-
金叶女贞Ligustrum × vicaryi又名英国女贞,新叶呈金黄色,在园林绿化中作为色叶树种和造型树种被广泛栽植[1-2]。为了达到观赏效果,在园林设计和施工中金叶女贞常被高密度栽植,从而使病害发生严重。由多主棒孢Corynespora cassiicola引起的叶斑病是金叶女贞上新见的一种严重病害,叶片感染后,出现轮纹病斑并伴随大量落叶,严重影响景观和生态价值[3]。多主棒孢是一种寄主范围广泛的重要植物病原菌,可侵染380个属内的530余种植物,包括黄瓜Cucumis sativus,番茄Solanum lycopersicum,辣椒Capsicum annuum和茄S. melongena等重要经济作物[4]。对不同寄主分离的棒孢菌株进行寄主范围及致病力测定,结果表明:多主棒孢种内存在寄生专化现象,来源于同一寄主的多主棒孢致病力具有高度的相似性,与其他来源的菌株存在显著的差异[5-6]。进一步的遗传多样性分析和系统发育分析表明:相同寄主来源菌株同源性较高,系统发育分析结果与各菌株间的致病力具有一定的相关性,说明多主棒孢的种内分化可能与其寄主来源有关[7-8]。病原菌寄生专化性的有无决定了不同寄主间是否存在交叉侵染的可能,对于制定防治策略具有重要意义。为研究金叶女贞上分离多主棒孢是否对其他经济作物有致病性,明确其与其他主要经济作物上分离棒孢的亲缘关系,本试验从河南省洛阳市分离的多主棒孢中选取代表菌株进行致病性测定并对其核糖体DNA内转录间隔区(rDNA-ITS),β-微管蛋白(β-tubulin)基因和延伸因子(ef-1α)基因进行扩增、测序及系统发育分析,为该病害的深入研究提供理论依据。另外,针对新见的金叶女贞叶斑病,对其病菌的生物学特性进行了较为系统的研究,并对病原菌形态描述补充了更为详实的研究方法和结果。
HTML
-
病害标本采自河南省洛阳市隋唐遗址植物园、洛浦公园及开元大道两旁绿化带,保存在河南科技大学植物病理学实验室;供试菌株(ST1,ST2,ST3)由采集的病叶分离、纯化,经柯赫氏法则确认后备用。
-
马铃薯葡萄糖琼脂(PDA)培养基和Czapek培养基,配方参考方中达[9]的方法。
-
病叶保湿培养后,刮取病原菌子实体在光学显微镜下观察其形态并拍照,随机分别选取100个分生孢子梗和分生孢子测量其大小,参照田雪亮等[10]方法观察孢子萌发。将分离获得纯培养供试菌株在PDA平板上培养2周后,显微观察产孢结构及孢子形态特征,并随机分别选取100个分生孢子测量其大小。
-
① 温度对菌丝生长的影响。将供试菌株ST1转接到PDA培养基上25 ℃培养6 d,用直径5 mm打孔器在菌落边缘打出菌饼,接种于PDA平板后分别置于5,10,15,20,25,30,35和40 ℃共8个温度处理下培养。每处理重复5次,6 d后用十字交叉法测量菌落直径。②pH值对病原菌菌丝生长的影响。PDA培养基灭菌后冷却至50 ℃左右时,用1 mol·L-1盐酸和1 mol·L-1氢氧化钠溶液将PDA培养基的pH值调为4,5,6,7,8,9,10和11并制成平板,转接ST1菌株菌饼后25 ℃培养6 d。每处理重复5次,6 d后用十字交叉法测量菌落直径。③光照对病原菌菌丝生长的影响。设置全黑暗、全光照(25 W日光灯)、黑暗光照交替(12 h黑暗+ 12 h光照)3种光照条件。其他同②。④碳源对病原菌菌丝生长的影响。以Czapek培养基为基本培养基,分别用麦芽糖、葡萄糖、甘露醇、可溶性淀粉、木糖和D-果糖替换Czapek培养基中的蔗糖,制成不同碳源的培养基平板,其他同②。⑤氮源对病原菌菌丝生长的影响。以Czapek培养基为基本培养基,分别用蛋白胨、酵母膏、尿素、L-天冬氨酸、硝酸铵和硫酸铵替换Czapek培养基中的硝酸钠,制成不同氮源的培养基平板,其他同②。
-
参考刘以道等[11]方法,选取金叶女贞、黄瓜、番茄、辣椒和茄的健康叶片(金叶女贞为枝条上部嫩叶,其他为2~4片真叶期叶片),用体积分数为0.8%次氯酸钠溶液消毒5 min,无菌水冲洗3次,正面向上平放在保鲜盒内,叶柄用少许灭菌棉花包住,滴加无菌水保湿。采用菌饼接种法接种供试植物的离体叶片,同一叶片主脉对称位置设有伤和无伤及对照,叶片左边上为有伤接种菌饼,下为有伤无菌对照(有伤接种用消毒后接种针刺伤);叶片右边上为无伤接种菌饼,下为无菌对照。接种时用直径5 mm的打孔器在生长6 d的菌落上距离中心相同位置打菌饼,再将菌饼接种于消毒后叶片上,对照为直径5 mm的PDA培养基圆碟,每种植物接种10片叶,试验重复1次。接种叶片放置在光照培养箱内25 ℃条件下保湿培养。接种后每天记录供试植物的发病情况,连续观察6 d。
-
采用改良CTAB法[12]提取供试菌株ST1,ST2和ST3基因组DNA。以引物ITS1/ITS4,tub-F1/tub-R2和EF1-728F/EF1-986R(表 1)分别扩增核糖体DNA内转录间隔区(rDNA-ITS),β-微管蛋白(β-tubulin)和延伸因子(ef-1α)基因片段。PCR扩增产物委托生工生物工程(上海)股份有限公司进行双向测序。
-
将得到序列拼接,在GenBank中利用BLAST进行序列分析,根据结果及相关文献[5],选择下载合适序列与本研究供试菌株序列共同构建系统发育树。用软件ClustalX 1.83对各菌株的rDNA-ITS,β-tubulin和ef-1α基因序列进行多重比较后,应用SequenceMatrix进行序列串联[15],以C. smithii的相应基因序列为外群,用MEGA 6.0软件进行系统发育进化分析[16],采用邻接法(neighbor-joining,NJ)构建系统发育进化树,最后应用自举法(bootstrap)重复抽样1 000次以评估各分支节点的可靠性。
-
用SPSS 18.0统计软件进行数据统计分析,用Duncan氏新复极差法进行差异显著性分析。
1.1. 材料
1.1.1. 供试材料
1.1.2. 供试培养基
1.2. 试验方法
1.2.1. 病原菌形态观察
1.2.2. 病原菌生物学特性研究
1.2.3. 不同寄主致病性测定
1.2.4. 病原菌DNA提取、扩增及核苷酸序列测定
1.2.5. 序列比对和系统发育树的构建
1.3. 数据分析
-
发病叶片保湿培养2~3 d后叶两面产生大量子实体,分生孢子梗褐色或浅褐色,单生或几根簇生,直立或略弯曲,有2~9个分隔,基部膨大成球形,孢梗长98.5~768.6 μm(平均423.4 μm),孢梗宽4.5~6.6 μm(平均5.3 μm)(图 1A);偶见有层出梗孢(图 1B);分生孢子浅褐色或浅橄榄色,形态以柱状为主,偶见倒棍棒状,直或稍弯曲,孢子长78.8~317.3 μm(平均173.5 μm),孢子宽5.3~12.6 μm(平均7.9 μm),横隔数3~19个(平均11.9个)(图 1C)。分生孢子萌发方式主要从孢子两端长出芽管,偶见只从一端长出芽管(图 1D)。
PDA培养基上通常培养7~8 d以后可产生少量分生孢子,2周后产生大量分生孢子,分生孢子浅褐色到褐色,长椭圆形或倒棍棒形,较直,有横隔(图 1E);孢子长14.8~147.8 μm(平均47.3 μm),孢子宽4.8~9.5 μm(平均6.9 μm),横隔数:0~10个(平均2.8个)(图 1E)。分生孢子产生于由菌丝特化的分生孢子梗上,单生或2~6个串生(图 1F)。
-
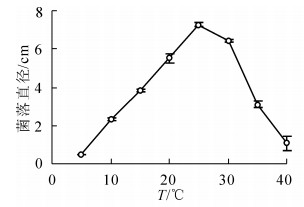
在10~25 ℃内,随着温度升高病原菌生长速度增加,25 ℃时菌落直径最大,达到(7.27 ± 0.10)cm,显著高于其他温度条件下菌落直径(P<0.05);30 ℃之后随着温度升高生长速度下降,40 ℃生长6 d后菌落直径仅为(1.08 ± 0.37)cm;病原菌在5 ℃不能生长(图 2)。
-
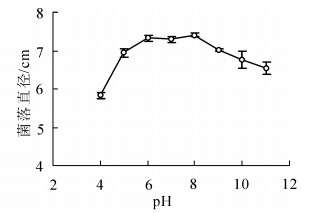
病原菌能在较广的pH值范围内生长。pH值为4~11时菌丝均能生长,以pH值为6~8时生长最好,菌落直径显著大于其他pH值条件下的菌落直径(P<0.05);在pH值为4时菌丝生长较缓慢,菌落直径为(5.84 ± 0.08)cm,显著小于其他pH值条件下菌落直径(P<0.05)(图 3)。
-
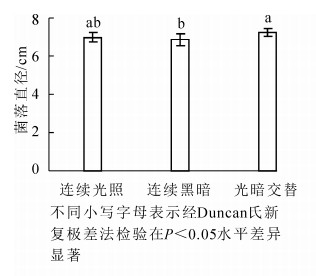
病原菌菌丝在光暗交替(12 h光照/12 h黑暗)下生长较好,菌落直径为(7.26 ± 0.21)cm,显著大于连续黑暗条件下菌落直径(P<0.05),连续光照和光暗交替条件下病菌菌落大小没有显著差异(P>0.05)(图 4)。
-
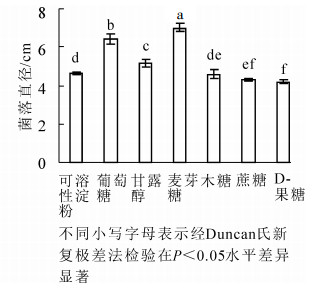
病原菌在供试的7种碳源上均可生长,但对各碳源利用程度不同(图 5)。以麦芽糖为碳源的培养基上菌丝生长最快,菌落直径为(7.04 ± 0.07)cm,显著大于其他碳源条件下菌落直径(P<0.05);其次是以葡萄糖为碳源的培养基。病原菌在D-果糖为碳源的培养基上菌丝生长较慢,其菌落直径显著低于以麦芽糖、葡萄糖、甘露醇、可溶性淀粉和木糖为碳源的菌落直径(P<0.05)(图 5)。
-
在氮素营养利用方面,蛋白胨作为氮源病菌菌丝生长最好,其次是酵母膏,两者显著优于尿素、L-天冬氨酸、硝酸钠、硝酸铵和硫酸铵(P<0.05);病原菌在以铵盐硝酸铵和硫酸铵为氮源培养基上生长受到抑制,菌落直径显著小于其他氮源(P<0.05)(图 6)。
-
接种试验结果表明:C. cassiicola菌株ST1除了侵染金叶女贞外,还可侵染黄瓜、番茄、辣椒和茄,不同植物叶片上的显症时间存在差异(表 2)。如图 7所示:金叶女贞、辣椒、番茄和黄瓜叶片有伤接种1~2 d后都可见病斑,金叶女贞上病斑扩展最快(图 7A)。辣椒叶片无伤接种2~3 d后出现褐色小斑点,近圆形或不规则,病斑周围有黄色晕圈(图 7B)。番茄叶片无伤接种5 d后出现水渍状病斑(图 7C)。黄瓜叶片无伤接种最早出现症状,病斑呈水渍状小斑点,扩展后略凹陷、近圆形或不规则,病斑后期变为褐色,周围有黄色晕圈(图 7D)。茄叶片无伤接种显症最晚,5~6 d后出现水渍状小斑点(图 7E)。
寄主 有伤接种 无伤接种 致病性 显症时间/d 致病性 显症时间/d 金叶女贞 + 1~2 + 2~3 黄瓜 + 1~2 + 1~2 辣椒 + 1~2 + 2~3 番茄 + 1~2 + 3~4 茄 + 2~3 + 5~6 说明:“ + ”表示致病 Table 2. Pathogenicity of C. cassiicola to leaves of different hosts
-
对源自于金叶女贞、黄瓜、辣椒、番茄和茄上的多主棒孢菌株进行系统发育研究,采用NJ法基于ITS,β-tubulin基因和ef-1α基因序列构建系统发育树(图 8)。图 8表明:同一寄主植物的分离物以较高的置信度相聚在一起,来自5个寄主植物上的多主棒孢菌株形成3个大分枝,寄主为辣椒和茄的分离物聚在1个分枝A(自展支持率为99%),番茄上的分离物独自聚为1个分枝B(自展支持率为99%)。本研究获得的3个分离物ST1,ST2和ST3在进化关系上与黄瓜上分离的所有菌株亲缘关系最近,以较高的自举值(自展支持率为100%)聚为一个分枝C。
2.1. 病原菌的形态特征及菌落特征
2.2. 不同温度对菌丝生长的影响
2.3. 不同pH值对菌丝生长的影响
2.4. 不同光照条件对菌丝生长的影响
2.5. 不同碳源对菌丝生长的影响
2.6. 不同氮源对菌丝生长的影响
2.7. ST1菌株对不同寄主致病性测定
2.8. 基于ITS,β-tubulin基因和ef-1α基因序列的系统发育分析
-
本研究观察发现:在自然发病叶片上多主棒孢形成的分生孢子与PDA培养基上形成的孢子在形态、大小方面有很大的差异,发病叶片上形成的分生孢子形态以柱状为主,偶见倒棍棒状,直或稍弯曲,孢子平均长173.5 μm,孢子平均宽7.9 μm,横隔膜平均11.9个;PDA培养基上形成的分生孢子形态为长椭圆形或倒棍棒形,较直,孢子较短,平均长仅为47.3 μm,孢子平均宽6.9 μm,横隔膜平均2.8个。这与潘羡心等[17]报道的结果较一致,自然侵染橡胶Hevea brasiliensis树叶片上产生的多主棒孢分生孢子比在PDA培养基上的形成分生孢子更长,具有更多的隔膜。由此可见,培养基质对多主棒孢分生孢子形态影响较大,由于棒孢属内种的划分主要依据分生孢子梗的形态、大小、层出梗的多少以及分生孢子的大小、颜色、形状及假隔膜数[18]。笔者建议多主棒孢的鉴定以自然寄主上形态特征为准。
本研究表明:金叶女贞叶斑病原菌多主棒孢可生长的温度为10~40 ℃,适宜生长温度为25 ℃,与橡胶[17],甜瓜Cucumis melo[19],广藿香Pogostemon cablin[20]等其他寄主植物上多主棒孢生长的适宜温度一致,这与该病在洛阳地区5月下旬开始发病,7-8月高温多雨季节有利于病害迅速传播蔓延的发病规律相吻合。病原菌在pH值为4~11的范围菌丝均能生长,在酸性条件pH值为4和5,碱性条件pH值为10和11时生长状况稍差,但仍有一定的生长量,可见该病菌既耐酸又耐碱,适应范围较广。
碳源和氮源在病原菌生长和繁殖过程中具有非常重要的作用,碳源是构成其细胞骨架的主要物质,也是生命活动所需能源的提供者,而氮源则是其用来进行核酸、蛋白质、酶类以及含氮代谢产物合成的物质[21]。不同菌物在代谢过程中产生的酶种类、活性存在差异,因此对碳源和氮源的利用也存在差异[22]。本研究表明:金叶女贞叶斑病病原多主棒孢对麦芽糖的利用最好,与张春霞等[23]和张贺等[24]的研究结果一致。在氮源利用上,病菌对无机氮源铵盐利用能力最低,有机氮源蛋白胨和酵母膏最适合该病菌,这可能是因为有机氮营养丰富且成分复杂,除含有氮素外还含有其他生长因子,更有利于微生物的生长。
多主棒孢寄主范围很广,可以寄生在许多不同科属的植物上[4, 7],但其中一些菌株却只能对某种植物致病,种内存在寄生专化现象[6, 8]。本研究结果表明:从金叶女贞上分离的多主棒孢菌株对供试植物都有致病性,但是致病力存在明显差异,ST1菌株对金叶女贞致病力最强,其他依次是黄瓜、辣椒、番茄和茄。据此结果,建议在黄瓜、辣椒、番茄和茄等蔬菜园周围不要栽植金叶女贞,避免交叉感染。进一步的系统发育分析表明,相同寄主来源菌株同源性较高,聚在一个分枝上,这一结果与前人的研究结果相一致[5, 7];另外,系统发育树显示金叶女贞上分离的多主棒孢菌株与黄瓜上分离的菌株亲缘关系很近,以较高的自举值(自展支持率为100%)和黄瓜上分离的所有菌株聚为一簇。SUMABAT等[8]对分离自美国东南部地区8个寄主植物上的多主棒孢进行系统发育分析,也发现同一寄主植物上分离的多主棒孢在遗传上往往具有更高的一致性;不同寄主上多主棒孢菌株间亲缘关系有差异,棉花上分离的多主棒孢菌株与大豆上的菌株亲缘关系更近,聚为一族(自举检验值94%/贝叶斯后验概率100%)。


















 DownLoad:
DownLoad: