-
随着工农业的发展及人类的活动加剧,重金属污染已经严重危害到人类的健康[1-2]。目前,植物修复因其绿色环保受到广泛的关注,但由于受到土壤中重金属生物有效性的影响,植物修复效率低下[3-4]。利用螯合剂来活化土壤中的重金属,促进植物对重金属的吸收,是提高植物修复效率的方法之一[5],其中,乙二胺四乙酸(EDTA)是使用最广泛的一种螯合剂,能够显著提高铅(Pb),铜(Cu),镉(Cd)等重金属的活性[6-8]。CHIGBO等[9]在铜污染的土壤中施加EDTA强化植物修复后显著提高了土壤中铜的有效态含量,同时显著促进了植物对铜的吸收积累。有研究表明:EDTA的生物降解性差,可能会对环境造成一定污染[10]。草酸、柠檬酸等生物降解性较好的小分子有机酸也被广泛用于植物修复中[11]。DUQUÈNE等[12]研究发现,使用5 mmol·kg-1柠檬酸、柠檬酸铵、草酸等有机酸显著增加了土壤溶液中重金属的质量浓度。相对于其他超积累植物或者重金属富集植物,毛竹Phyllostachys edulis拥有巨大的生物量,并且已被证明作为植物修复材料的可能性[13-14]。本研究采用镀锌厂重金属污染土壤和毛竹苗进行土柱淋洗试验,研究施加螯合剂EDTA和有机酸后毛竹叶片叶绿素荧光特性,毛竹重金属质量分数变化,土壤溶液重金属质量分数及EDTA质量浓度的动态变化,为螯合剂强化重金属污染土壤植物修复提供科学依据。
HTML
-
供试土壤来自浙江省杭州市富阳区云纳热镀锌厂附近农田(29°53′17″N,119°53′24″E),采集0~20,20~40,40~60 cm等3个土层的重金属污染土壤,风干过5 mm筛,其土壤基本理化性质见表 1。供试植株:由种子培养生长在基质中的毛竹幼苗,选择长势相似且无病虫害的幼苗,用蒸馏水去除附着的基质,在含有1/2 Yoshida营养液(pH 5.8)的黑色塑料盆中预培养。营养液隔5 d更新1次,保持24 h通气。
土层/cm pH值 w碱解氮/(mg·kg-1) w有效磷/(mg·kg-1) w速效钾/(mg·kg-1) w锌/(mg·kg-1) w铜/(mg·kg-1) w镉/(mg·kg-1) 0~20 1 367.10 296.20 2.21 20~40 7.16 30.10 4.41 98.64 300.00 58.90 0.56 40~60 133.78 34.11 0.24 Table 1. Physicochemical properties of soil
-
将不同土层的土壤装入土柱中,并且在5,20,40,60 cm土层处装入土壤溶液收集器[15]。栽植毛竹苗3株·土柱-1,并且控制土柱土壤含水率,整个实验培养周期为2个月,在实验结束前10 d,一次性分别添加人工螯合剂和有机酸。所添加的人工螯合剂为质量摩尔浓度1.5 mmol·kg-1(E1.5)和3.0 mmol·kg-1(E3.0)的EDTA-Na2;有机酸根据毛竹苗根系分泌物配制,为m(草酸):m(柠檬酸):m(乳酸)=5:1:1的混合物[16],质量摩尔浓度为15.0 mmol·kg-1(S15)和30.0 mmol·kg-1(S30),以不添加螯合剂和有机酸为对照(ck),共5个处理,重复3个·处理-1,并随机排放。整个试验在浙江农林大学温室大棚内完成。为避免pH值不同所引起的活化差异,所添加的EDTA和有机酸的pH值均调节成供试土壤pH值[17]。
-
在添加螯合剂前1 d,收集土柱各土层的土壤溶液,作为初始溶液,然后一次性加入EDTA和有机酸,并在第1,3,5,7,9天收集土壤溶液[18],共6次。收集到的土壤溶液取10 mL酸化,利用ICP-OES测定土壤液中重金属质量分数,其余冷冻保存冰箱备用。
-
在试验结束前2 d,选择晴朗天气,利用便携式叶绿素荧光仪在9:00-12:00测定每株毛竹第3片叶片叶绿素荧光特征。Fo为初始荧光;Fm为最大荧光产量;Fv/Fm为PSⅡ最大光化学量子产量;Fv/Fo为PSⅡ潜在光化学效率。
-
收获的毛竹用20.0 mmol·L-1EDTA-Na2浸泡20 min,去除附着的重金属,将毛竹分为根茎叶3部分,粉碎,称取0.1 g的样品,用硝酸/高氯酸消煮定容至50 mL并过滤,用ICP-OES测定重金属质量分数。
-
EDTA质量浓度测定参考郭晓方等[19]、郑睿行等[20]方法:取1.0 mL样品至管中,加1.0 mL氯化铁溶液(2.5 mmol·L-1)和1.0 mL抗坏血酸(含4.0 mg抗坏血酸),稀释至5.0 mL,水定容摇匀,经0.22 μm微孔滤膜过滤,立即液相色谱法测定。液相色谱为日本LC-20AT,分析柱:C18 Inertsil ODS-SP色谱柱(4.6 mm × 250 mm,5 μm),岛津;保护柱:Inertsil ODS-SP(5 μm,4.0 mm × 10 mm),岛津;流动相:水-甲醇(80+20)含有0.02 mol·L-1四丁基溴化铵,0.03 mol·L-1乙酸钠缓冲液(磷酸调pH 4);流速:0.8 mL·min-1,检测波长:258 nm。
-
采用Excel 2013和SPSS 21.0软件进行数据处理,用SigmaPlo 12.5软件作图。采用方差分析和最小差异显著法(LSD)对数据进行统计分析,差异显著性水平为P < 0.05。
1.1. 供试材料
1.2. 实验设计与处理
1.3. 土壤溶液的收集
1.4. 植物叶绿素荧光的测定
1.5. 毛竹重金属质量分数测定
1.6. 土壤溶液中EDTA质量浓度的测定
1.7. 数据分析
-
由表 2可知:施加EDTA和有机酸后,Fo略微上升,与对照组之间无显著差异;而Fm,Fv /Fm和Fv /Fo不同程度地下降,且添加3.0 mmol·kg-1EDTA和有机酸后,Fm,Fv /Fm和Fv /Fo显著降低,在30.0 mmol·kg-1有机酸下Fm,Fv /Fm和Fv /Fo下降幅度最大,分别降低了11.7%,5.3%和19.2%。可见,30.0 mmol·kg-1的有机酸处理对毛竹叶片叶绿素荧光特性抑制效果最为显著。
处理 Fo Fm Fv/Fm Fv/Fo ck 178.11±6.61 a 801.44±38.43 a 0.779±0.007 a 3.502±0.201 a E1.5 182.75±7.34 a 762.63±31.06 ab 0.760±0.013 ab 3.179±0.237 ab E3.0 187.33±14.91 a 733.17±49.10 bc 0.743±0.027 bc 2.941±0.448 b S15 181.63±14.38 a 718.50±36.91 c 0.747±0.018 bc 2.973±0.273 bc S30 185.13±7.44 a 707.75±35.21 c 0.737±0.018 c 2.830±0.265 c 说明:同列相同字母表示差异不显著(P>0.05),同列不同字母表示差异显著(P<0.05) Table 2. Changes of chlorophy fluorescence parameter of moso bamboo leaves after adding EDTA and organic acids
-
从图 1可以看出:添加EDTA后毛竹对3种重金属的吸收显著增加,且随着EDTA质量摩尔浓度增加而增加;添加有机酸后,毛竹对重金属的吸收无显著差异。毛竹从土壤吸收的重金属主要集中在根部,其次在茎,最少在叶,并且毛竹对镉的转运效率最高,对铜的转运效率最低。
-
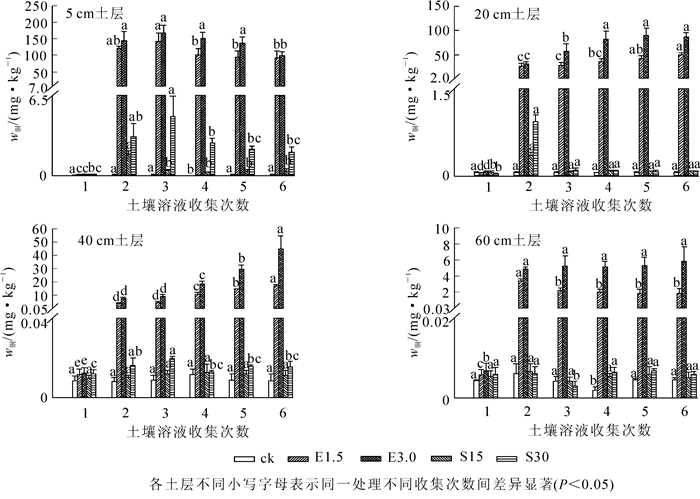
由图 2所示:在对照处理中,土壤溶液中铜质量分数随着采集时间的增加始终在低于0.1 mg·kg-1的范围内波动。在土壤中添加螯合剂后,土壤溶液中铜的质量分数显著增加。在EDTA处理中,3.0 mmol·kg-1EDTA对铜的活化作用较强,在添加EDTA后第1天各土层土壤溶液中铜质量分数显著上升,且5 cm土层处土壤溶液中铜质量分数变化最大,与对照相比分别增加了1 215和1 639倍。随着时间的推移,5 cm表层土层土壤溶液中铜质量分数先升高,在第3天的时候达到最大,然后再随时间的增加而降低;在20 cm及更深的土层中,土壤液中的铜不断累积。添加有机酸后,只有表层土壤表现出明显的活化作用。在5 cm土层中,15.0 mmol·kg-1有机酸处理后,土壤溶液中铜质量分数随着时间增加逐渐减少,15.0 mmol·kg-1有机酸处理后,土壤溶液中铜质量分数随着时间增加先增加后减少,在20 cm处,仅在添加有机酸的第1天铜质量分数显著增加。在整个土柱试验中,土壤溶液中铜主要集中在表层土壤。
-
由图 3可知:对照土壤溶液中锌质量分数随着采集时间无显著变化,添加EDTA螯合剂后,土壤溶液中锌质量分数显著增加,且相比添加有机酸作用更加明显。在EDTA处理中,高质量浓度EDTA对锌的活化作用较强,在添加1.5和3.0 mmol·kg-1EDTA后第1天各土层土壤溶液中锌质量分数显著上升。随着时间的增加,5和60 cm土层处锌质量分数逐渐降低,在20和40 cm土层,土壤溶液中的锌质量分数随着时间的增加而增大。添加有机酸后,在5和20 cm土层土壤溶液中锌质量分数显著增加。
-
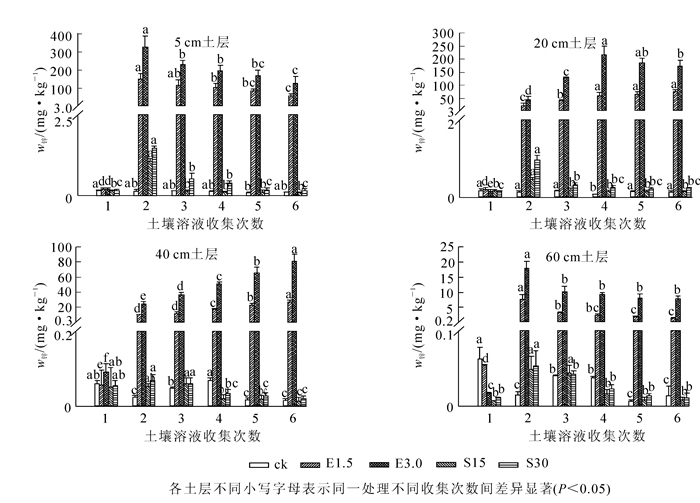
由图 4可见:对照土壤溶液中镉质量分数始终在很低的范围内波动。在土壤中添加螯合剂EDTA后,各土层土壤溶液中镉质量分数显著增加,对镉的活化作用显著强于有机酸处理。在添加EDTA后第1天各土层土壤溶液中镉质量分数显著上升,且5 cm土层土壤溶液中镉质量分数变化最大,相比对照分别增加了353和1 012倍。随时间的增加,各土层土壤溶液中镉与锌的质量分数变化趋势一致。添加有机酸对各土层土壤镉都有活化作用,且表层土壤最显著,随时间的增加镉质量分数不断降低。在整个淋洗柱中,土壤溶液中镉质量分数随土壤深度的增加而减少。
-
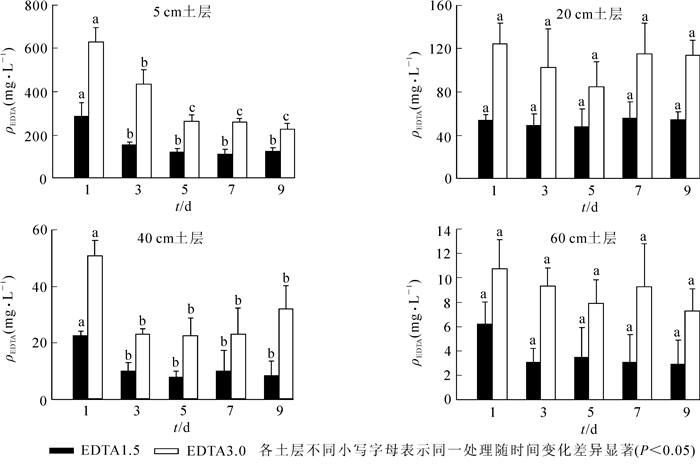
由图 5所知:添加1.5和3.0 mmol·kg-1EDTA后,土壤溶液中EDTA主要集中在5 cm土层。随着时间的增加,5 cm土层处EDTA质量浓度显著降低,5 d后达到稳定,分别为110.9~122.9 mg·L-1和257.8~263.3 mg·L-1;20 cm土层EDTA质量浓度为47.7~55.6 mg·L-1和84.4~124.5 mg·L-1,无显著变化;40 cm土层EDTA施加第1天达到最高,分别为22.3和50.7 mg·L-1,之后显著下降达到稳定;60 cm土层EDTA质量浓度相对较小,分别为2.9~6.2 mg·L-1和7.3~10.7 mg·L-1,差异不显著。
2.1. EDTA及有机酸对毛竹叶绿素荧光特性的影响
2.2. EDTA及有机酸强化毛竹吸收重金属
2.3. EDTA及有机酸对重金属在不同土层内迁移的影响
2.3.1. EDTA及有机酸对铜在不同土层内迁移的影响
2.3.2. EDTA及有机酸对锌在不同土层内迁移的影响
2.3.3. EDTA及有机酸对镉在不同土层内迁移的影响
2.4. 土壤溶液中EDTA质量浓度的变化
-
叶绿素荧光是反映植物光合生理的一个指标[21]。本研究中,添加3.0 mg·L-1EDTA和有机酸后毛竹叶片最大荧光Fm,PSⅡ最大光化学效率Fv /Fm和PSⅡ潜在光化学效率Fv /Fo显著降低,表明螯合剂的添加降低了植物的光合生物素,使植物的光合系统遭到破坏。EDTA处理后的毛竹叶片荧光参数降低,主要原因可能是EDTA活化了土壤中的重金属,提高了重金属的生物有效质量分数,高质量分数的重金属损害了毛竹的生长,对毛竹细胞内叶绿体、线粒体及细胞壁等结构产生了毒害作用[13, 22]。同样添加有机酸后也降低了毛竹叶片的叶绿素荧光参数,可能有机酸分解后改变了土壤的基本理化性质,从而改变了毛竹苗的生长状况。
螯合剂可以活化土壤中难溶态重金属,改善其生物有效性[7, 27],提高植物对土壤中重金属的吸收效率[23-24]。EDTA是一种普遍使用的螯合剂,添加EDTA显著提高了毛竹对土壤中锌、铜和镉的吸收,作用效果显著高于有机酸。这与CAY等[25]研究EDTA强化蜀葵Althaea rosea修复重金属污染土壤得出的结果相似。添加有机酸并没有明显促进毛竹对重金属的吸收,可能是由于添加的有机酸分解后改变了土壤的基本理化性质,抑制了毛竹吸收重金属,这与吴龙华等[26]的研究结果类似。本研究中,EDTA淋洗显著增加了0~20 cm土层土壤中可溶性铜、锌和镉的质量分数,由于EDTA活化效果强,土壤溶液中铜、锌和镉的质量分数相对比较高。添加EDTA增强了重金属的迁移性,导致20和40 cm处土壤溶液中铜、锌和镉的质量分数随着时间的增加而增加。YLIVAINIO等[28]研究表明:在石灰性土壤中添加EDTA可持续提高镉、铅和镍在土壤中的溶解性。在本研究中,添加有机酸初期对土壤中铜和锌的活化效果比较明显,随着时间的推移,活化效果消失甚至产生了钝化效果。可能是由于有机酸在土壤中很快被土壤微生物分解,对重金属的活化效果消失,而且可能改变了土壤的pH值,从而产生钝化效果。吴龙华等[26]研究表明:施加3.0 mmol·kg-1,pH 6.3的草酸、柠檬酸或苹果酸对土壤铜含量及其形态分配无显著影响。添加有机酸后,土壤溶液中铜和锌质量分数在40和60 cm土层处变化不显著,说明添加有机酸后铜和锌在土壤中的纵向迁移能力弱;而有机酸处理对镉具有一定的活化和迁移能力。在整个土柱试验中,EDTA处理对重金属的活化和迁移效果始终强于有机酸。李艳丽等[29]采用土柱模拟淋洗试验,研究了EDTA,柠檬酸和草酸3种淋洗剂对污染土壤中镉纵向迁移的影响,结果表明:EDTA对镉的迁移能力强于柠檬酸和草酸。
EDTA添加到土壤后会长时间残留,并迁移到更深的土壤中,从而对环境造成一定的风险[30-31]。有研究表明:可根据土壤类型,通过控制EDTA的施用量及施用时间来控制EDTA在强化修复重金属污染土壤中的残留和迁移[19]。相比EDTA,有机酸在自然界中很容易被生物降解,因此它的环境风险更小[32-33]。本研究中,EDTA主要集中在表层土壤,但第1天在40 cm土壤溶液中检测到相对较高的质量浓度,说明EDTA在土壤中迁移能力强。在第5天到第9天EDTA质量浓度相对稳定且相对较高,也说明EDTA很难被分解。
-
乙二胺四乙酸(EDTA)和有机酸处理抑制了毛竹的叶绿素荧光特性,且30.0 mmol·kg-1有机酸处理的抑制程度最大,3.0 mmol·kg-1EDTA处理其次,15.0 mmol·kg-1有机酸处理最小。在整个土柱试验中,EDTA处理显著提高了土壤溶液中重金属质量分数,加重了重金属向下迁移的风险;有机酸处理对重金属的活化效果及在土壤中的迁移能力不明显。添加EDTA促进了毛竹对铜、锌、镉的吸收,而有机酸则无促进作用。添加1.5和3.0 mmol·kg-1EDTA后,土壤溶液中EDTA质量浓度主要集中在5 cm土层,且短期内很难被分解。















 DownLoad:
DownLoad: