcene C10H16 — — 1.51 ± 0.01 — 14 10.28 苯丙烯phenyl propylene C9H10 — 1.48 ± 0.02 1.37 ± 0.06 — 15 10.46 7-辛烯-2-酮7-octen-2-one C8H14O — 2.59 ± 0.55 2.21 ± 0.27 — 16 10.53 β-蒎烯β-pinene C10H16 7.22 ± 0.55 12.55 ± 2.02 147.06 ± 26.79 2.68 ± 0.29 17 10.80 辛醛octanal C8H16O 1.67 ± 0.25 2.32 ± 0.38 1.44 ± 0.37 — 18 10.86 水芹烯phellandrene C10H16 — 2.77 ± 0.42 1.49 ± 0.19 8.12 ± 0.29 19 10.99 乙酸-3-己烯酷3-hexenyl acetate C8H14O2 5.60 ± 0.21 4.63 ± 0.51 15.05 ± 2.77 6.57 ± 0.34 20 11.20 α-萜品烯α-terpinene C10H16 — 1.76 ± 0.31 18.33 ± 2.24 14.34 ± 3.30 21 11.53 柠檬烯limonene C10H16 4.48 ± 0.35 4.52 ± 0.68 65.51 ± 1.76 0.91 ± 0.02 22 11.60 辛醇ethyl hexanol C8H18O 2.74 ± 0.65 5.64 ± 0.48 3.45 ± 0.50 6.89 ± 0.34 23 11.80 反式-罗勒烯trans-ocimene C10H16 2.43 ± 0.60 7.65 ± 0.34 41.03 ± 1.43 1.48 ± 0.29 24 12.07 顺式-罗勒烯cis-ocimene C10H16 3.24 ± 0.26 3.45 ± 0.32 68.08 ± 1.52 2.38 ± 0.38 25 12.33 γ-萜品烯γ-terpinene C10H16 — 1.38 ± 0.34 1.29 ± 0.34 6.10 ± 0.34 26 12.74 顺式氧化芳樟醇cis-linalool oxide C10H18O2 1.67 ± 0.23 1.39 ± 0.37 52.14 ± 2.06 1.25 ± 0.27 27 13.43 芳樟醇linalool C10H18O 1.51 ± 0.39 1.87 ± 0.42 57.10 ± 0.29 3.46 ± 0.46 28 13.48 萜品醇terpineol C10H18O 34.88 ± 5.52 35.08 ± 3.43 487.21 ± 63.73 2.69 ± 0.32 29 13.52 壬醛nonanal C9H18O 3.51 ± 0.69 4.93 ± 0.52 3.82 ± 0.66 — 30 13.88 紫苏烯perillene C10H14O — — 1.32 ± 0.31 2.53 ± 0.19 31 14.18 香芹醇carveol C10H16O 1.28 ± 0.30 — 0.41 ± 0.03 4.49 ± 0.39 32 15.32 环氧芳樟醇epoxylinalol C10H18O2 1.14 ± 0.14 — 0.44 ± 0.05 7.18 ± 0.25 33 15.88 二氢香茅醇dihydrocitronellol C10H22O — — 2.50 ± 0.50 — 34 16.14 癸醛decanal C10H22O 2.33 ± 0.44 1.66 ± 0.37 2.40 ± 0.46 — 35 17.77 正丙烯酸己酷hexyl acrylate C9H16O2 — — 3.52 ± 0.62 — 36 19.35 二氢甲基紫罗兰酮dihydro-methylionon, C14H24O 1.52 ± 0.48 2.09 ± 0.06 — — 37 21.16 紫罗烯ionene C13H18 — 2.41 ± 0.45 — — 38 22.08 绿叶烯patchoulene C15H24 1.30 ± 0.00 1.72 ± 0.35 1.67 ± 0.28 — 39 22.58 长叶烯longifolene C15H24 — 6.38 ± 0.41 12.06 ± 1.75 — 40 22.96 石竹烯caryophyllene C15H24 — — 1.31 ± 0.33 — 41 23.17 α-紫罗兰酮α-ionone C13H20O 8.92 ± 0.25 19.09 ± 2.00 7.30 ± 0.37 17.19 ± 1.69 42 23.45 二氢紫罗兰酮dihydro-ionone C13H22O 3.68 ± 0.34 5.61 ± 0.39 0.76 ± 0.05 13.60 ± 3.10 43 24.48 兔耳草醛cyclamen aldehyde C13H18O 1.45 ± 0.29 5.57 ± 0.19 — 9.14 ± 0.16 44 24.64 β-紫罗兰酮β-ionone C13H20O 26.22 ± 4.05 33.61 ± 3.37 163.80 ± 18.93 0.60 ± 0.02 45 25.27 甲基紫罗兰酮methylionone C14H22O — — 1.48 ± 0.33 — 总峰面积 150.72 227.37 1 202.14 124.33 说明:“—”代表未检测到VOCs Table 4. The VOCs from the fresh flowers of Asiaticus cultivar in blooming process
综上所述,同个桂花品种在整个花期的不同阶段,鲜花释放的VOCs成分和相对含量存在差异。均表现为花蕾期种类少、相对含量低;盛开期种类多、相对含量高,释放量表现为单峰曲线。4个桂花品种进行比较,四季桂在盛花期释放的VOCs量最大;而丹桂在盛花期释放的VOCs种类最多,即为52种。在每种桂花花蕾期、初花期、盛花期和衰败期VOCs的成分中均含有萜类、烃类、醛类、醇类、酮类、酯类及芳香族类化合物,其中萜类化合物占大多数(均高于70%以上)。金桂、银桂、丹桂及四季桂盛花期萜类物质相对含量分别为78.2%,93.8%,65.7%及94.4%,其中四季桂中萜烯物质相对含量最高。
-
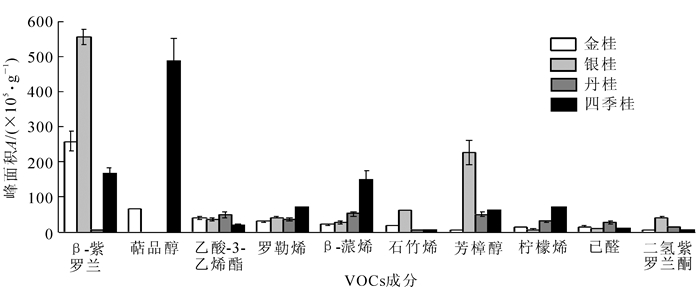
4个桂花品种在整个开花过程中盛花期所释放的VOCs种类和相对含量均达到最高值。对比盛花期高相对含量的前几种物质发现,不同品种的桂花,VOCs组成及相对含量存在显著的差异(图 2)。金桂在盛花期阶段相对含量最高的物质成分为β-紫罗兰酮(46.2%),萜品醇(11.5%),乙酸-3-己烯酯(6.7%),罗勒烯(4.8%),β-蒎烯(3.3%),占盛花期总量的72.5%。银桂在盛花期阶段含量最高的物质成分包括β-紫罗兰酮(50.5%),芳樟醇(20.4%),石竹烯(5.3%),二氢-紫罗兰酮(3.5%),罗勒烯(3.5%),占盛花期总量的83.1%。丹桂在盛花期阶段含量最高的物质成分包括β-蒎烯(10.2%),芳樟醇(9.8%),乙酸-3-己烯酯(9.6%),罗勒烯(7.2%),柠檬烯(5.8%),占盛花期总量的42.6%。四季桂在盛花期阶段含量最高的物质成分为萜品醇(40.5%),β-紫罗兰酮(13.6%),β-蒎烯(12.2%),罗勒烯(5.7%),柠檬烯(5.4%),占盛花期总量的77.5%。

Figure 2. Contrast of major VOCs from the fresh flowers of four Osmanthus fragrans cultivars at full-bloom stage
通过对比4个桂花品种盛花期鲜花释放的VOCs主要成分和相对含量,发现它们存在很大的差异,虽然萜烯类是它们释放的主要物质,但是每种萜烯在4种桂花中的组成和相对含量相差较大,构成了4个桂花品种各自具有独特的主要VOCs组分。例如莰烯、2-蒈烯、大马酮(盛花期无)是金桂的特有成分;桉树脑、脱氢芳樟醇、4-庚烯-1-醇是银桂的特有成分;萜品油烯、柠檬醛、反式-2-癸烯醇、去氢白菖烯、葑烯、杜松烯、甲基紫罗兰烯、戊醛、羟丙酮、水杨酸甲酯、安息香醛、顺式-3-己烯异戊酸是丹桂的特有成分;二氢香茅醇、二氢甲基紫罗兰酮(盛花期无)、辛醛、山梨醛、辛醇、正丙烯酸己酯、间乙基甲苯、苯丙烯是四季桂的特有成分。
2.1. 4个桂花品种不同开花阶段鲜花VOCs分析
2.1.1. 金桂
2.1.2. 银桂
2.1.3. 丹桂
2.1.4. 四季桂
2.2. 4个桂花品种盛花期VOCs主要物质比较
-
蜡梅、日本紫藤、‘西伯利亚’百合Lilium ‘Siberia’等观赏植物在花发育过程中VOCs的种类和含量均发生显著的变化,随着花的开放,释放VOCs的种类及含量逐渐增多,并随着花瓣的展开释放量增加[7, 12, 16]。本研究表明:4个桂花品种开花进程中鲜花所释放的VOCs成分种类和相对含量总体呈现单峰曲线状态,并在盛花期达到顶峰。花香VOCs成分和释放量的变化主要取决于不同发育阶段花香成分相关合成酶的活性及基因表达的差异[17]。所以,可能是酶活性和基因表达的差异导致了桂花在开花过程中,鲜花VOCs成分及含量的不同。
辣薄荷Mentha × piperita,金鱼草Antirrhinum majus中的萜类物质含量及组分随着其发育阶段的不同而发生显著的差异:薄荷中单萜快速合成阶段主要集中在叶片发育初期,随着叶片的生长发育,主要成分也不断发生变化,依次为柠檬烯、薄荷酮[18];览香烯和罗勒烯作为金鱼草花香的主要成分,在花期的第2~6天快速上升直至最大值,随后逐步减少[19]。与上述类似,伴随着桂花鲜花的不断发育,萜类物质迅速增加,盛开期含量最高且种类最多,衰败期又有所减少,同时醛类、酯类、醇类、烃类及芳香类物质也基本呈现出相同的变化规律。这可能是受相关生物合成酶的影响所致。萜类化合物是萜类合酶以异戊烯二磷酸为底物,合成了半萜、单萜、倍半萜、二萜及多萜,其中萜类合酶催化机制复杂[3],但萜类合酶的数量和活性决定了植物萜类化合物结构的多样性[20]。这可能导致了桂花在开花过程中VOCs成分和释放量的多样化。另外,这也有可能是受开花季节外源环境的影响所致,如光、温度、湿度和营养平衡等都可以很大程度地影响VOCs的合成和释放[21]。如在提高相对温度、湿度及光照条件下,薄荷会释放出更多的VOCs[18]。5-磷酸脱氧木酮糖还原异构酶(DXR)只在光照条件下参与磷酸脱氧木酮糖(DXP)途径,从而影响DXP途径的活性,影响单萜的生物合成[22]。随着气温的升高和紫外线的增强,雌性欧洲杨树Populus tremula的VOCs释放量要高于雄性[23];KARBAN等[24]发现蒿属Artemisia植物VOCs作为植物彼此间沟通的一种语言,在受到损伤后释放的VOCs可以通过空气传播方式增强邻近植物的诱导性防御应答。因此一个随意的机械损伤,会长期影响菊科Compoistae植物VOCs的释放[25]。
-
植物释放的VOCs种类和含量决定其自身的气味,在不同植物中VOCs的种类、数量、含量存在一定的比例,因而构成了其特征香气。如侯丹等[26]通过对桂花香气成分及释放节律的研究,发现3个桂花品种中含量最高的物质成分均为芳樟醇,其中‘堰虹桂’‘Yanhong Gui’‘杭州黄’‘Hangzhou Huang’中的芳樟醇相对含量从蕾期至盛开末期先增大后减小,并在盛开期达到最大值;而‘玉玲珑’‘Yu Linglong’中的芳樟醇相对含量最高峰则出现在半开期。本研究结果显示:金桂在开花进程中β-紫罗兰酮和萜品醇相对含量较高,银桂开花进程中β-紫罗兰酮和芳樟醇相对含量较高,丹桂开花进程中是β-蒎烯和乙酸-3-己烯酯相对含量较高,四季桂开花进程中是萜品醇和β-紫罗兰酮相对含量较高,这些物质均在盛花期达到顶峰。这与前者的研究存在差异,究其原因可能实验方法不同、苗木的生长状态及其环境因素中的温度光强差异所致,也有可能是本研究是以自然条件下的15~20年生桂花为实验对象,有别于扦插繁殖的5~7年生植株。林富平等[27]通过对4个桂花品种盛花期VOCs成分分析发现,银桂中的α-紫罗兰酮、β-紫罗兰酮相对含量最高;而(z)-β-罗勒烯在金桂中的相对含量最高;四季桂中β-紫罗兰酮含量最高,丹桂中β-蒎烯含量最高,与本研究结果十分相似。金荷仙等[28]通过实验证明了4个桂花品种的VOCs中,普遍存在着芳樟醇、氧化芳樟醇、α-紫罗兰酮、β-紫罗兰酮、罗勒烯等成分,但不同品种间的气体组分存在差异;LI等[29]对湖北咸宁3个桂花品种鲜花的精油进行分析,发现其中紫罗兰酮和芳樟醇的含量较高,这均与本实验部分研究结果类似。另外,CAI等[9]通过对桂花品种的香气活性物质分析,发现香气活性物质并非都是含量较高的挥发性物质,有些含量较低的挥发性物质也对桂花香气形成有贡献。这也都将值得我们进一步去研究探讨。
-
VOCs中尤其是一些具有香味的成分对人体身心健康可产生一定影响,芳香植物的挥发物对舒缓情绪、缓解压力、消炎镇痛、提高免疫力等方面起到了积极作用,对人体有着很好的保健作用[30]。例如GAO等[31]对油松Pinus tabulaeformis,白皮松P. bungeana,桧柏Sabina chinensis,红皮云杉Picea koraiensis,雪松Cedrus deodara所释放的柠檬烯、β-蒎烯、壬醛等物质进行分析,发现柠檬烯、β-蒎烯等物质,能明显抑制细菌生长。林富平等[32]通过实验证明了桂花可以有效地降低林地空气中微生物的数量,对改善空气质量具有显著的作用。张风娟等[33]也发现了乙酸-3-己烯酯同样具有显著的抑菌效果。YAMADA等[34]研究也表明了薰衣草Lavandula burnatii释放的芳樟醇可以缓解人们紧张的情绪。另外,霍丽妮等[35]发现芳樟醇还能清除自由基和脂质过氧化物,减少自由基对组织细胞的损伤,有利于老年退行性疾病的预防和治疗。而本实验显示芳樟醇、β-蒎烯、乙酸-3-己烯酯在4个桂花品种整个开花进程中的相对含量较高。同理可推,金桂、银桂、丹桂及四季桂的鲜花VOCs在抑制微生物生长、加大空气清新感、增强人体健康方面具有重要意义。










 DownLoad:
DownLoad: