-
近年来,受全球气候变化的影响,极端降水事件频繁发生,严重危害经济社会发展、人类生命安全以及生态系统稳定[1]。降水是自然生态系统中水分调节的重要过程,为植物、动物和微生物的生理活动提供水分,也影响着土壤中营养元素迁移和转化[2-3]。氮是植物生长发育必需的营养元素之一[4],土壤氮素是植物可利用氮素的主要来源,它的有效性受微生物介导的土壤氮转化控制[5],而水分则通过影响土壤氧气(O2)、底物扩散、微生物活性及其群落结构等进而影响土壤氮转化[6-8]。CURTIN等[9]发现土壤水分对氮素矿化作用的影响包括对微生物生物量的直接影响和扩散底物的间接影响;MANZONI等[10]发现土壤水分变化显著影响微生物群落结构,如真菌和放线菌比细菌更耐受干旱胁迫、干旱土壤中革兰氏阳性菌比革兰氏阴性菌更占优势等。适量的土壤水分含量能促进氮素迁移和转化,提高氮素有效性,有利于植物生长,而一旦水分含量超过临界范围会引起生态系统负面效应,如硝态氮(
${\rm{NO}}_3^{-} $ )淋溶造成的水体富营养化、反硝化增强造成的气体污染、营养元素不足造成的生物多样性降低等[11-12]。因此,研究土壤水分变化如何影响氮转化对评估全球气候变化背景下土壤氮素有效性及其生态效应具有十分重要的意义。已有研究认为:土壤水分变化对氮净转化速率有影响[13-14],氮净转化速率能够表征土壤供氮能力和氮损失风险[15],但仅代表单位时间内某种特定形态氮素的净含量变化,是与氮库相关的多个氮初级转化速率的综合结果;氮初级转化速率是指土壤氮素从一种形态转化为另一种形态的实际转化速率[16-17]。土壤净矿化速率低并不意味着土壤未发生矿化和硝化作用,也可能是因为矿化和硝化作用产生的铵态氮(${\rm{NH}}_4^{+} $ )和${\rm{NO}}_3^{-} $ 被微生物同化而抵消[18-19],因此氮初级转化速率更能反映土壤真实的氮素动态变化。土壤不同氮转化过程,如有机氮矿化、${\rm{NH}}_4^{+} $ 微生物同化、自养硝化、异养硝化、${\rm{NO}}_3^{-} $ 微生物同化、反硝化等,是同时发生的,这些过程的初级速率决定了土壤无机氮的主导形态、含量变化和去向[5]。当前定量研究土壤水分变化对氮初级转化速率影响的报道很少,且主要集中于土壤氮初级矿化和硝化速率[19-20],氮转化对土壤含水量变化的响应机制还不是很清楚。明确不同氮转化过程的初级速率对水分变化的响应,能更好地评估全球气候变化背景下土壤氮素动态及其有效性。KIRKHAM等[21]在1954年提出了运用15N稀释技术结合算术分析方法定量土壤氮初级转化速率的方法;随着计算机分析技术的快速发展,一些数值优化模型取代了较为简单的算术分析计算方法,使得土壤氮转化研究更接近于真实过程[22-23]。MÜLLER等[23]提出,利用土壤氮转化模型如15N示踪模型,可同时计算出有机氮矿化、${\rm{NH}}_4^{+} $ 微生物同化、自养硝化、异养硝化、${\rm{NO}}_3^{-} $ 微生物同化等10个过程的氮初级转化速率。红壤约占中国土地面积的21%,广泛分布在热带和亚热带地区,气候条件优越,开发利用潜力巨大,在农业、经济、生态可持续发展中占有重要地位和作用[24]。明确红壤氮转化对土壤水分变化的响应,能够为全球气候变化背景下红壤氮素动态及其生态效应的研究提供基础数据。本研究在江西双圳林场采集典型的红壤,采用15N成对标记技术,结合数值优化模型(NtraceBasic),量化红壤不同水分条件(最大持水量的20%、60%、80%、100%) 下土壤不同氮转化过程的初级速率,为探明红壤氮转化对土壤水分变化的响应机制提供基础。
HTML
-
研究区江西省鹰潭市双圳林场(27°59′N,117°25′E)属亚热带季风气候,年平均气温为17.6 ℃,年平均降水量为1 778 mm,主要集中在4−6月。土壤为花岗岩母质发育的红壤,优势植被是马尾松Pinus massoniana和杉木Cunninghamia lanceolata。于2018年11月在研究区随机选择5个1 m×1 m地块,除去凋落物层,采集0~20 cm土壤样品。所有样品混合均匀,过2 mm筛,1份存于4 ℃冰箱用作培养实验,1份风干后用于理化性质测定。供试土壤pH 5.07,有机碳和全氮质量分数分别为19.78和1.92 g·kg−1,可溶性有机碳质量分数为236.5 mg·kg−1。
-
称取相当于20 g烘干土的鲜土置于250 mL三角瓶中,用带孔保鲜膜封住瓶口,放入25 ℃恒温培养箱中预培养24.0 h。取出预培养样品,分2组,1组加入2 mL15N丰度为10.20%的15NH4NO3,另1组加入2 mL15N丰度为10.25%的NH415NO3,每组氮添加量均为30 mg·kg−1。设置4个土壤水分梯度,即土壤最大持水量的20% (W20,干燥状态)、60% (W60,水分最适合的好氧状态[25])、80% (W80,好氧状态与厌氧状态的转折点[26])、100% (W100,水分饱和状态),用带孔保鲜膜封住三角瓶口,继续置于25 ℃恒温培养箱中培养144.0 h。每隔2 d补充土壤水分。在加入15NH4NO3或NH415NO3后0.5、48.0、96.0、144.0 h进行破坏性取样,测定土壤
${\rm{NH}}_4^{+} $ 、${\rm{NO}}_3^{-} $ 质量分数及15N丰度。 -
土壤pH采用DMP-2型pH计(Quark)测定,土水质量比为1.0∶2.5。土壤有机碳质量分数采用高温重铬酸钾-外加热法测定。土壤全氮质量分数使用半微量开氏消煮法测定。土壤可溶性有机碳质量分数采用TOC仪(Multi N/C)测定。用2 mol·L−1的氯化钾(KCl)溶液浸提土壤
${\rm{NH}}_4^{+} $ 和${\rm{NO}}_3^{-} $ ,使用流动分析仪(SA1000, Skalar)测定氮质量分数。用氧化镁(MgO)和戴维斯合金微扩散结合草酸吸附法分离浸提液中的${\rm{NH}}_4^{+} $ 和${\rm{NO}}_3^{-} $ ,使用同位素质谱仪(Europa Scientific Integra)测定15N丰度。 -
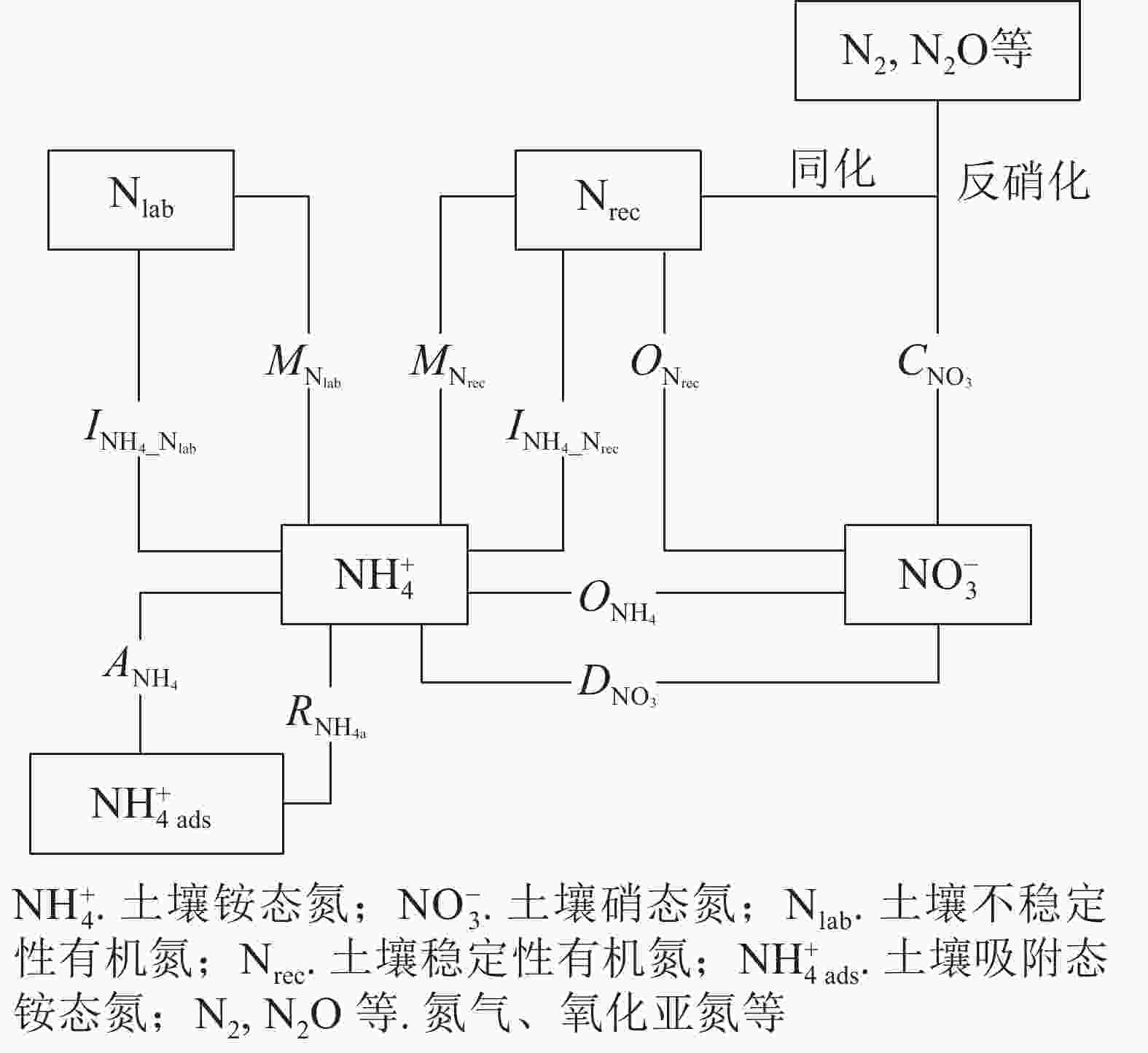
采用NtraceBasic模型定量土壤中主要氮转化过程的初级速率[23](图1),将测定的土壤
${\rm{NH}}_4^{+} $ 和${\rm{NO}}_3^{-} $ 质量分数及15N丰度输入模型,通过优化运算,计算土壤中10种氮转化的初级速率。其中$M_{{\rm{N}}_{\rm{lab}}} $ 表示易分解有机氮库初级矿化速率,$M_{{\rm{N}}_{\rm{rec}}} $ 表示难分解有机氮库初级矿化速率,$O_{{\rm{N}}_{\rm{rec}}} $ 表示初级异养硝化(难分解有机氮库氧化为${\rm{NO}}_3^{-} $ )速率,$O_{{\rm{NH}}_{4}} $ 表示初级自养硝化(${\rm{NH}}_4^{+} $ 氧化为${\rm{NO}}_3^{-} $ )速率,$I_{{\rm{NH}}_{4}{\_{{\rm{N}}_{\rm{lab}}}}} $ 表示微生物同化${\rm{NH}}_4^{+} $ 到易分解有机氮库速率,$I_{{\rm{NH}}_{4}{\_{{\rm{N}}_{\rm{rec}}}}}$ 表示微生物同化${\rm{NH}}_4^{+} $ 到难分解有机氮库速率,$C_{{\rm{NO}}_3} $ 表示初级${\rm{NO}}_3^{-} $ 消耗(微生物同化${\rm{NO}}_3^{-} $ 和反硝化)速率,$D_{{\rm{NO}}_3} $ 表示${\rm{NO}}_3^{-} $ 异化还原成${\rm{NH}}_4^{+} $ 速率,$A_{{\rm{NH}}_4} $ 表示${\rm{NH}}_4^{+} $ 的吸附速率,$R_{{\rm{NH}}_{\rm{4a}}} $ 表示${\rm{NH}}_4^{+} $ 的释放速率。ZHANG等[27]、MÜLLER等[23]和RÜTTING等[28]详细介绍了该模型,包括参数设置和具体操作等。总氮初级矿化速率计算方法:M=$M_{{\rm{N}}_{\rm{lab}}} $ +$M_{{\rm{N}}_{\rm{rec}}} $ 。总氮初级硝化速率计算方法:N=$O_{{\rm{NH}}_{4}} $ +$O_{{\rm{N}}_{\rm{rec}}} $ 。总初级${\rm{NH}}_4^{+} $ 微生物同化速率计算方法:$I_{{\rm{NH}}_4} $ =$I_{{\rm{NH}}_4\_{{\rm{N}}_{\rm{lab}}}} $ +$I_{{\rm{NH}}_4\_{{\rm{N}}_{\rm{rec}}}} $ 。土壤氮净矿化和净硝化速率的计算方法参照文献[29]:MN=24×(MN1−MN0)/t,NN=24×(NN1−NN0)/t。其中,MN表示净矿化速率(mg·kg−1·d−1),NN表示净硝化速率(mg·kg−1·d−1),MN1表示培养结束时土壤${\rm{NH}}_4^{+} $ 和${\rm{NO}}_3^{-} $ 质量分数(mg·kg−1),MN0表示培养开始时土壤${\rm{NH}}_4^{+} $ 和${\rm{NO}}_3^{-} $ 质量分数(mg·kg−1),NN1表示培养结束时土壤${\rm{NO}}_3^{-} $ 质量分数(mg·kg−1),NN0表示培养开始时土壤${\rm{NO}}_3^{-} $ 质量分数(mg·kg−1),t为培养时间(h)。采用单因素方差分析(ANOVA)检验4个水分处理间各土壤氮转化速率的差异。根据实际实验重复次数,计算各个氮转化过程速率在5%显著性水平下的最小显著性差异[30]。观测到的误差与实际重复次数有关,反映在各参数的概率密度函数中[23]。使用Sigma Stat 4.0(Systat Software)进行统计分析。方程拟合和图形绘制均在Sigma Plot 14.0上完成。
1.1. 研究区概况
1.2. 15N成对标记实验
1.3. 土壤理化学性质测定
1.4. 数据计算与统计分析
-
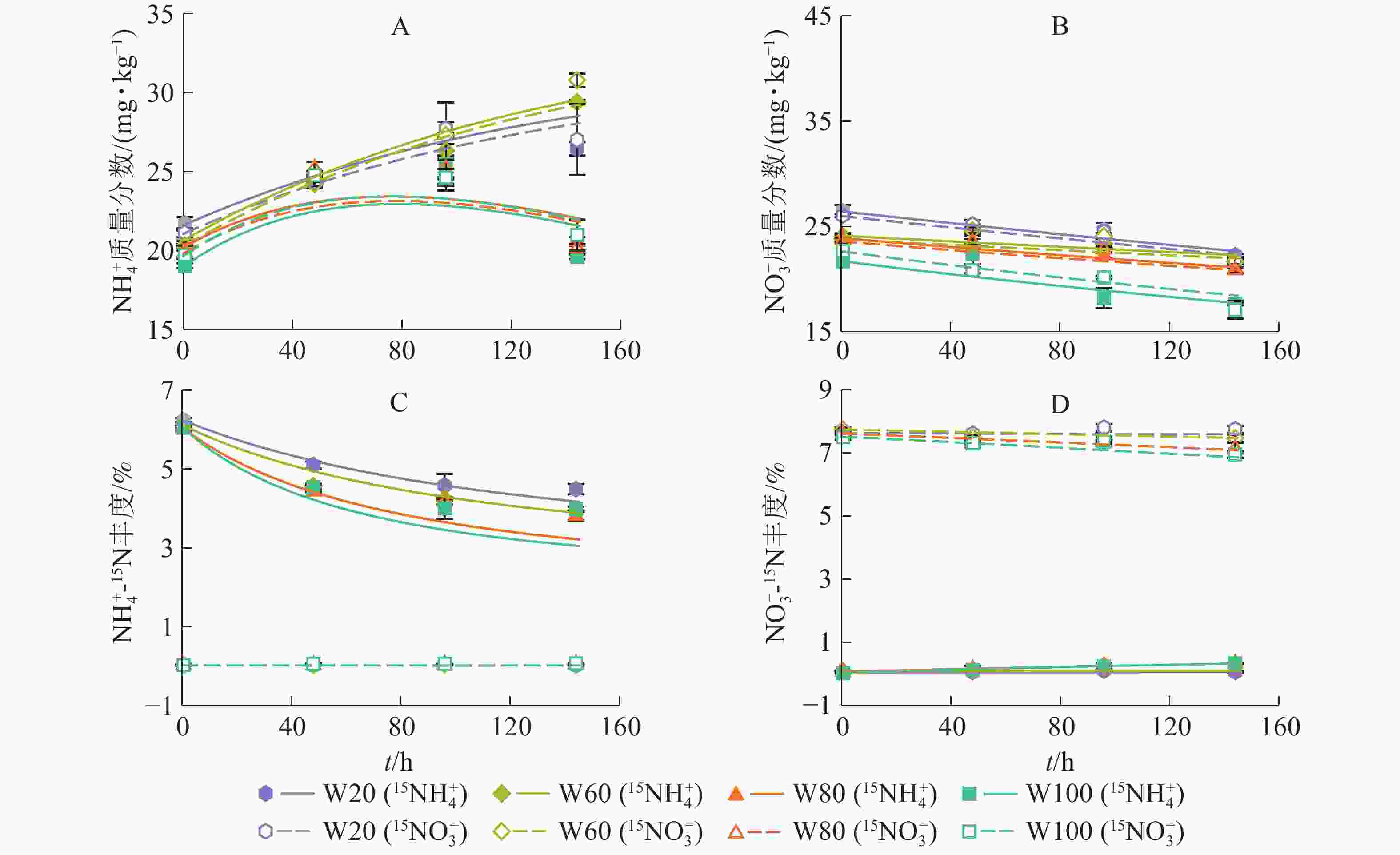
如图2所示:土壤
${\rm{NH}}_4^{+} $ 、${\rm{NO}}_3^{-} $ 质量分数及15N丰度的模型拟合结果与实测结果吻合较好(R2>0.84)。培养期间,W20和W60处理下${\rm{NH}}_4^{+} $ 质量分数显著增大;随培养时间增加,W80和W100处理下${\rm{NH}}_4^{+} $ 质量分数逐渐增大,96.0 h后缓慢减小,并且在整个培养时间小于W20和W60处理(图2A)。随培养时间增加,土壤${\rm{NO}}_3^{-} $ 质量分数逐渐下降,且土壤含水量越大,${\rm{NO}}_3^{-} $ 质量分数下降越快,说明增加土壤含水量促进了红壤${\rm{NO}}_3^{-} $ 消耗(图2B)。随培养时间和土壤水分含量增加,添加15NH4NO3处理的土壤${\rm{NH}}_4^{+} $ 库15N丰度降低(图2C),而${\rm{NO}}_3^{-} $ 库15N丰度有上升趋势(图2D),说明发生了有机氮矿化和${\rm{NH}}_4^{+} $ 氧化作用,土壤含水量越大,该作用越强;相反,添加NH415NO3处理的土壤${\rm{NH}}_4^{+} $ 库15N丰度变化不显著(图2C),而${\rm{NO}}_3^{-} $ 库15N丰度逐渐降低(图2D),表明有自然丰度或者更低丰度的${\rm{NO}}_3^{-} $ 进入该库。 -
土壤氮净矿化速率(MN)和净硝化速率(NN)均随含水量增加先上升后下降,且在W60处理时达到最大,分别为1.227和−0.385 mg·kg−1·d−1,W80和W100处理的氮净矿化速率和所有处理的氮净硝化速率均是负值(图3)。
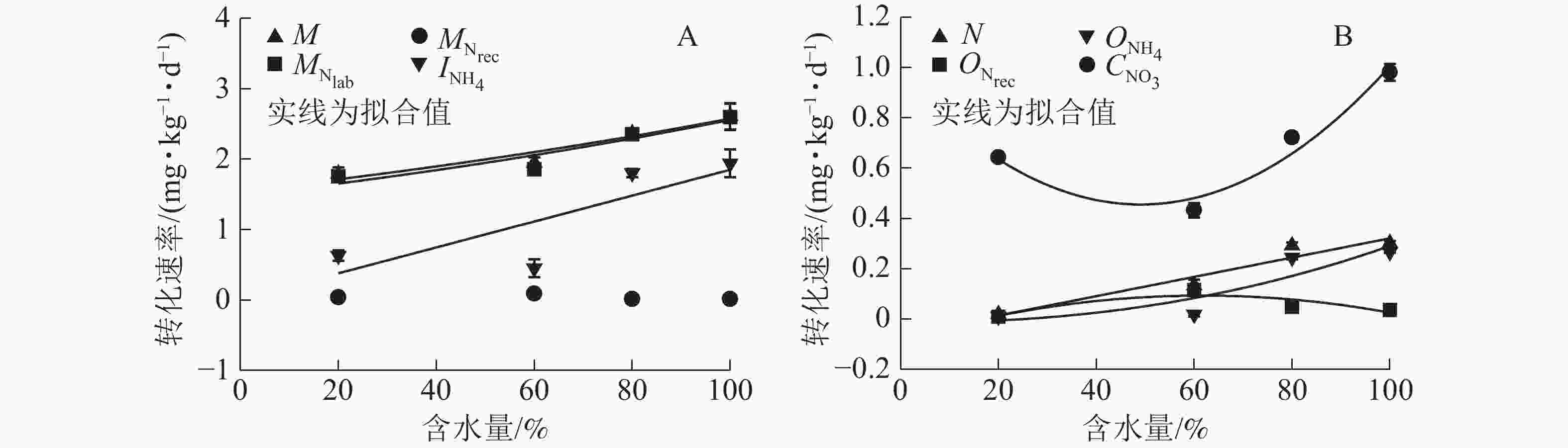
${\rm{NO}}_3^{-} $ 异化还原成${\rm{NH}}_4^{+} $ ($D_{{\rm{NO}}_3} $ )、${\rm{NH}}_4^{+} $ 的吸附速率($A_{{\rm{NH}}_{\rm{4}}} $ )和释放速率($R_{{\rm{NH}}_{\rm{4a}}} $ )极低,可忽略不计,其他氮转化过程初级转化速率受水分变化影响显著。由图4A可知:当土壤含水量增加,$M_{{\rm{N}}_{\rm{lab}}} $ 从1.757 mg·kg−1·d−1 (W20)增大到2.598 mg·kg−1·d−1(W100),$M_{{\rm{N}}_{\rm{rec}}} $ 变化不显著,因此M呈指数上升(R2=0.92)。总初级${\rm{NH}}_4^{+} $ 微生物同化速率($I_{{\rm{NH}}_4} $ )随土壤含水量增加显著增加,在W100处理时达最大值(1.941 mg·kg−1·d−1);${\rm{NH}}_4^{+} $ 主要被微生物同化到难分解有机氮库,以W80和W100处理最明显(图5)。土壤$O_{{\rm{NH}}_4} $ 随含水量增加而增大,在W100处理下达到最大值(0.266 mg·kg−1·d−1);$O_{{\rm{N}}_{\rm{rec}}} $ 随含水量增加呈现先上升后下降的变化特点,W60处理时达到最大值(0.115 mg·kg−1·d−1);W80和W100处理下$O_{{\rm{NH}}_4} $ 明显大于$O_{{\rm{N}}_{\rm{rec}}} $ ,所以N随土壤含水量增加而增加(图4B)。土壤含水量由最大持水量的20%(W20)升到100%(W100)时,$C_{{\rm{NO}}_3} $ 先缓慢下降,之后迅速增加,因此总无机氮消耗速率($I_{{\rm{NH}}_4} $ 和$C_{{\rm{NO}}_3} $ )随土壤含水量增加显著增大。土壤不同氮过程的初级转化速率随土壤含水量梯度变化的拟合方程见表1。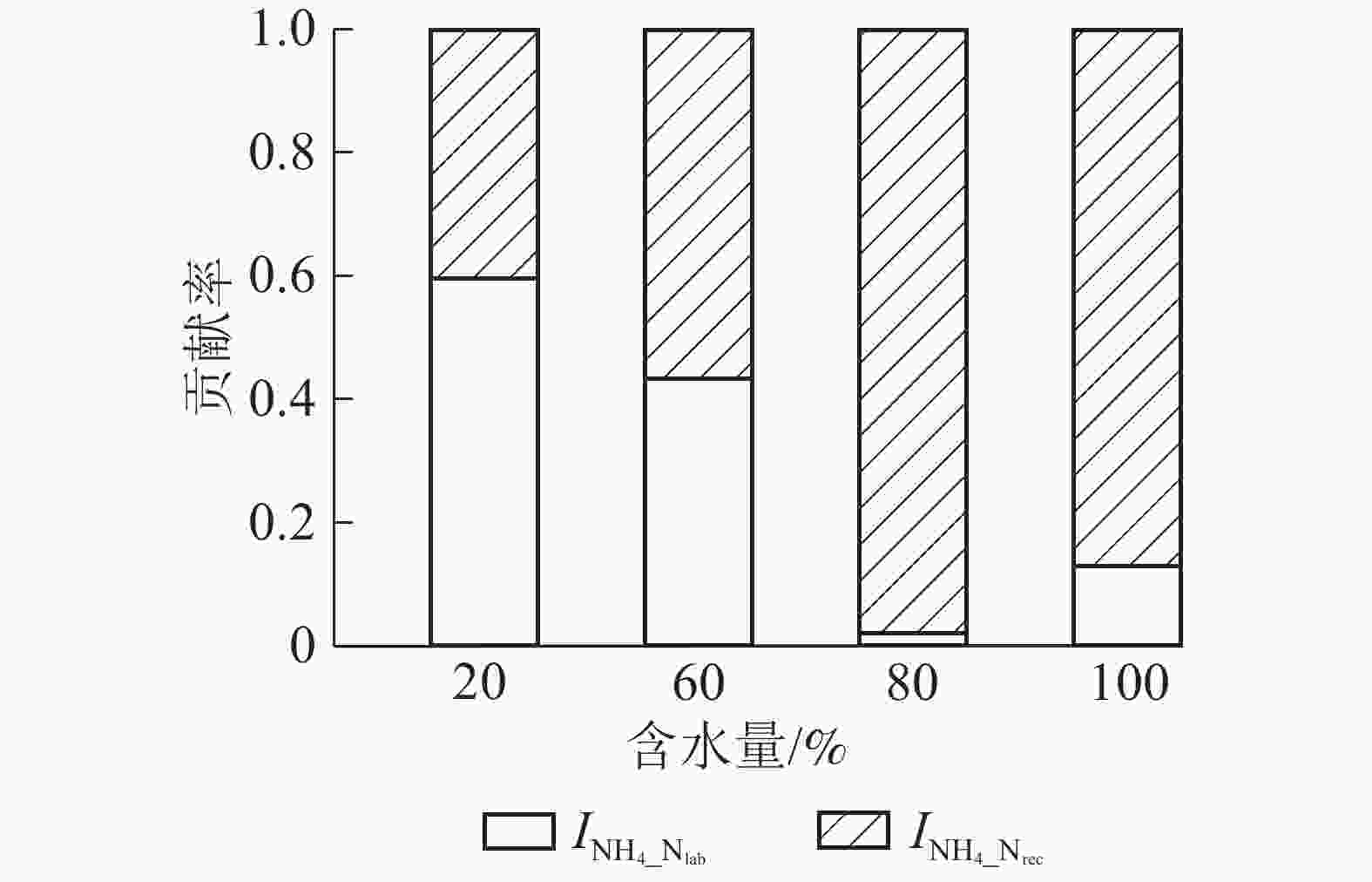
Figure 5. Contribution ratio of
$I_{{\rm{NH}}_{4}{\_{{\rm{N}}_{\rm{rec}}}}} $ and$I_{{\rm{NH}}_{4}{\_{{\rm{N}}_{\rm{lab}}}}} $ to$I_{{\rm{NH}}_4} $ under various moistures参数 方程 参数 方程 M y=1.547 5exp(0.005 1x),R2=0.922 0,P<0.05 ${O_{{\rm{N}}_{\rm{rec}}}} $ y=−0.083 0+0.005 7x−(4.630 7E−5)x2,R2=0.755 2,P<0.05 ${M_{{\rm{N}}_{\rm{lab}}}} $ y=1.483 2exp(0.005 5x),R2=0.885 0,P<0.05 ${O_{{\rm{NH}}_4}} $ y=−0.014 4+(1.059 6E−5)x2.229 3,R2=0.821 1,P<0.05 ${M_{{\rm{N}}_{\rm{rec}}} }$ − ${C_{{\rm{NO}}_3}} $ y=0.966 4−0.020 8x+0.000 2x2,R2=0.954 5,P<0.05 ${I_{{\rm{NH}}_4}} $ y=0.009 2+0.018 4x,R2=0.658 2,P<0.05 MN y=−0.880 8+0.068 5x−0.000 7x2,R2=0.684 7,P<0.05 N y=−0.062 9+0.003 8x,R2=0.930 4,P<0.05 NN y=−1.204 0+0.028 8x−0.000 2x2,R2=0.994 3,P<0.05 说明:−表示没有数值 Table 1. Fitting curve equations of soil N tansformation rates (y) with changing moistures (x)
2.1. 无机氮质量分数和15N丰度
2.2. 土壤含水量对氮转化速率的影响
-
土壤水分通过影响O2、底物扩散、微生物活性及其群落结构等影响氮转化过程,控制土壤氮素有效性,影响生态系统稳定[5-6, 8]。本研究表明:土壤不同活性有机氮库初级矿化速率对水分变化的响应不同。CHEN等[31]发现:30%土壤孔隙含水量(WFPS)处理的黑土易分解有机氮库初级矿化速率(
$M_{{\rm{N}}_{\rm{lab}}} $ )显著高于70%WFPS处理,可能与低水分条件下微生物渗透压调节有关。本研究中$M_{{\rm{N}}_{\rm{lab}}} $ 随土壤含水量增加(从土壤最大持水量的20%到100%)显著增加,说明影响红壤易分解有机氮库初级矿化速率的机制与黑土不同。增加土壤含水量可促进反应底物扩散[29, 32],提高微生物与底物接触机率,进而刺激土壤易分解有机氮库矿化过程。一般来讲,当土壤含水量达到最大持水量的100%时,土壤处于厌氧状态,不利于除厌氧微生物外的其他微生物生存。本研究$M_{{\rm{N}}_{\rm{lab}}} $ 在W100时表现出最大响应,可能与土壤性质有关;供试土壤为针叶林红壤,有机质含量适中,砂砾/黏砾比例偏高[19],即使土壤含水量达到最大持水量的100%,土壤仍有未充满水的大孔隙,通气性较高,使微生物活性维持在较高水平,进而刺激易分解有机氮库矿化过程。土壤水分变化对氮元素矿化作用的影响可能还取决于土壤温度[33]。一般来讲,土壤矿化作用的温度敏感性取决于有机物分解的温度敏感性[15, 34]。本研究区的优势植被是马尾松和杉木,凋落物难分解有机质含量高,而难分解有机质的温度敏感性低[35],即矿化作用的温度敏感性低,因此需要更高温度才能刺激难分解有机氮矿化。DAN等[36]在相同研究区研究温度变化(5~45 ℃)对氮转化的影响发现:难分解有机氮库初级矿化速率($M_{{\rm{N}}_{\rm{rec}}} $ )仅在45 ℃时有显著响应(2.102 mg·kg−1·d−1),与本研究条件相同的常温(25 ℃)处理下$M_{{\rm{N}}_{\rm{rec}}} $ 很低,仅0.089 mg·kg−1·d−1,这可能是$M_{{\rm{N}}{\rm{rec}}} $ 在不同水分状态下都很低且无显著差异的主要原因。由此可见,本研究红壤${\rm{NH}}_4^{+} $ 主要通过易分解有机氮库矿化产生,且土壤含水量越大,${\rm{NH}}_4^{+} $ 产生量越多。土壤矿化产生的
${\rm{NH}}_4^{+} $ 为微生物同化作用提供底物。随土壤含水量增加,M呈指数上升,${\rm{NH}}_4^{+} $ 质量分数增加,导致$I_{{\rm{NH}}_4} $ 显著增大。此外,提高水分含量促进了反应底物(${\rm{NH}}_4^{+} $ )扩散[29, 32]也可能是$I_{{\rm{NH}}_4} $ 显著增加的原因之一。${\rm{NH}}_4^{+} $ 被微生物同化到有机氮库,而有机氮可以短期或长期储存后再矿化产生${\rm{NH}}_4^{+} $ [37],给土壤可利用氮素的供应提供一种有效的缓冲机制,有利于缓解潜在的${\rm{NO}}_3^{-} $ 淋溶和NH3挥发等氮损失风险,提高土壤保氮能力。$I_{{\rm{NH}}_4} $ 包括$I_{{\rm{NH}}_{4}{\_{{\rm{N}}_{\rm{lab}}}}} $ 和$I_{{\rm{NH}}_{4}{\_{{\rm{N}}_{\rm{rec}}}}} $ ,本研究表明微生物同化${\rm{NH}}_4^{+} $ 以$I_{{\rm{NH}}_{4}{\_{{\rm{N}}_{\rm{rec}}}}} $ 为主,与以往的研究结果一致[27],在土壤最大持水量的80%(W80)和100%(W100)时最明显。土壤${\rm{NH}}_4^{+} $ 产生主要通过易分解有机氮库矿化过程,而${\rm{NH}}_4^{+} $ 主要被微生物同化到难分解有机氮库,说明长期强降雨导致的土壤高水分含量条件(如本研究W80和W100处理)不利于有机氮再矿化产生${\rm{NH}}_4^{+} $ ,这样虽然能缓解潜在的氮损失风险,但降低了植物可利用的氮含量,不利于本就受氮素限制的森林生态系统植物生长,影响生态系统生产力和稳定性。自养硝化作用主要是自养硝化细菌或古菌将NH3氧化成
${\rm{NO}}_3^{-} $ ,异养硝化作用则主要是异养硝化细菌或真菌将NH3或有机氮氧化成${\rm{NO}}_3^{-} $ ,被认为是${\rm{NO}}_3^{-} $ 产生的2种重要途径。本研究表明:随含水量增加,上述2个过程对总${\rm{NO}}_3^{-} $ 产生速率(N)的贡献不同。CHEN等[31]整合文献时发现:对于所有生态系统(特别是森林生态系统)而言,土壤初级异养硝化速率($O_{{\rm{N}}_{\rm{rec}}} $ )与降水量显著正相关。但他们的实验结果却发现:增加农田黑土水分含量(从最大持水量的30%到70%)抑制了异养硝化作用[31]。LIU等[38]也观测到农田土壤在50%WFPS处理时$O_{{\rm{N}}_{\rm{rec}}} $ 显著高于70%WFPS处理。本研究也发现土壤含水量由最大持水量的60%(W60)升至100%(W100),$O_{{\rm{N}}_{\rm{rec}}} $ 逐渐下降,这可能与微生物性质有关。真菌是亚热带酸性针叶林土壤异养硝化作用的主要驱动者[39],一般适应偏干旱环境[40],高水分含量条件下活性低,不利于异养硝化作用进行。此外,高水分含量限制了O2向土壤扩散,降低真菌活性,进而抑制真菌驱动的异养硝化过程。本研究中W60处理的$O_{{\rm{N}}_{\rm{rec}}} $ 显著大于W20处理,可能是该水分条件有利于异养硝化微生物活动。本研究发现:随含水量增加,M呈指数上升,
${\rm{NH}}_4^{+} $ 质量分数增加,为自养硝化作用提供更多的底物,进而刺激自养硝化过程。此外,氮矿化速率增加也提高了土壤微域pH,土壤初级自养硝化速率($O_{{\rm{NH}}_4} $ )与pH呈显著正相关[41],因此提高水分含量会刺激土壤自养硝化作用。底物扩散效率也会影响土壤$O_{{\rm{NH}}_4} $ 。增加水分含量可促进反应底物扩散[29,32],提高微生物与底物接触机率,进而刺激土壤自养硝化过程。微生物对土壤水分含量变化的响应也是影响自养硝化过程的关键因素之一。一般来讲,森林土壤的自养硝化作用主要由氨氧化古菌(AOA)驱动[42-43]。有研究表明:随土壤含水量增加,温带森林有机质层土壤中AOA丰度增加[44]。本研究中$O_{{\rm{NH}}_4} $ 在W80和W100处理下显著升高,可能是由于AOA丰度或活性升高,但背后的机制还需进一步研究。随土壤含水量增加,$O_{{\rm{NH}}_4} $ 不断上升,$O_{{\rm{N}}_{\rm{rec}}} $ 先上升后下降,在W60处理下达最大值;此外,在W80和W100处理时$O_{{\rm{NH}}_4} $ 明显大于$O_{{\rm{N}}_{\rm{rec}}} $ ,因此N不断增大。${\rm{NO}}_3^{-} $ 微生物同化和反硝化过程是${\rm{NO}}_3^{-} $ 消耗的主要途径,它们对水分变化的响应对土壤氮素保存具有重要意义。本研究采用的NtraceBasic模型不能同时定量这2个过程,只能测定${\rm{NO}}_3^{-} $ 总消耗速率,即$C_{{\rm{NO}}_3} $ 。已有研究报道了上述2个过程对水分变化的响应,例如,当酸性针叶林和阔叶林土壤含水量从最大持水量的70%上升至90%[19],农田土壤含水量从最大持水量的30%上升至70% [29-31],林地和森林土壤含水量从最大持水量的60%上升至100%[18],土壤初级${\rm{NO}}_3^{-} $ 微生物同化速率无显著变化。林地和森林土壤含水量从最大持水量的60%上升至100%,反硝化速率分别从0上升至2.47和4.91 mg·kg−1·d−1[18]。因此,本研究$C_{{\rm{NO}}_3} $ 随水分含量增加(最大持水量的60%到100%)显著增加,可能是因为反硝化作用不断增大。土壤水分含量低于最大持水量的60%时,反硝化过程基本消失,${\rm{NO}}_3^{-} $ 消耗以微生物同化作用为主[18]。一般认为,即使土壤中${\rm{NH}}_4^{+} $ 质量分数较低也会抑制微生物同化${\rm{NO}}_3^{-} $ [45-46],而本研究W60处理下${\rm{NH}}_4^{+} $ 质量分数达到30 mg·kg−1,${\rm{NO}}_3^{-} $ 微生物同化作用依然存在,表明所研究红壤的${\rm{NH}}_4^{+} $ 质量分数可能不是调控${\rm{NO}}_3^{-} $ 微生物同化作用的主要因素。综上,本研究在W20和W60处理下$C_{{\rm{NO}}_3} $ 主要是${\rm{NO}}_3^{-} $ 微生物同化速率。与W60处理相比,W20处理的$C_{{\rm{NO}}_3} $ 更高,可能与风干效应有关。W20处理的土壤进行了风干处理,而鲜土风干过程具有部分灭菌作用[47-48],即风干过程导致部分微生物死亡,其体内的养分释放,使得土壤中能被微生物利用的物质增加,进而导致风干加水培养后微生物大量繁殖增长,从而刺激W20处理的${\rm{NO}}_3^{-} $ 微生物同化过程。由此可见,当土壤含水量不超过最大持水量的60%时,${\rm{NO}}_3^{-} $ 消耗以${\rm{NO}}_3^{-} $ 微生物同化过程为主;这一过程将${\rm{NO}}_3^{-} $ 保持在土壤有机质中,对受氮素限制的亚热带酸性森林生态系统有更重要的意义。当土壤含水量超过最大持水量的60%时,${\rm{NO}}_3^{-} $ 消耗以反硝化过程为主,这一过程会导致氮素损失,并产生N2O,危害生态环境。本研究中,
$I_{{\rm{NH}}_4} $ 随含水量增加显著增加,在W100处理下观测到最大值,$C_{{\rm{NO}}_3} $ 在W80和W100处理时也明显增加;认为总无机氮消耗速率随水分含量增加而增加,并在W80处理时超过M。因此,MN随水分含量增加先上升到最大值,然后迅速下降到负值。说明适当增加土壤水分含量(如将土壤含水量从最大持水量的20%增加到60%)会提高红壤氮素的可利用性;如果土壤水分含量继续增加,土壤氮循环将进入净消耗状态,有效氮的供应受到限制。虽然N随含水率增加不断增加,但始终小于$C_{{\rm{NO}}_3} $ ,即不同处理中NN始终是负值。
-
红壤各种氮转化对水分变化的响应存在显著差异。随着土壤含水量增加,易分解有机氮库初级矿化速率显著上升,难分解有机氮库初级矿化速率无显著变化,总氮初级矿化速率显著上升。初级
${\rm{NH}}_4^{+} $ 微生物同化速率随含水量增加线性增加;${\rm{NO}}_3^{-} $ 总消耗速率在W80和W100处理时也明显增加,使得总无机氮消耗速率随水分含量增加而增加,并在W80处理时超过总氮初级矿化速率。因此,随含水量增加氮净矿化速率先上升到最大值,然后迅速下降到负值,说明适当增加土壤水分含量会提高红壤氮素的可利用性;但如果水分含量继续增加,土壤氮循环将进入净消耗状态,有效氮的供应会受到限制。












































































































































































 DownLoad:
DownLoad:



