-
城市园林绿化事业的快速发展导致园林废弃物的数量日益增多[1]。堆肥已成为园林废弃物资源化利用的主要方式之一[2-5]。园林废弃物中的木本植物残体存在大量难降解的木质素。这些木质素溶解性差,没有任何易被水解的键,分子结构复杂且不规则,含有各种稳定的复杂键型,微生物及其分泌的酶不易与之结合[6]。这些木质素还包裹着纤维素,即使微生物可分解单独存在的纤维素,但细胞壁中木质素对纤维素起到保护作用,纤维素的降解仍受到限制[7-8],严重影响堆肥进程,因此促进木质素降解是加快堆肥进程和提高堆肥产品品质的重要环节[9]。自然界中的真菌、细菌及相应微生物群落可通过产生分解木质素的酶系统(漆酶、锰过氧化物酶和木质素过氧化物酶)将木质素完全降解,且大多数真菌降解效果强于细菌[6, 10]。堆肥中添加微生物菌剂可显著提高木质素降解率,加快堆肥进程[8, 11]。YU等[12]通过二次回归正交设计研制出了一种园林废弃物专用复合菌剂,其木质素降解能力强于有效微生物复合菌(EM菌)。何慧中等[13]开发出一种复合功能菌剂,添加到桉树Eucalyptus皮堆肥中,木质素降解效果显著,与对照相比木质素含量下降了78.78%。目前,有关木质素降解的菌剂研究多集中在液体菌剂,但液体菌剂存在生产工序复杂,易污染,易失活,不便于保存等缺点。因此,有必要将木质素降解菌制成固体菌剂,弥补液体菌剂的不足。微生物固定化技术是指通过物理或化学的手段将游离的微生物限定在一定的空间区域,保持其生物活性并能反复利用的方法[14]。将菌株运用固定化方式制成的固体菌剂,具有生产成本低,耐储存,不易失活,便于运输等优点,有利于菌剂在更大范围内推广和应用[15]。然而,固体菌剂的产品质量受多种因素影响,如接菌量、保护剂浓度和含水率可直接影响菌剂产品的稳定性和应用效果,而且国内外关于木质素降解菌固定化的研究尚不充分,有关园林废弃物堆肥的报道更为鲜见。鉴于此,本研究将1株木质素降解菌通过固定化的方式制成固体菌剂,以有效活菌数为评价指标,对菌剂制作过程中的主要影响因素进行优化,再通过正交试验获得最佳固体菌剂的制备条件,将其应用到园林废弃物堆肥中进行效果检验,以期为该类菌剂的研制与应用提供理论依据。
HTML
-
菌种为曲霉属Aspergillus sp.真菌No.11[1],目前保存于北京林业大学林学院土壤生物学实验室。堆肥原料来源于北京植物园,主要为花草树木的人工修剪物和自然生长产生的枯枝落叶,粉碎成1~2 cm粒径。培养基:马铃薯葡萄糖肉汤(PDB)培养基和马铃薯葡萄糖琼脂(PDA)培养基。载体与保护剂:通过预实验确定生物质炭和米糠为固定化载体,海藻糖为保护剂,载体混合质量比为1∶1。
-
将4 ℃保存的菌株No.11接种到PDA培养基上,28 ℃下培养3 d完成活化。将活化后的菌株No.11挑取至装有100 mL PDB培养基的摇瓶中,置于28 ℃、200 r·min−1的摇床中培养48 h(对数生长期末)获得种子液备用。
-
接菌量试验:按照载体质量的5%、10%、15%、20%和25%接种种子液,调节料水质量比为1.0∶0.8,搅拌均匀,28 ℃培养48 h。培养完成后放在40 ℃烘箱中完全烘干,在室温下干燥密封保存30 d后,测定有效活菌数。
保护剂体积分数试验:种子液中分别添加体积分数为0、4%、8%、12%、16%和20%的保护剂,按载体质量的10%接种到载体中,调节料水质量比为1.0∶0.8,混匀后,28 ℃培养48 h。培养完成后放在40 ℃烘箱中完全烘干,在室温下干燥密封保存30 d后,测定有效活菌数。
含水率试验:向载体中接种质量分数为10%的种子液,调节料水质量比为1.0∶0.8,混合均匀,28 ℃培养48 h之后,放在40 ℃烘箱中烘至含水率为5%、10%、15%、20%和25%,在室温下干燥密封保存30 d后,测定有效活菌数。
-
根据1.2.2节试验结果确定优化范围,其中接菌量为5%、10%、15%,保护剂体积分数为0、4%、8%,含水率为10%、15%、20%,进行3因素3水平正交试验设计。具体方案见表1。根据表1,向载体中接种相应水平的种子液和保护剂,调节料水质量比为1.0∶0.8,混合均匀,28 ℃培养48 h,之后放在40 ℃烘箱中烘至该处理对应的含水率。将制备好的固体菌剂在室温环境下干燥密封保存30 d,测定有效活菌数,确定最佳菌剂的制备条件。
水平 因素 接菌量/% 保护剂/% 含水率/% 1 5 0 10 2 10 4 15 3 15 8 20 Table 1. Design of the orthogonal experiment
-
堆肥模拟试验。将粉碎后的园林废弃物分别装入500 mL锥形瓶,装80 g·瓶−1,调节含水率达60%,共4组处理,分别为不添加菌剂(ck)、添加质量分数为0.5%市售EM菌剂(T1)、添加质量分数为0.5%自制固体菌剂(T2)、添加质量分数为1.0%自制固体菌剂(T3)。各组处理3次重复。搅拌均匀后用8层纱布封好瓶口,置于恒温培养箱中避光培养。为模拟堆肥过程中的升温、高温和降温阶段,弥补堆肥模拟试验中因堆体较小,无法自主升温的缺陷,人工进行培养箱温度的调节:温度从25 ℃逐渐上升至50 ℃,再逐渐降至30 ℃。各阶段经历时间分别为5、30、5 d。
样品采集。采集第1、8、16、24、32、40 天的堆肥样品鲜样1 g,测定木质素降解相关酶的酶活力;采集第1 天和第40 天堆肥样品测定pH、电导率(EC)、D(465)/D(665)[样品滤液在465 nm处吸光度D(465)和665 nm处吸光度D(665)的比值]、种子发芽指数(IG)、木质素质量分数和纤维素质量分数等指标。
木质素降解相关酶活力测定。漆酶、锰过氧化物酶和木素过氧化物酶活力测定参照田林双[16]的木质素降解相关酶类测定标准方法。
堆肥腐熟指标测定。pH和EC测定:称取待测样品5 g,置于100 mL塑料瓶中,加入50 mL蒸馏水,200 r·min−1振荡1 h,过滤其上清液,用pH 400防水型笔式pH计和EC 400防水型笔式电导率/TDS/盐度计分别测定各样品的pH和EC;样品滤液在465 nm处吸光度D(465)和665 nm处吸光度D(665)测定:用UV-6100紫外可见分光光度计 (上海元析仪器有限公司)测定样品滤液在465 nm处吸光度D(465)和665 nm处吸光度D(665);IG测定:取5 g鲜样置于100 mL塑料瓶中,加入50 mL蒸馏水,振荡1 h后获取上清液,将2张滤纸平铺到直径为9 cm的培养皿中,滤纸上加入5 mL上清液,以蒸馏水为空白对照,播撒白菜Brassica chinensis种子20粒·皿−1,置于25 ℃培养箱中培养48 h后记录发芽率和根长。计算IG,IG=(上清液处理的发芽率×根长)/(空白组的发芽率×根长)×100%。木质素、纤维素降解率测定:木质素、纤维素质量分数分别用硝酸-乙醇法和72%硫酸法进行测定[17]。
-
采用Excel 2010 和SPSS 22.0 软件对数据进行分析处理。
1.1. 材料
1.2. 方法
1.2.1. 种子液的制备
1.2.2. 单因素优化试验
1.2.3. 正交试验设计
1.2.4. 固体菌剂堆肥效果验证
1.2.5. 数据分析
-
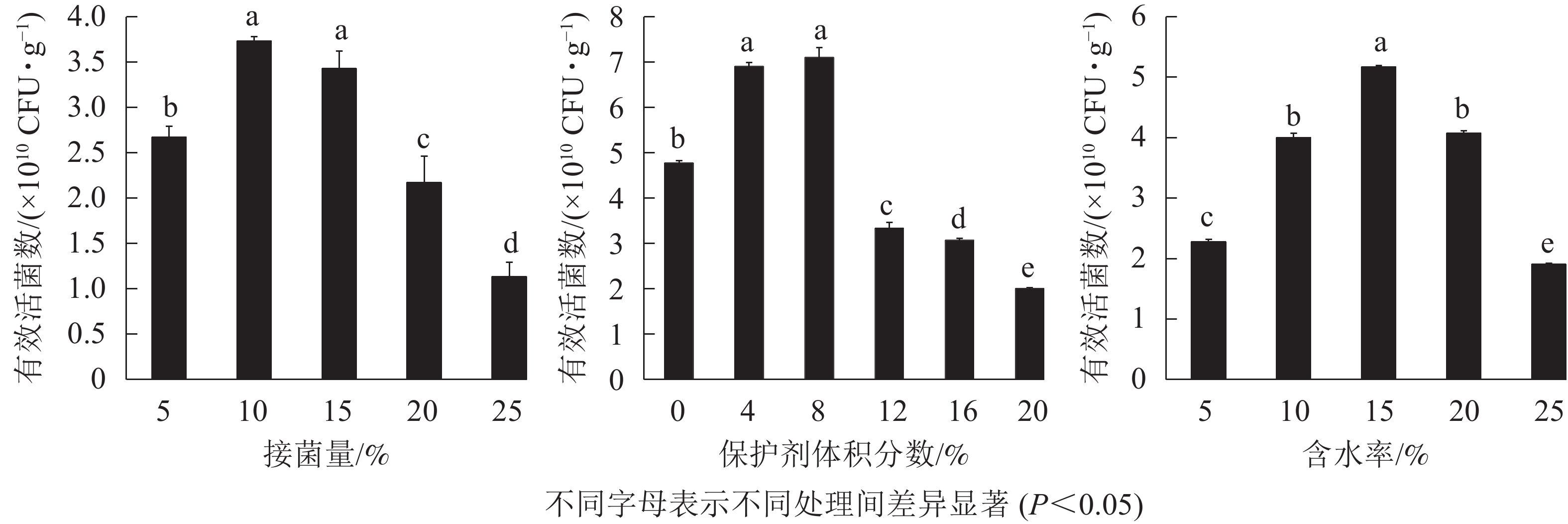
接菌量可直接影响固体菌剂的质量。接菌量过少会延长菌株的生长停滞期,过大会增加生产成本,也会增强微生物之间的竞争作用[18]。由图1A可知:固体菌剂中的有效活菌数随接菌量的增加呈先增加后减少的趋势,接菌量为10%时有效活菌数最高,达3.73×1010 CFU·g−1;其次是接菌量为5%和15%时,有效活菌数达2.50×1010 CFU·g−1以上;当接菌量超过15%时,菌剂中的有效活菌数逐渐降低,低于2.50×1010 CFU·g−1。因此,选用接菌量5%、10%和15%作为正交试验的3个水平。

Figure 1. Effect of inoculation amount, protective agent concentration and water content on living bacteria count
微生物菌剂中添加一定量的保护剂可以增强其耐储藏性和稳定性,能直接影响菌剂的产品质量与应用效果[19]。由图1B可看出:当保护剂体积分数为8%时,活菌数最高达7.10×1010 CFU·g−1,保护剂体积分数<8%时,有效活菌数随保护剂体积分数升高而增多;当保护剂体积分数>8%后,随着保护剂体积分数的升高,有效活菌数显著(P<0.05)降低,低于4.00×1010 CFU·g−1。因此,选用0、4%和8%作为正交试验的3个水平。
含水率对固体菌剂的储存有很大影响。含水率过高容易滋生杂菌,使固体菌剂受到污染影响应用效果,含水率过低不利于菌株的生存,一段时间后有效活菌数会大幅度降低[20]。由图1C可知:随着含水率的提高,固体菌剂中有效活菌数呈先升高后降低的趋势,含水率为15%时有效活菌数最高,可达5.17×1010 CFU·g−1;含水率为10%和20%时,固体菌剂中有效活菌数可达4.00×1010 CFU·g−1以上;含水率为5%和25%时,固体菌剂的储存效果最差,有效活菌数仅为2.00×1010 CFU·g−1左右。综上可知,当固体菌剂含水率为10%~20%时,有效活菌数较高,因此,选用10%、15%、20%作为正交试验中的3个水平。
-
由表2可知:在接菌量、保护剂体积分数和含水率等3种因素中,影响程度最大的是接菌量,其次是含水率,影响程度最小的是保护剂体积分数。固体菌剂制备的最佳配方为A2B3C2,即:接菌量10%、保护剂体积分数8%、含水率15%。该条件下,菌剂中有效活菌数达1.26×1011 CFU·g−1,符合GB 20287−2006《农用微生物菌剂》的标准(>0.50×108 CFU·g−1)。
处理 接菌量
(A)/%保护剂
(B)/%含水率
(C)/%有效活菌数/
(×1010 CFU·g−1)1 5 0 10 5.87±0.17 b 2 5 4 15 1.03±0.09 d 3 5 8 20 2.23±0.12 c 4 10 0 20 0.53±0.05 e 5 10 4 10 6.23±0.17 b 6 10 8 15 12.57±0.05 a 7 15 0 15 2.30±0.36 c 8 15 4 20 0.50±0.08 e 9 15 8 10 1.17±0.31 d K1 9.13 8.70 13.27 K2 19.33 7.76 15.90 K3 3.97 15.97 3.26 平均K1 3.04 2.90 4.42 平均K2 6.44 2.59 5.30 平均K3 1.32 5.32 1.09 极差R 5.12 2.74 4.21 说明:不同小写字母表示不同处理间差异显著(P<0.05) Table 2. Range analysis of orthogonal test
-
pH是评价堆肥腐熟程度的指标之一。堆肥腐熟后,pH一般为7.0~8.5[21]。由表3可知:堆肥结束时各处理pH均在8.0左右,符合NY 525−2002《有机肥料》标准。
处理 pH EC/(mS·cm−1) D(465)/D(665) IG/% ck 7.89±0.04 a 0.35±0.00 a 5.85±0.53 a 106.00±15.31 a T1 8.01±0.13 a 0.34±0.02 a 6.22±0.06 a 114.00±21.44 a T2 8.01±0.03 a 0.34±0.01 a 6.26±0.11 a 118.00±3.55 a T3 7.97±0.06 a 0.32±0.01 a 6.46±0.09 a 112.00±10.93 a 说明:相同小写字母表示不同处理间差异不显著(P>0.05) Table 3. Determination results of composting maturity index
EC可以表示堆肥中可溶性总盐的含量,其大小能影响植物的生长,EC过高的堆肥产品可以影响土壤理化性质,使植物生长受到毒害。EC小于4.00 mS·cm−1,表明堆肥已达到腐熟,对植物生长无毒害[22]。由表3可知:ck处理EC为0.35 mS·cm−1,各处理的EC相近,均小于4.00 mS· cm−1,在腐熟标准之内。
D(465)/D(665)能反映出胡敏酸分子的稳定程度,D(465)/D(665)较大说明胡敏酸相对稳定,较小说明胡敏酸结构简单,因此可用来分析评价堆肥的腐殖化作用大小[9]。由表3可知:堆肥结束时,各处理的D(465)/D(665)均为6.0左右。
IG是判断堆肥产品是否腐熟的生物学指标。堆肥产品未腐熟时会产生对植物生长有毒有害的物质,抑制植物的生长。一般情况下,当IG大于80%就可认为产品已达到腐熟[23]。由表3可知:各处理的IG均超过80%,堆肥产品对植物无毒。
综上可知,堆肥进行40 d后,各处理的pH和EC均达到腐熟标准且无显著差异,但添加菌剂后可以缩短堆肥腐熟的时间[24]。各处理的D(465)/D(665)均为6.0左右,说明其缩合度和芳构化仍很低,这也从另一方面表明腐殖质活性较强[9]。各处理的IG均超过80%,这与ZHANG等[25]测定的园林废弃物堆肥产品IG一致。
-
堆肥过程中微生物会分泌各种酶,从而将木质素类大分子物质转化成腐殖质等促进植物生长的物质[26]。与木质素降解相关的生物酶主要包括漆酶、锰过氧化物酶和木质素过氧化物酶[27]。
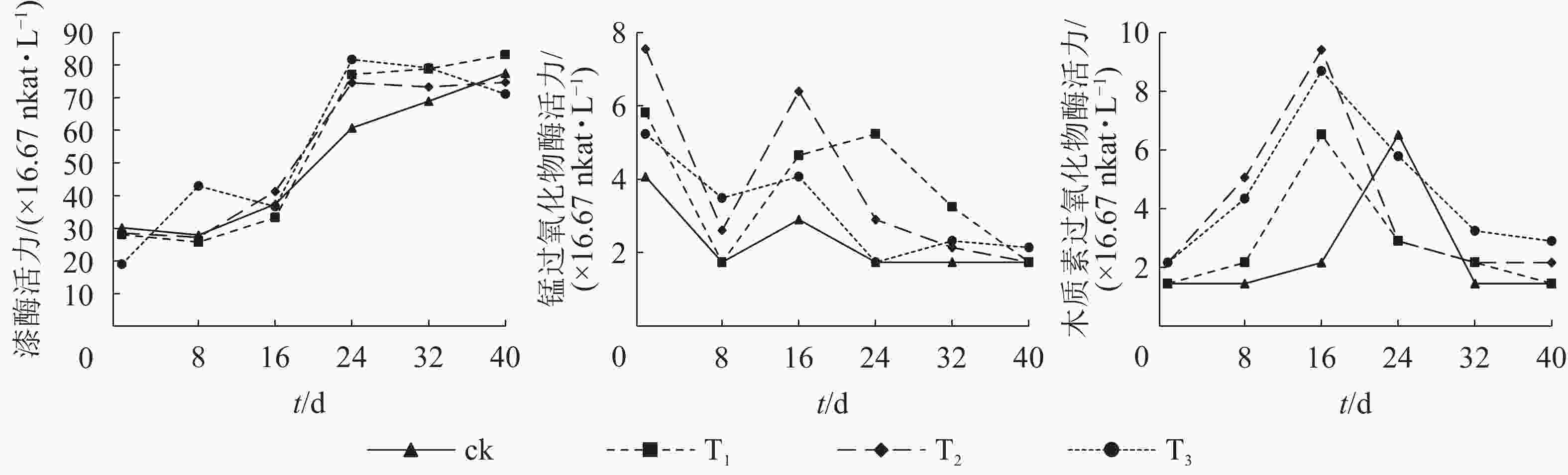
在堆肥过程中,漆酶对木质素的降解起着非常重要的作用,研究漆酶活力的变化对评价堆肥进程及微生物活动强度至关重要[28]。由图2A可看出:除T3外,其余处理漆酶活力在初始阶段相差不大,呈现先降后升趋势,与陈建军等[29]研究结果一致。可能是堆肥材料中某些小分子物质先降解,之后微生物再降解木质素类难降解的大分子物质。T3酶活力先升后降,可能与其开始微生物数量较多,分解速率较高有关。堆肥进行到24 d时,T1、T2和T3漆酶活力远超过ck,说明添加自制菌剂与市售菌剂都可大大增强堆肥中微生物的活动强度,随着微生物菌落增多,产酶能力也增加。第24 天之后,T3酶活力下降,ck酶活力上升,T1与T2酶活力变化不大,且大小相当,均达80 U·L−1(1 U=16.67 nkat)左右。菌株No.11的研究结果也显示:与可高效降解木质素的黄孢原毛平革菌Phanerochaete chrysosporium相比,此菌株有更强的产酶能力,也进一步说明自制固体菌剂有更大的应用潜力。
锰过氧化物酶是一种酚氧化物酶,可与其他酶共同作用提高对木质素的降解作用[10]。由图2B可看出:添加菌剂的处理组锰过氧化物酶活力均高于ck,说明添加菌剂可以提高堆肥中锰过氧化物酶的酶活力。堆肥初始阶段,所有处理组的锰过氧化物酶活力均出现先降后升趋势,可能与堆肥中的氮素含量有关,氮素含量会影响微生物分泌锰过氧化物酶[27]。第8 天后所有处理组酶活力又出现了上升趋势,说明微生物代谢活动增强,开始分泌锰过氧化物酶,T2与T3在第16天时达到峰值,T1的峰值出现在第24天左右。这表明添加自制固体菌剂后菌株可较快适应环境分泌锰过氧化物酶。有研究表明:锰过氧化物酶在限氮高锰培养基中产量较高[10],因此制备此类菌剂时,可通过优化含氮量提高产锰过氧化物酶的能力。
木质素过氧化物酶是一种含亚铁血红素的过氧化物酶,可直接与芳香底物发生反应,也可通过氧化低分子量的中介体而间接地发挥作用[30]。由图2C可看出:所有处理组木质素过氧化物酶活力均呈现先升后降趋势,添加菌剂的处理组酶活力的峰值出现时间均早于ck,且峰值高于ck,表明加入菌剂后可明显提高微生物分泌木质素过氧化酶的速率[31]。堆肥的后期,木质素过氧化酶显著降低,分析原因可能与此时碳氮比的变化有关。
-
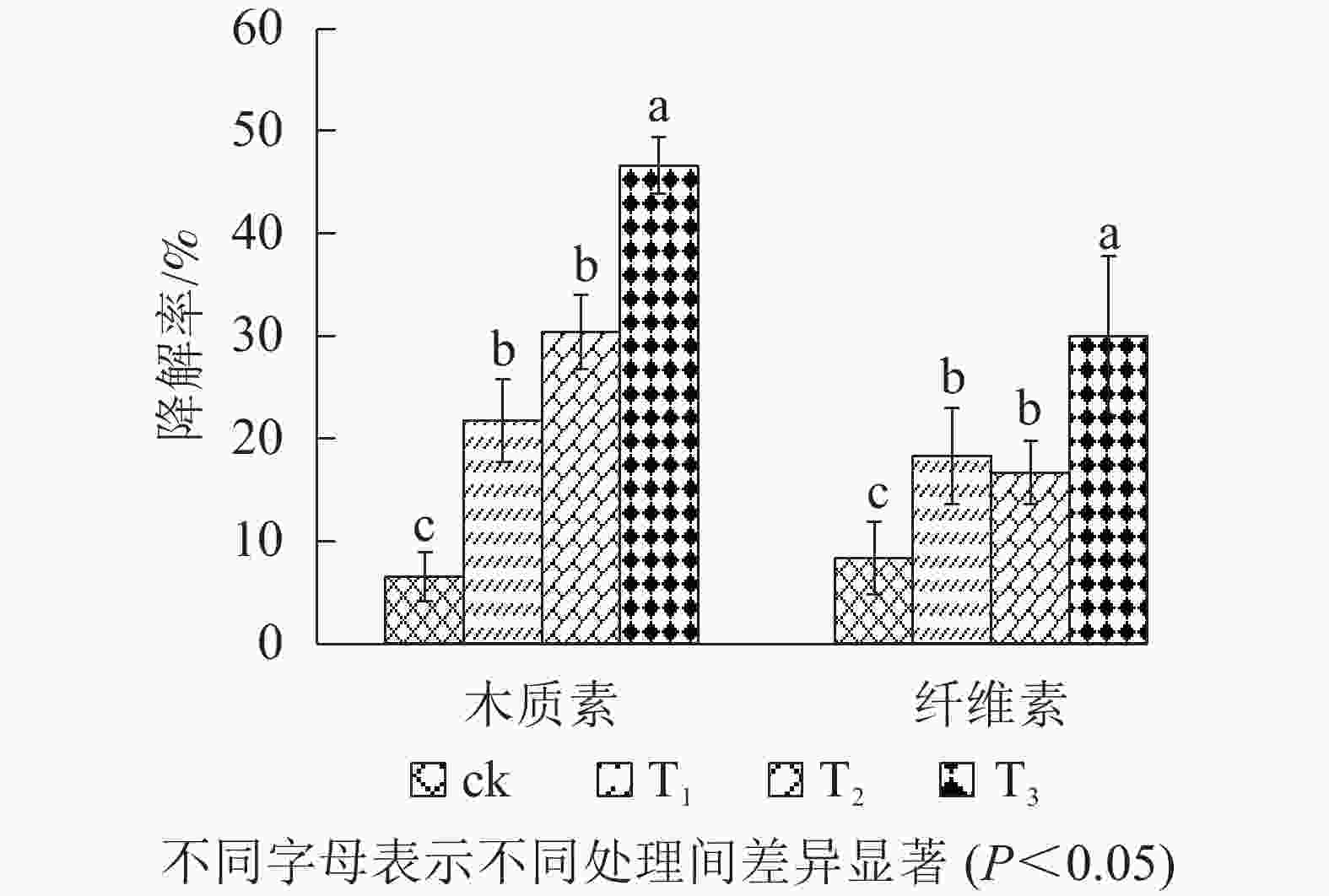
木质素是一种在自然界中广泛存在的有机高分子化合物,多存在于植物的细胞壁中[32]。木质素的完全降解由细菌、放线菌和真菌共同参与,其中真菌起重要作用[33]。由图3可看出:添加菌剂的处理木质素与纤维素降解率均高于ck,T3木质素降解率达46.65%,其次是T2,木质素降解率为30.43%,而T1的木质素降解率仅为21.74%。
纤维素是植物细胞壁的主要结构成分,通常与半纤维素和木质素结合在一起[34]。自然界中有许多微生物可以通过酶的作用分解植物残体中的纤维素,但细胞壁中木质素对纤维素起到保护作用,所以木质素和纤维素的分解都受到限制[6]。由图3可知:添加菌剂后可提高园林废弃物堆肥中纤维素降解率,其中T1降解率为18.33%,T2降解率为16.67%,T3纤维素降解率最高,达30.00%。
综上可知,T2木质素降解率高于T1,说明自制固体菌剂对园林废弃物中木质素的降解效果较好。纤维素降解率结果显示:T1略强于T2,这可能是因为市售菌剂中的菌株对纤维素降解能力较好,而自制固体菌剂中的菌株主要产生木质素降解相关酶,对木质素的降解效果较好。T3的木质素降解率与纤维素降解率均高于T2,说明在考虑成本的前提下,需进一步研究自制固体菌剂的添加量,以获得最大经济效益。与王顺利等[35]制备出堆肥菌剂CC-1相比,接菌量相当的情况下添加自制固体菌剂可使纤维素降解率提高11.68%,木质素降解率提高46.65%。这可能与菌株No.11的特殊菌丝结构有关,同时说明自制固体菌剂可高效降解木质素和纤维素。与尹爽等[36]研制的复合菌剂相比,添加自制固体菌剂木质素降解率较高,可能是因为自制固体菌剂更易于微生物在堆肥中均匀生长,能极大程度地发挥降解作用。自制固体菌剂可以较好地分解园林废弃物中的木质素,并能提高纤维素降解率。
2.1. 单因素优化试验结果
2.2. 正交设计试验结果
2.3. 堆肥试验结果
2.3.1. 堆肥腐熟指标
2.3.2. 木质素酶活力测定结果
2.3.3. 木质素和纤维素降解率测定结果
-
木质素降解菌No.11的最佳固定化条件为:接菌量10%、保护剂体积分数8%、含水率15%。在此条件下,获得的固体菌剂成品保存30 d后,其有效活菌数达1.26×1011 CFU·g−1,符合GB 20287−2006《农用微生物菌剂》的要求。
添加自制固体菌剂的堆肥产品pH为8.01,达到NY 525−2002《有机肥料》标准,EC为0.34 mS·cm−1,D(465)/D(665)为6.26,IG达118%,对植物无毒。
堆肥中添加自制木质素降解固体菌剂有利于木质素降解酶系的产生,漆酶、锰过氧化物酶和木质素过氧化物酶的酶活力均得到提升。与不添加菌剂相比,木质素降解率提高23.91%,纤维素降解率提高8.34%;0.5%接种比例下,与EM菌相比,纤维素降解率未提高,木质素降解率提高8.69%。









 DownLoad:
DownLoad:

