-
骨骼肌组织的发生和发育是肉品质形成的重要因素。肌肉生成是一个复杂的程序,包括肌肉先祖细胞的增殖和迁移(或卫星细胞的激活),成肌细胞的生成、增殖和分化,成肌细胞的融合以及多核肌纤维的形成等过程。骨骼肌的先祖细胞在基因调控下迁移入肢芽间充质,分裂形成肌肉的始祖细胞库,在基因调控下形成成肌细胞进入分化。成肌细胞在基因的调控下分泌特异因子,导致成肌细胞发生融合形成多核肌管。肌小管是初级肌肉纤维的前体细胞,随后形成肌纤维,最后构成复杂的骨骼肌[1-2]。在胚胎发育后期阶段,肌肉进入后期增长,主要包括肌纤维长度的增加和直径的增大[3]。肌肉重生和自我更新来源于骨骼肌中的正常沉默的卫星细胞[4]。在这些过程中,多个调控因子进行双向调节。目前,已确定与肌纤维生长发育相关的基因有MRFs(myogenic regulatory factors)基因家族、PAX(paired box)基因家族和肌肉生成抑制因子基因(Myostatin,MSTN)[5]等,MRFs家族包括肌分化因子MyoD,肌细胞生成素MyoG(Myogenin),生肌决定因子Myf6和Myf5[6]。它们调控前体肌细胞定型、细胞增殖和肌纤维的形成以及机体出生以后肌肉成熟和功能完备等肌肉发生和发育的各个环节[7-9],对肌细胞的增殖和分化、肌纤维的数量和大小起着关键的调控作用,若该基因家族表达沉默则会导致生肌节形成的失败,最终无法形成肌肉[10]。而PAX家族蛋白为一类重要的转录调控因子,在胚胎发育过程中对组织和器官的分化起重要的调控作用。其中,Pax3和Pax7等2个转录因子通过调控MRFs基因家族和其他相关基因,被认为在肌纤维的生长发育过程中,指导众多过程的形成[11]。鹅Anser cygnoides是鸟纲雁形目Anseriformes鸭科Anatidae的一种动物。鹅肉是理想的高蛋白、低脂肪、低胆固醇的营养健康食品。因此,目前对鹅肉的需求量也日趋上涨,鹅肌肉生长和肉质形成的研究受到一定的关注。如前所述,MRFs基因家族和PAX基因家族参与肌肉的发育过程,与肌纤维的数量和大小有着密切关系。但鹅该基因家族在胚胎期肌肉发育过程中的表达规律及在肌肉发育过程中的调控作用未见报道。中国鹅品种资源非常丰富,记载于《中国禽类遗传资源》上的地方品种共36个,培育鹅种5个。本研究以浙东白鹅Anser cygnoides domestica ‘Zhedong’为材料,该品种分布于浙江东部地区,为中等体型白色鹅种,其身躯有2种,分别是长方形和长尖形,全身羽毛白色,额部有肉瘤,颈部细长腿部粗壮,是中国肉鹅的著名地方品种,可作为研究肌肉生长发育的理想动物模型。采用实时荧光定量聚合酶链式反应(qRT-PCR)分析技术,研究物种胚胎期和胚胎后早期胸肌(breast muscle, BM),腿肌(leg muscle, LM)中MRFs家族以及Pax3和Pax7的表达规律,以探索这些基因在肌肉发育过程中的作用。
HTML
-
试验所用鹅个体由浙江天鸿鹅业提供(浙江绍兴),选取相同批次且孵化条件相同的胚龄分别为7,11,15,19,23,27 d的鹅胚各3枚(分别以E7,E11,E15,E19,E23,E27表示);同时选取同一批次出壳,并在相同饲养管理条件下饲养的7日龄鹅3只(以P7表示)。迅速分离出鹅胚以及出生后小鹅的胸肌和腿肌组织,置于液氮速冻,然后转入-80 ℃冰箱保存。
-
肌肉总RNA采用RNAiso plus(TaKaRa,大连)提取,RNA样品经质量分数为1%的琼脂糖凝胶电泳检测质量,紫外分光光度计检测计算浓度。采用PrimeScriptTMreagent Kit(TaKaRa,大连)合成cDNA。
-
根据已发表的鸭Anas platyrhynchos和鹅MRFs家族、Pax3和Pax7序列分别设计qRT-PCR引物(表 1),GAPDH作为内参基因。引物由上海英骏生物工程有限公司合成。qRT-PCR采用SYB®PrimeScrip®RT-PCR Kit(TaKaRa,大连),反应体系和反应条件按试剂盒说明进行,设置技术平行的3个·样品-1。采用CT值(循环数)比较法计算表达量,采用SASS分析表达差异是否显著。
基因 参考序列 序列(5′→3′) MyoD chicken:NM_204214; F(上游):TGCCGTCGGAGCAGTTGGAG duck:JN408699, JN408700 R(下游):DCAACGCCATCCGCTACATCG MyoG chicken:NM204184, XM_005013713; F:CGCCTGAAGAAGGTGAACGAAGC duck:NM_001310376 R:GTCCCTCTGCTCCCGCTCCTG Myf5 chicken: NM_001030363; F:TGAGGAACGCCATCAGATACATCG R:AGCTGGAGGTGGGGCTGGTC Myf6 duck:NM_001310793 F:AGCAGGCAAATGGCTCGGACTTC R:GCTTGGGCTCGTCGGAGGAAAT Pax3 chicken:AB080581; F:AGCCATCCTACCAGCCCACCTC duck:JQ070187 R:CGAAGGGAGGCTGCTTTGGTGT Pax7 chicken:NM_205065, DQ471304; F:GCTCAGCGGTGAAAGTGGTTCG duck:JQ070188; R:CGGCATCCTGGGCGACAAAG GAPDH 参考文献[12] F:GCCAAAAGGGTCATCATCTC R:GTAGAG GCAGGGATGATGTTC Table 1. Primers used for qRT-PCR
1.1. 试验样品
1.2. 总RNA提取和cDNA的合成
1.3. qRT-PCR及分析
-
在胸肌和腿肌组织发育的不同阶段均能检测到MyoD(图 1A),Myf5(图 1B),MyoG(图 1C)和Myf6(图 1D)的表达,两者表达模式相似,前3种基因均呈现反“√”的模式,而Myf6的表达则均呈“wave”模式。
MyoD:腿肌(LM)在E11~E23阶段维持高表达,显著高于其他阶段(P<0.05),在E15表达量达到最高;胸肌(BM)则在E15~E23阶段维持高表达,显著高于其他阶段(P<0.05),同样在E15表达量达到最高。
Myf5:腿肌在E11表达量显著上升(P<0.05),达到最高,E15~E19阶段维持高表达,E19~E23后显著下降(P<0.05),但在E23~E27表达量略微回升,出壳后表达量低。胸肌同样在E11表达量显著上升(P<0.05),达到最高,E15~E19阶段维持高表达,在E19~P7阶段表达量逐渐降低。
Myf6:E7~E15表达量逐渐上升,E15~P7表达量逐渐下降。腿肌第15胚龄日表达量显著高于其他胚龄日(P<0.05),胸肌中E15表达量显著高于其他阶段(P<0.05),出壳后表达量降低。
Myf6:腿肌在E7~E19阶段表达量呈上升趋势(E15和E11比较有小回落),在E23显著下降(P<0.05),而在E27~P7又显著上升(P<0.05)。胸肌的表达模式类似,E7~E15阶段表达量呈上升趋势,在E15达到表达最高峰,E15~E23显著下降(P<0.05),而E27~P7有所上升。该基因在胸肌中早于腿肌达到表达高峰期,并且P7阶段达到第2高峰阶段时,腿肌的表达显著高于胸肌(P<0.05)。
-
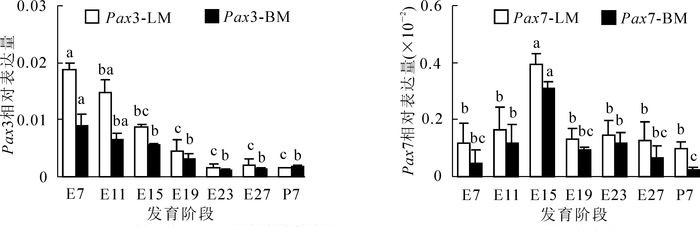
在胸肌和腿肌组织发育的不同阶段均可检测到Pax3的表达(图 2A),其表达呈逐渐降低模式,在胸肌和腿肌的表达模式相似。在胸肌和腿肌中,均在E7检测到表达量最高,随着生长发育,该基因的表达逐渐降低,在E15~E19阶段,该基因的表达已显著低于E7(腿肌在E15后表达显著低于E7,P<0.05;胸肌在E19后表达显著低于E7,P<0.05),E23后该基因表达量持续在较低水平。
在胸肌和腿肌组织发育的不同阶段均可检测到Pax7的表达(图 2B),在2个肌肉组织中的表达模式形似,均呈反“√”型。在腿肌中Pax7基因在E11出现上升,并于E15达到表达最高时期,显著高于其他阶段(P<0.05),在E15之后,基因表达保持较低水平。在胸肌中,该基因同样在E15出现表达高峰后,保持在较低的表达水平,胸肌中该基因的表达整体低于腿肌。
2.1. 胸肌和腿肌组织中生肌调节因子家族的表达变化
2.2. 胸肌和腿肌组织中Pax家族基因表达变化
-
MRFs家族成肌因子均属于碱性螺旋-环-螺旋(bHLH)转录因子,转移非分化的细胞进入生肌系统,通过与参与骨骼肌分化的E12,E47,HEB等E蛋白形成异源二聚体识别DNA序列,从而激活肌酸激酶、肌球蛋白等与肌肉生成相关的特异性基因[13-15]。在肌肉生成过程中,MRFs家族并不同时表达,其成员在功能上表现出特有的表达模式。初级肌肉发育阶段,涉及到了成肌前体细胞的命运决定和成肌细胞的大量增殖。该过程与MyoD和Myf5的作用有关。在次级肌肉发育过程中涉及到了成肌细胞融合为肌管和肌管进一步分化为肌纤维的过程,该过程中MyoG和Myf6扮演了重要的角色。有研究认为:在Pax3基因调控肌源性祖细胞迁移到目标位置后,Myf5率先开始表达,调节体节在轴下部位和肢芽中分化、增殖;接着MyoD在Myf5和Pax3的协调下被刺激表达,使成肌细胞退出细胞周期;MyoG同样也在早期激活,作为肌肉分化因子调控肌肉进入分化形成阶段;Myf6最终表达,并且在出生后成为主要转录产物,促使肌小管的形成[16-20]。
本研究发现:鹅胚胎期MRFs家族基因表达为(1)Myf5基因在胸肌和腿肌中有相似的表达规律,为先上升后下降的模式,并且其在胸肌和腿肌中均在E11到达表达的最高峰,并维持4~7 d,但也有不同之处,腿肌中发育的后期阶段Myf5的表达略有回升,然而胸肌中Myf5呈现出持续下降的模式。一些研究认为,不同肌纤维类型的肌组织中,Myf5的表达模式有所差异。这是由于胸肌和腿肌在胚胎早期发生的起源中存在一定差异,胸肌主要起源于轴下生肌节的成肌细胞,腿肌虽然也主要起源于轴下生肌节,但是主要来源于迁移到肢芽处的成肌细胞[21]。高邮鸭Anas platyrhynchos ‘Gaoyou Breed’和金定鸭Anas platyrhynchos domestica‘Jinding’的相关研究中也表明:Myf5在胚胎发育早期出现高表达[21]。(2)MyoD在鹅胸肌腿肌中的表达规律跟Myf5极为相似,也是先升高后降低的趋势,但也存在明显的不同。MyoD在胸肌和腿肌中均在E15到达表达的最高峰,比Myf5到达最高峰的时间晚。该结果说明,虽然MyoD和Myf5均在肌肉发育的初级阶段发挥作用,但还是存在不同的作用模式。朱文奇等[22]研究发现:在鸭中MyoD基因在E13~E21维持高表达,这和我们的研究结果基本保持一致的。(3)Myf6基因在E15(甚至腿肌E19)中出现表达高峰,并且在出壳后也出现较高的表达,这和朱文奇[22]报道的Myf6在E21出现表达高峰保持一致,该现象和Myf6基因参与肌小管的形成有关。MyoG和Myf6基因在成肌细胞的融合和分化中起作用, 但是其表达规律不尽相同,这说明MyoG和Myf6有不同的功能。MyoG和Myf6在表达规律与它们的功能密切相关。
-
对于Pax3,首先,非分化的前体细胞转化成肌细胞过程中,受到Pax3和Foxc2的互惠调控。其次,在肌肉肌细胞形成过程中,Pax3通过控制Myf5基因的增强子区域,从而控制MRFs基因家族的转录,调节细胞分化进入成肌细胞和肌丝层[23-24]。该现象表明:Pax3基因在胚胎发育早期就开始发挥作用。研究显示,鸭在胚胎早期,Pax3基因表达最强,随着时间增加而递减。本研究中无论在胸肌还是在腿肌中,Pax3均是在E7表达量最高,随着肌肉的生长发育,该基因的表达逐渐降低,这与已有的研究结果保持一致。
Pax7被认为是出生后肌肉发育即损伤肌肉修复过程中的标志基因。该基因通过与细胞生长、黏连等相关的特定基因结合来促进细胞增殖,抑制其分化[25]。在胚胎期,肌卫星细胞增殖并融合到已存在的肌管中,促进出生后肌纤维的增长。本研究中发现在鹅胸肌和腿肌发育过程中,该基因表达高峰期出现在E11~E15阶段。可以认为,Pax7在调控肌祖细胞进入骨骼肌发生的过程中具有重要的作用。
本研究通过qRT-PCR研究了MRFs,Pax3和Pax7在鹅骨骼肌胚胎期以及出生早期的表达规律,发现这些基因均与鸟类的骨骼肌生成密切相关,并且总体上胸肌发育晚于腿肌,这可能与鸟类腿肌的特殊性有关,也和陈禧[12]的报道相一致。肌肉生成是一个复杂的程序,在胚胎期包括肌母细胞的增殖、退出细胞周期、最终分化导致生成多核肌纤维等步骤。本研究的结果,有助于更加具体和深入地了解鹅胚胎期肌肉发育相关基因的表达规律,为鹅骨骼肌发育过程中的调控机理提供理论依据。












 DownLoad:
DownLoad: