-
短枝木麻黄Casuarina equisetifolia作为木麻黄科Casuarinaceae木麻黄属Casuarina的代表树种,是世界各国引种最早、人工栽培面积最大的木麻黄植物[1],也是中国东南沿海地区重要的防护林树种[2],发挥着巨大的生态效益和经济价值。木麻黄是典型的热带植物,生长适温为22.1~26.9 ℃,耐寒性较差[3]。引种的木麻黄常遭受较严重冻害,给沿海防护林建设造成较大损失。因此,研究木麻黄对低温的响应机制,开展耐寒品种的选育,对于扩大木麻黄种植范围和推进沿海防护林建设尤为重要。在低温诱导下,植物形成了其各自特有的生理生化特征,研究植物的耐寒生理机制,对于解析植物的耐寒性具有重要意义。目前,国内外对木麻黄耐寒生理研究大多集中在抗性生理指标的测定分析上[2, 4-6],但从全面或更深入揭示木麻黄的低温适应的机制还远远不够。本研究针对木麻黄生产实践中存在的低温冻害问题,在前期对短枝木麻黄耐寒性评价的基础上,以短枝木麻黄耐寒无性系ZS7和不耐寒无性系HN1幼苗为材料,通过测定这2种无性系幼苗在低温胁迫下的叶绿素、渗透调节物质、酶类和非酶类抗氧化剂等耐寒相关生理指标,比较分析耐寒性不同无性系生理指标变化的趋势与差异,为深入阐明短枝木麻黄耐寒机理,促进木麻黄耐寒品种选育奠定基础。
HTML
-
根据前期试验结果,选定2个耐寒性差异较大的短枝木麻黄无性系:耐寒无性系ZS7和不耐寒无性系HN1,采用盆栽方式,于2016年10月在浙江省林业科学研究院温室进行扦插繁殖。塑料盆尺寸为13 cm(高)× 20 cm(直径)。盆栽基质为泥炭和蛭石混合物(4:1),装盆前进行灭菌处理。培养条件为光照16 h/黑暗8 h,温度25 ℃,光合光子照度180 mol·m-2·s-1,相对湿度60%~70%。在温室中培养2个月后,扦插苗高度达到15~20 cm时,选取生长良好、长势较一致的植株,开始低温处理。低温梯度处理设计:将ZS7和HN1无性系置于低温人工气候箱(PRXD-300,上海乔跃)中,从常温预冷至10 ℃左右,之后逐步降温至-2,-5,-8,-11 ℃,在降至每个低温节点持续2 h后,各取出5株幼嫩枝条用锡箔纸包裹后迅速放入液氮中,置-80 ℃超低温冰箱中保存,用于生理指标测定。以在常温25 ℃下同步培养的ZS7和HN1无性系作为2种低温处理的对照(ck),取样方法同上。每处理均设3次重复。连续低温处理设计:将ZS7和HN1无性系各40株移入低温人工气候箱内,在-5 ℃下连续处理1,2,5,8,16,24,48,72 h,并于每个时间点分别取ZS7和HN1的5株幼嫩枝条,用锡箔纸包裹后迅速放入液氮中,置于-80 ℃超低温冰箱中保存备用。
-
叶绿素质量分数的测定采用分光光度计法[7],过氧化氢(H2O2)质量摩尔浓度测定按照高洪波等[8]的方法,丙二醛(MDA)质量摩尔浓度测定采用硫代巴比妥酸(TBA)法[9],可溶性蛋白质测定采用考马斯亮蓝法[10],脯氨酸(Pro)质量分数测定采用酸性茚三酮比色法[11],超氧化物歧化酶(SOD)活性测定采用氮蓝四唑(NBT)光化还原法测定[12],过氧化氢酶(CAT)活性测定采用紫外吸收法[13],过氧化物酶(POD)活性测定采用愈创木酚显色法[14],抗坏血酸过氧化物酶(APX)活性测定参照程玉静等[15]的方法,谷胱甘肽还原酶(GR)活性测定参照PINHEIRO等[16]方法。还原型谷胱甘肽(GSH)和氧化型谷胱甘肽(GSSG)质量摩尔浓度参照JIANG等[17]的方法。
-
采用Excel 2010和SPSS 18.0对数据进行统计分析,采用邓肯(Duncan)新复极差法进行5%水平的差异显著性分析。
1.1. 试验材料与方法
1.2. 生理指标测定
1.3. 数据处理
-
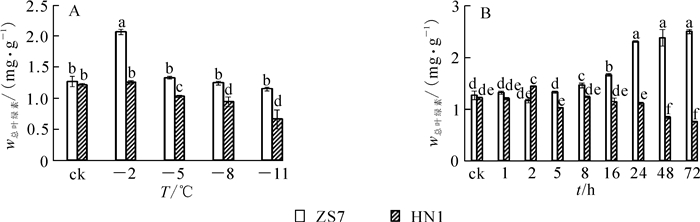
耐寒无性系ZS7幼苗在低温梯度胁迫下叶绿素质量分数随着胁迫的加剧,先升高后下降并趋于平缓。在降至-2 ℃时,ZS7的叶绿素质量分数迅速升高并达到峰值,升幅达63.2%;降至-11 ℃时,虽有所下降,但和对照相比差异不显著。不耐寒无性系HN1幼苗的叶绿素质量分数则呈显著下降趋势(图 1A),至低温胁迫结束,叶绿素质量分数降幅达45.7%,受到低温胁迫的伤害相对较重。在各胁迫温度下,ZS7的叶绿素质量分数相对稳定,始终显著高于HN1。

Figure 1. Effect of successive low temperatures and low temperature (-5 ℃) conditions for 1-72 h on chlorophyll content in Casuarina equisetifolia
在-5 ℃持续低温胁迫下,ZS7和HN1的叶绿素质量分数变化趋势如图 1B所示。ZS7叶绿素质量分数在1~5 h内与常温对照没有明显差异,8 h后叶绿素质量分数开始显著升高。HN1在5~72 h内,叶绿素质量分数呈逐渐下降趋势,且始终显著低于ZS7,说明-5 ℃的持续低温胁迫对HN1的叶片产生了破坏作用,而在短期内对耐寒无性系ZS7叶片的叶绿体结构破坏不明显。
-
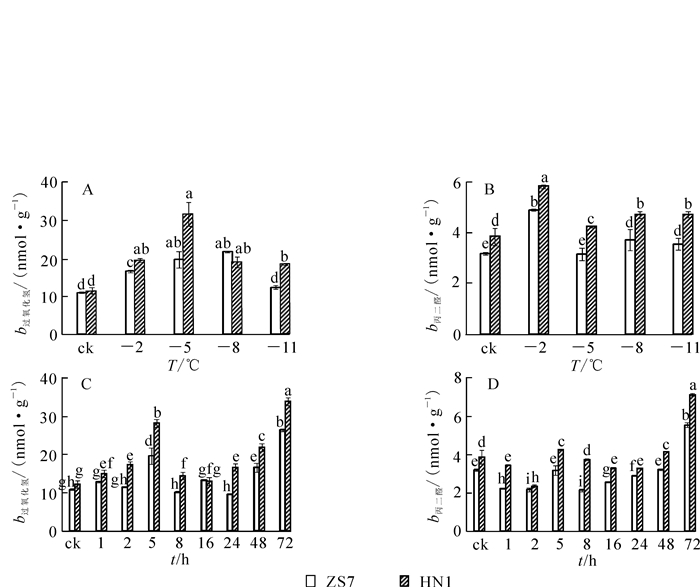
随着低温梯度胁迫的增强,ZS7和HN1幼苗叶片的H2O2质量摩尔浓度都呈现先升高后降低的变化趋势(图 2A)。在降至-5 ℃时,2种无性系的H2O2质量摩尔浓度均达到最高值,其中ZS7的H2O2质量摩尔浓度升高80.1%,HN1升高130.7%;在-5~-11 ℃,ZS7和HN1的H2O2质量摩尔浓度呈下降趋势,在低温梯度胁迫期间HN1的H2O2质量摩尔浓度一直较高。ZS7和HN1的MDA质量摩尔浓度呈升降升的趋势变化(图 2B),ZS7的MDA质量摩尔浓度在低温胁迫过程中始终显著低于HN1。至胁迫结束,ZS7的MDA质量摩尔浓度增幅为11.2%,HN1的增幅为23%,结果显示低温胁迫对耐寒性强的无性系ZS7的质膜损伤程度较低。在-5 ℃持续低温胁迫下,ZS7和HN1的H2O2,MDA质量摩尔浓度均随处理时间的延长呈现波动变化,且在胁迫结束时达到最高值(图 2C和2D)。从低温胁迫处理开始至结束,ZS7的H2O2和MDA质量摩尔浓度的增幅分别为142.4%和73.2%,HN1增幅分别为176.9%和84.7%。与不耐寒无性系相比,低温下耐寒无性系叶片中MDA质量摩尔浓度一直较低,且差异显著。
-
无论在常温条件下还是在低温梯度胁迫下,ZS7中可溶性蛋白质质量分数一直维持较高水平,并始终显著高于HN1(图 3A和3B)。至低温胁迫结束,ZS7和HN1的可溶性蛋白质质量分数的涨幅和降幅分别为13.9%和12.8%,和耐寒性强弱表现一致。在-5 ℃持续低温胁迫下,ZS7和HN1的可溶性蛋白质质量分数总体先升后降。-5 ℃处理24 h后,ZS7和HN1的可溶性蛋白质质量分数均达到最高,增幅分别为80.8%和43.2%;72 h后,都达到最低值,降幅分别为5.7%和57.5%。

Figure 3. Effect of successive low temperatures and low temperature (-5 ℃) conditions for 1-72 h on protein and proline content of in C. equisetifolia
在低温梯度胁迫下,ZS7和HN1中脯氨酸质量分数先上升后下降(图 3C)。处理结束后,2种无性系的脯氨酸质量分数均有所降低,降幅分别为36.3%和16.5%。在-5 ℃持续低温胁迫下,ZS7和HN1的脯氨酸质量分数呈波动变化(图 3D)。低温处理72 h后,2种无性系中脯氨酸质量分数的涨幅分别为37.1%和37.3 %。ZS7的脯氨酸质量分数在低温梯度胁迫和连续低温胁迫下始终显著高于HN1。
-
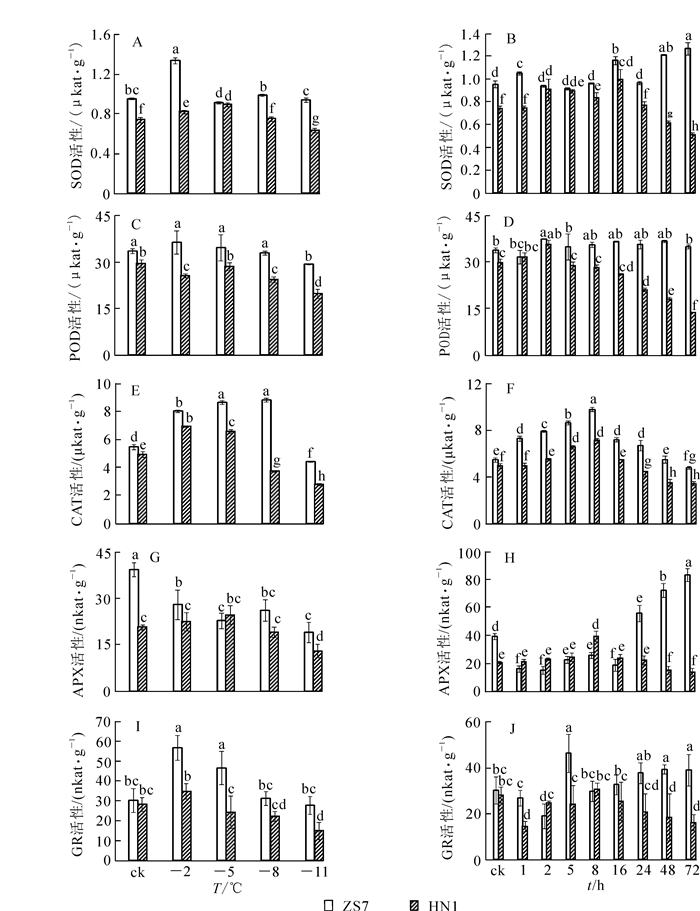
由图 4可以看出:耐寒无性系ZS7和不耐寒无性系HN1幼苗在低温梯度处理下SOD,POD,CAT,GR活性变化规律基本一致,呈先上升后下降的变化趋势;ZS7的APX活性呈下降趋势,HN1的APX活性则先上升后下降。在常温条件下,除ZS7和HN1的GR活性没有显著差异外,ZS7的SOD,POD,CAT,APX活性均显著高于HN1,且在整个低温胁迫过程中ZS7的保护酶活性始终高于HN1,说明耐寒无性系ZS7对过氧化伤害有较高忍耐能力。低温梯度处理结束后,ZS7和HN1的SOD,POD,CAT,APX,GR活性的降幅分别为1.3%,12.6%,18.9%,51.5%,8.6%和14.3%,33.2%,43.5%,36.9%,47.4%。

Figure 4. Effect of successive low temperatures and low temperature (-5 ℃) conditions for 1-72 h on antioxidant enzyme activity in Casuarina equisetifolia
在-5 ℃持续低温胁迫下,与HN1相比,ZS7叶片中的保护酶活性在整个低温胁迫过程中始终较高,且两者达到了显著性差异(图 4)。ZS7中SOD活性的最高值集中在低温胁迫48~72 h,且达到常温对照的32.7%;HN1的最高值集中在2~16 h,并在第72小时降至对照的33.9%。ZS7中POD活性在整个胁迫期间始终维持较高水平;HN1的POD活性在第72小时达到最低,并降至对照的53.7%。ZS7和HN1的CAT活性的最高值都出现在第8小时,至胁迫结束,与常温对照相比,降幅分别为12.1%和30.9%。ZS7的APX活性在低温胁迫24~72 h时显著上升,并升至常温对照的110%;HN1的APX活性一直较低,在胁迫结束时降至对照的32.6%。此时,ZS7的APX活性是HN1的6倍。至胁迫结束,ZS7的GR活性与对照相比涨幅为28.9 %,HN1的降幅为42.7 %。说明耐寒无性系ZS7从保护酶活性水平增强了其低温下的适应能力。
-
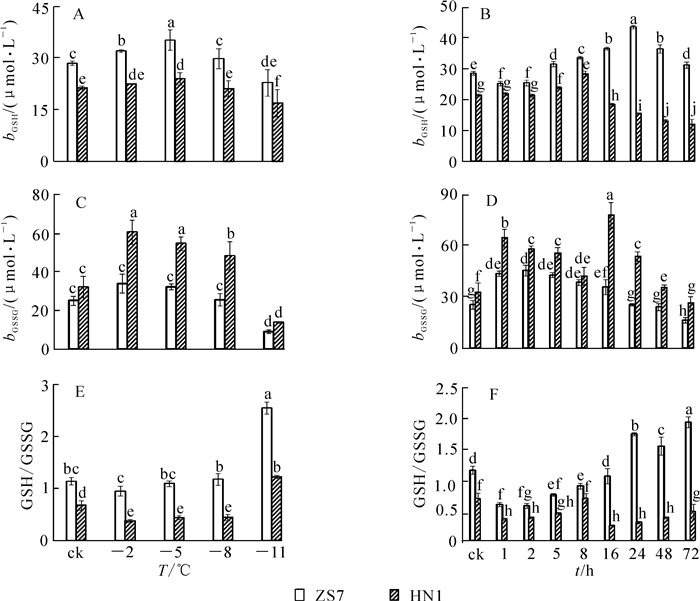
低温梯度胁迫下,2种无性系幼苗的GSH质量摩尔浓度均呈先升后降的趋势(图 5A),且都在-5 ℃时达到最高值,涨幅分别为23.6%和12.3%,说明GSH在2种无性系中均大量累积。胁迫结束时,ZS7和HN1的GSH质量摩尔浓度均达到各自最低值,降幅分别为19.8%和20.7%。在-5 ℃持续低温胁迫下,2种无性系的GSH质量摩尔浓度也都呈现先升后降的趋势(图 5B),但达到峰值的时间不同。ZS7的GSH质量摩尔浓度在第24小时达到最大值,HN1的GSH质量摩尔浓度在第8小时达到最高,涨幅分别为52.7%和32.5%。ZS7中的GSH质量摩尔浓度从第8小时开始一直显著高于对照,而HN1仅在第5和第8小时时显著高于对照。72 h后,ZS7和HN1的GSH质量摩尔浓度分别上升9.4%和下降4.5%。低温处理下,耐寒无性系ZS7的GSH质量摩尔浓度始终显著高于不耐寒无性系HN1。由图 5C可知:ZS7和HN1幼苗中的GSSG质量摩尔浓度都在-2 ℃时达到最高值,分别为对照水平的1.4倍和1.9倍;至低温胁迫结束,GSSG质量摩尔浓度比对照分别降低64.2%和57.1%。在-5 ℃低温持续胁迫下,2种无性系中的GSSG质量摩尔浓度总体呈现先上升后下降的趋势,且HN1中的GSSG质量摩尔浓度在整个胁迫过程中始终显著高于ZS7。ZS7和HN1中的GSSG质量摩尔浓度,分别于第2小时和第16小时达到最高值,涨幅分别为79.0%和139.0%;至胁迫结束时,分别比对照下降20.1%和19.2%(图 5D)。低温梯度胁迫下,ZS7的GSH/GSSG比值呈上升的趋势,HN1则先下降后上升,至胁迫结束时,都显著高于常温对照(图 5E)。在-5 ℃持续低温胁迫下,ZS7的GSH/GSSG比值总体呈上升趋势,HN1则呈下降趋势(图 5F)。
2.1. 低温胁迫对不同耐寒性短枝木麻黄幼苗叶绿素质量分数的影响
2.2. 低温胁迫对不同耐寒性短枝木麻黄幼苗活性氧水平及丙二醛质量摩尔浓度的影响
2.3. 低温胁迫对不同耐寒性短枝木麻黄幼苗可溶性蛋白质和脯氨酸质量分数影响
2.4. 低温胁迫对不同耐寒性短枝木麻黄幼苗无性系抗氧化酶活性的影响
2.5. 低温胁迫对不同耐寒性短枝木麻黄幼苗谷胱甘肽循环的影响
-
随着低温胁迫程度的增强,叶绿素质量分数下降的幅度和速度也加大,可作为植物耐受低温的重要生理指标[18]。本研究中,低温梯度胁迫下,不耐寒短枝木麻黄无性系幼苗的叶绿素质量分数呈下降趋势。在低温梯度胁迫和-5 ℃持续低温胁迫下,耐寒无性系的叶绿素质量分数分别表现出先升高后降低和逐渐上升的趋势,这说明一定程度的低温对耐寒植物叶绿素的合成有一定程度的刺激作用,而耐寒植物较强的耐寒性减缓了叶绿素质量分数的下降,增强了适应低温环境的能力,使叶绿素始终维持较高水平。这在杨树Populus spp.,金叶女贞Ligustrum vicaryi,紫薇Lagerstroemia indica,福禄考属Phlox等植物对干旱、低温等胁迫的生理响应研究中得到相似的结果[19-21]。
H2O2水平升高是低温下氧化胁迫的标志。它一方面可引起脂质过氧化,对膜造成伤害,另一方面可引起光合系统各种酶的失活[22]。MDA作为膜脂过氧化产物,其质量摩尔浓度的高低与细胞膜受伤害的程度直接相关,与植物的抗寒性呈负相关,是鉴别低温胁迫对植物膜危害程度的重要指标之一[23]。本研究中,耐寒无性系和不耐寒无性系幼苗的H2O2和MDA质量摩尔浓度都显著升高,说明低温胁迫下,植物体内产生大量的活性氧(ROS),细胞膜的结构受到损伤,膜脂过氧化作用加强。但耐寒无性系的MDA和H2O2质量摩尔浓度都显著低于不耐寒无性系,说明耐寒性较强的无性系能保持细胞内的氧化还原平衡,降低了脂质过氧化程度。
逆境胁迫下,植物细胞能通过主动积累脯氨酸等渗透调节物质来调节渗透势,阻止细胞脱水,从而维持植物组织内各种酶活性和正常的细胞膜结构[24]。研究发现,脯氨酸在杠柳Peroploca sepium生境适应性上有重要作用,且脯氨酸代谢对植物维持组织细胞内ROS水平密切相关[25]。抗寒性强的油橄榄Olea europaea品种在低温胁迫下,累积的渗透调节物质含量,如脯氨酸和可溶性蛋白质,逐渐增加且显著高于其他不耐寒品种[26]。本研究中,耐寒无性系ZS7幼苗在常温下的脯氨酸和可溶性蛋白质质量分数就显著高于HN1,且随温度的降低或低温胁迫时间的延长,脯氨酸和可溶性蛋白质质量分数也始终显著高于HN1。说明在低温处理下,耐寒无性系通过增强渗透调节能力和降低膜脂过氧化作用,提高对外界环境变化的适应力。
SOD,POD,CAT,APX和GR是植物体内活性氧自由基清除系统的保护酶[27-29],通过协同作用抑制膜脂过氧化,防御活性氧自由基对细胞膜的损伤,缓解低温胁迫对膜系统的伤害[30]。GR活性和GSH质量分数大小被认为是有机体抗氧化状态的重要标志。在低温梯度胁迫下,耐寒无性系和不耐寒无性系的SOD,POD,CAT,GR活性以及GSH质量摩尔浓度均呈先上升后下降的趋势,表明植物通过调节酶和非酶类活性的高低来适应低温。GSH/GSSG的比值与蛋白质的生物合成有关,维持较高的比值可以增强植物对低温的抵抗力。在低温胁迫下,耐寒无性系中的GSH/GSSG比值呈上升趋势,不耐寒无性系中则呈下降趋势,说明GSH和GSSG对于短枝木麻黄耐寒性的提高具有重要作用。耐寒无性系中的APX活性在低温梯度胁迫下逐渐下降,但在-5 ℃持续低温胁迫下,表现出了强烈的诱导作用。这可能与适应低温的过程中,植物在代谢补偿性调节上发挥作用有关,某些代谢过程的增强抑制或降低了另外的代谢途径,暗示植物在适应低温环境时发生了积极的代谢调节[31]。低温胁迫处理下,耐寒无性系的酶类和非酶类抗氧化剂含量高,且始终显著高于不耐寒无性系,更有利于维持其细胞结构的稳定性。
本研究结果反映了耐寒性强的短枝木麻黄无性系通过在低温胁迫下保持较高的脯氨酸和可溶性蛋白质质量分数,增强SOD,CAT,POD,APX和GR等抗氧化酶的活性以及提高非酶抗氧剂质量摩尔浓度,抑制植物细胞中叶绿素质量分数的下降及膜脂过氧化程度的加剧,进而适应和抵御低温。











 DownLoad:
DownLoad: