-
植物化感作用是一种活或死的植物通过适当的途径向环境释放特定的化学物质而直接或间接影响邻近或下茬植物萌发或生长的效应[1]。在自然条件下,水溶性的化感物质主要随着雨水等的冲刷从凋落物淋溶进入土壤中,对周围植物生长产生影响。凋落物的化感作用是影响林木自然更新的一个重要因子,凋落物产生的化感物质对种子萌发和胚生长具有重要影响[2]。刺槐Robinia pseudoacacia为落叶乔木,具有适应性强、耐干旱贫瘠、易繁殖、生长快、固氮等特性,是中国广泛栽培的阔叶乔木树种之一。伐后萌蘖更新是刺槐人工林的主要更新方式[3],利用刺槐具有根系发达、萌蘖能力强等特性,快速更新成为林分。但随着皆伐后萌蘖再皆伐再萌蘖的多代连作,在三代、四代及以后刺槐萌生形成的次生林中出现生产力逐代下降、生长迟缓以及树干弯曲等连栽障碍[4],影响刺槐林的生产效率,因此刺槐的实生更新问题受到人们关注。有研究表明,在近自然条件下,刺槐实生更新困难,林下种子天然萌发率及胚生长存活率均较低[5]。森林内的林木种子主要分布在凋落物以及腐殖质层[6],刺槐的凋落物层会加速种子损失率[6],凋落物分解过程中会产生一些次生代谢产物[7],对刺槐种子的萌发和胚生长可能会产生影响。因此,本研究主要探讨连栽刺槐人工林下凋落物是否具有化感作用影响自身种子萌发。研究表明杨树Populus的化感作用随着连栽代数的增加而增加[8],随着马尾松Pinus massoniana连栽代数以及林龄的增加,浸提液中化感物质的种类增加[9]。
刺槐化感作用的研究集中在刺槐凋落物与根际土壤对其他的植物生长的影响。已有研究表明:刺槐凋落叶的浸提液对绿豆Vigna radiata具有较强的抑制作用,对甜瓜Cucumis melo具有较弱的促进作用[10];随刺槐凋落物浸提液质量浓度的升高,对马尾松生长的促进作用转变为抑制作用,刺槐凋落物的浸提液对马尾松胚的地径、苗高、主根长、地上部鲜质量和地下部鲜质量均表现为促进效应[11−12];刺槐根际土壤的水浸提液对5种园林植物的生长表现为抑制作用[13],刺槐叶片混合土壤对食用植物以及杂草生长具有抑制作用[14];刺槐根际土壤以及刺槐凋落物的浸提液对刺槐种子萌发呈低促进高抑制的趋势,刺槐根际土的浸提液对刺槐苗木生长也呈现低促高抑的趋势[15];在黄土地区,刺槐凋落物的浸提液对刺槐自身种子萌发和胚生长具有抑制作用,刺槐根系、根际土壤的浸提液对刺槐的生长呈现低促进高抑制的化感作用,并检测出37种化感物质[16−17]。
不同经营世代和不同林龄刺槐凋落物对自身种子萌发的影响研究极少。本研究针对不同刺槐林下凋落物的浸提液,研究其对种子萌发及胚生长的影响,以期揭示刺槐多代连作培育经营下实生更新困难与刺槐自身化感效应关系,为刺槐人工林高效培育与可持续经营提供科学依据。
-
刺槐人工林位于河南省洛阳市洛宁县马店镇国有吕村林场(34°06′~34°38′N,111°08′~111°50′E),选取刺槐一代幼龄林(F1-1)、一代中龄林(F1-2)、一代成熟林(F1-3)、二代幼龄林(F2-1)、二代中龄林(F2-1)、二代成熟林(F2-3)、三代幼龄林(F3-1)、三代中龄林(F3-2)、三代成熟林(F3-3)进行设置样地(表1),样地面积为20 m×20 m,每种林分设置3个重复样地,共计27块刺槐样地。对样地进行基本林分调查,包括海拔、坡度、胸径、树高、活枝下高、郁闭度、植被等。刺槐一代林是实生苗,二代林和三代林分别是一代林经过1次和2次皆伐后萌蘖形成的林分,幼龄林、中龄林、成熟林分别为6~7 a、12~15 a、15 a以上的林分。林下植被主要包括荆条Vitex negundo、夏至草Lagipsis supina、堇菜Viola verecunda、求米草Oplismenus undulatifolius、白屈菜Chelidonium majus、葎草Humulus scandens等。
林分 海拔/
m坡度/
(°)胸径/
cm树高/
m活枝
下高/m密度/
(株·hm−2)郁闭度 F1-1 611 24 6.55 8.17 5.24 2258 0.57 F1-2 665 18 9.71 10.11 5.59 1558 0.47 F1-3 712 15 20.50 11.13 6.44 625 0.33 F2-1 817 16 6.78 8.17 5.56 2517 0.53 F2-2 646 14 10.02 9.86 4.51 1267 0.37 F2-3 644 25 14.92 10.35 6.16 983 0.37 F3-1 656 22 4.84 6.46 3.87 4250 0.53 F3-2 671 15 6.80 8.47 5.93 3742 0.43 F3-3 677 22 7.25 8.61 4.65 3292 0.53 Table 1. Basic situation of the sample plots
-
2020年11月在不同林分的刺槐样地中收集林下凋落物(当年新鲜凋落的叶片和小枝,外表轮廓完整,可以辨认出叶片和小枝的形态和质地),在每块样地随机设立3个1 m×1 m的小样方收集样品,放入密封袋带回实验室,去除杂质,自然风干。供试种子为当地刺槐种子。
-
刺槐林下凋落物浸提液质量浓度设置需考虑凋落物与降雨量比例[18]。根据调查,刺槐人工林凋落物的现存量为80~220 g·m−2,该地区年平均降水量为570 mm,换算得凋落物浸提液质量浓度为1.0∶2.5~1.0∶10.0。将自然风干的凋落物按质量浓度1.0∶2.5加入适量蒸馏水,在180 r·min−1回旋式震荡1 h,使用滤纸进行过滤,得原液。考虑野外在非雨季时,凋落物处于干燥未被浸提的状态[19],将原液稀释2、4、8、16倍,得0.400、0.200、0.100、0.050、0.025 kg·L−1的浸提液,分别标记为C1~C5,将浸提液放入4 ℃冰箱保存备用。
-
种子萌发实验采用培养皿滤纸法。选取大小均匀一致、颗粒饱满的刺槐种子,用质量浓度为0.5%的高锰酸钾溶液浸泡消毒2 h后,使用蒸馏水冲洗干净,再用75~80 ℃的热水至自然冷却浸泡24 h,以破除种子硬实。发芽床由直径9 cm的双层定性滤纸和培养皿构成。取5 mL不同处理的浸提液,加入培养皿中,每个培养皿整齐均匀放置50粒处理后的刺槐种子,每个处理设置3个重复,加入等量蒸馏水为对照(ck)。将培养皿放置于人工培养箱,设置光周期为16 h光/8 h暗,温度为25 ℃,相对湿度为80%。每天定量补充相应处理浸提液使滤纸保持湿润。
-
种子置床当天为第1天,之后每天同一时间观察记录发芽种子数,以胚根露出种皮1~2 mm为发芽标准,大部分处理连续3 d无种子萌发时,结束发芽试验,记录腐烂种子和硬实种子的数量。计算发芽率、发芽势、发芽速度指数、活力指数,并使用精度0.01 cm的游标卡尺测量胚根长和胚芽长,用精度0.0001 g的电子天平称量每皿胚根和胚芽的鲜质量和干质量。发芽率=(发芽种子数/供试种子总数)×100%;发芽势=(日发芽种子数达到最高峰/供试种子总数)×100%;发芽速度指数I=2×(6x1+5x2+4x3+3x4+2x5+x6)[20],x指每天发芽的种子数,解释化感物质对种子发芽的延缓作用,高于对照表示促进作用。活力指数=发芽指数×苗长,其中,发芽指数=
$ \displaystyle\sum \dfrac{\mathrm{每}\mathrm{天}\mathrm{的}\mathrm{发}\mathrm{芽}\mathrm{数}}{\mathrm{相}\mathrm{应}\mathrm{的}\mathrm{发}\mathrm{芽}\mathrm{天}\mathrm{数}} $ ,种子活力指数是衡量种子发芽期长短,胚是否健壮的重要指标,其值越大,表示胚越健壮,生长越好。化感综合效应指数反映化感效应的强弱,指同一处理下对同一受体各测试项目化感效应指数的算术平均值。种子萌发化感综合效应指数指发芽率、发芽势、发芽速度指数、活力指数等指标的化感效应指数的平均值。胚生长化感综合效应指数指胚根长、胚芽长、胚根鲜质量、胚芽鲜质量、胚根干质量、胚芽干质量等指标的化感效应指数的平均值。化感效应指数(IR)[21]:IR=1−Ick/ IT(IT≥Ick);IR= IT/Ick−1(IT<Ick)。其中,Ick为对照值,IT为凋落物浸提液的处理值。当IR>0时,表示促进作用;当IR<0时,表示抑制作用。IR绝对值越大,化感效应强度也越大。
-
用Excel 2019对数据进行基本汇总和处理,用SPSS 23软件进行分析,采用方差分析和多重比较分析不同处理之间的差异性,各林分之间的多重比较结果是同一质量浓度下多重比较的平均值。所有图使用Origin 2017进行绘制。数据均为平均值±标准误。
-
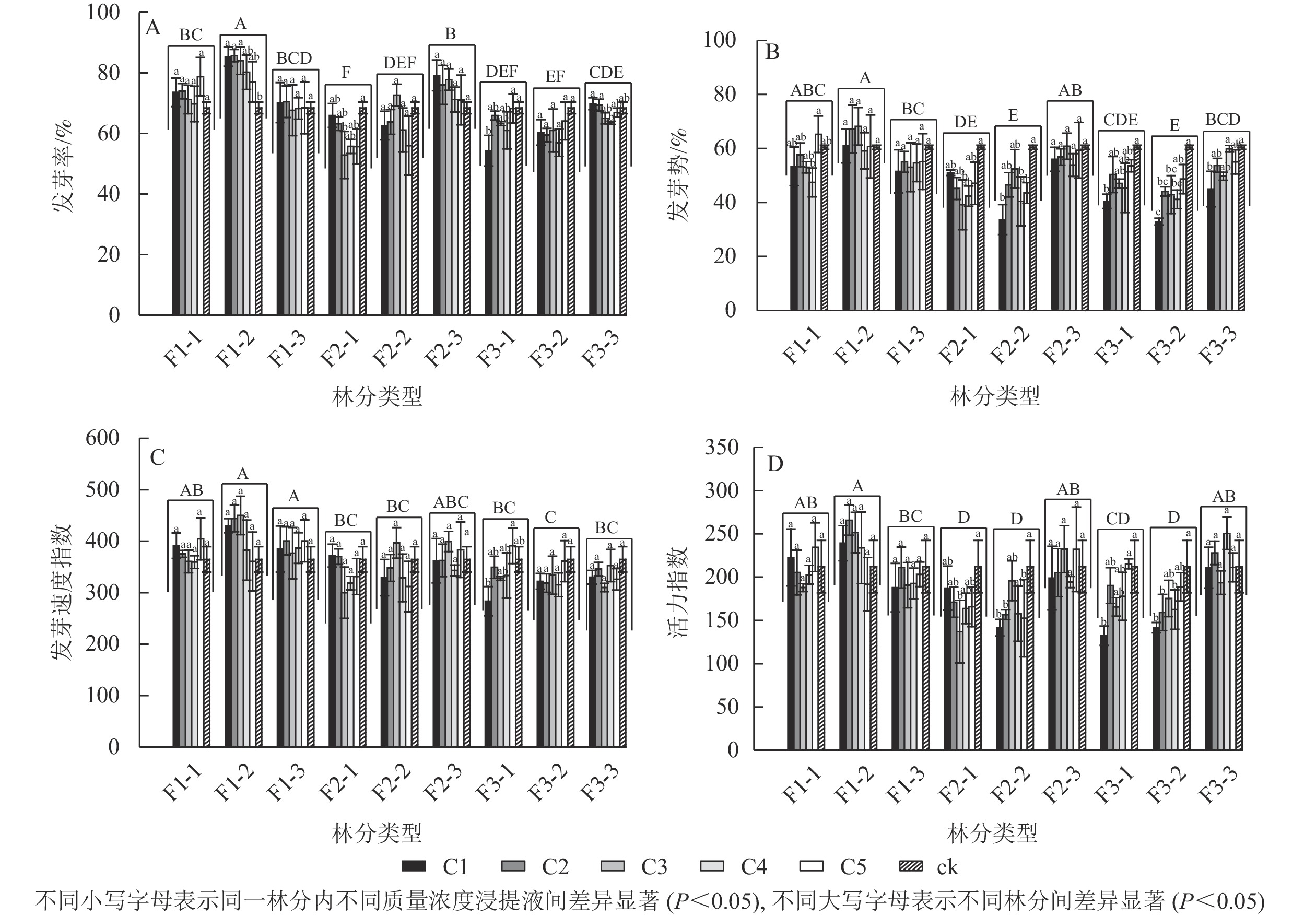
由图1A可见:不同林分凋落物浸提液处理下的刺槐种子发芽率具有显著差异(P<0.05),其中一代中龄林的凋落物浸提液处理下的刺槐种子发芽率最高,二代幼龄林发芽率最低。大多数林分不同质量浓度浸提液处理下的发芽率之间无显著差异。一代中龄林中,不同浸提液质量浓度之间的发芽率有显著差异(P<0.05),随着浸提液质量浓度的降低,其发芽率呈下降趋势,与对照相比,C1、C2、C3处理对发芽率具有显著促进作用(P<0.05);二代幼龄林中,发芽率随浸提液质量浓度的降低呈先下降后上升的趋势,C3对发芽率的抑制作用最强,发芽率与对照相比显著减少了22.74%(P<0.05);三代幼龄林中,随着浸提液质量浓度的降低,发芽率呈上升趋势,C1的发芽率与对照相比显著降低了20.66%(P<0.05);三代成熟林中,C4的发芽率比C1、C2分别显著下降9.95%、9.18%(P<0.05),随着浸提液质量浓度的下降,发芽率呈下降趋势。
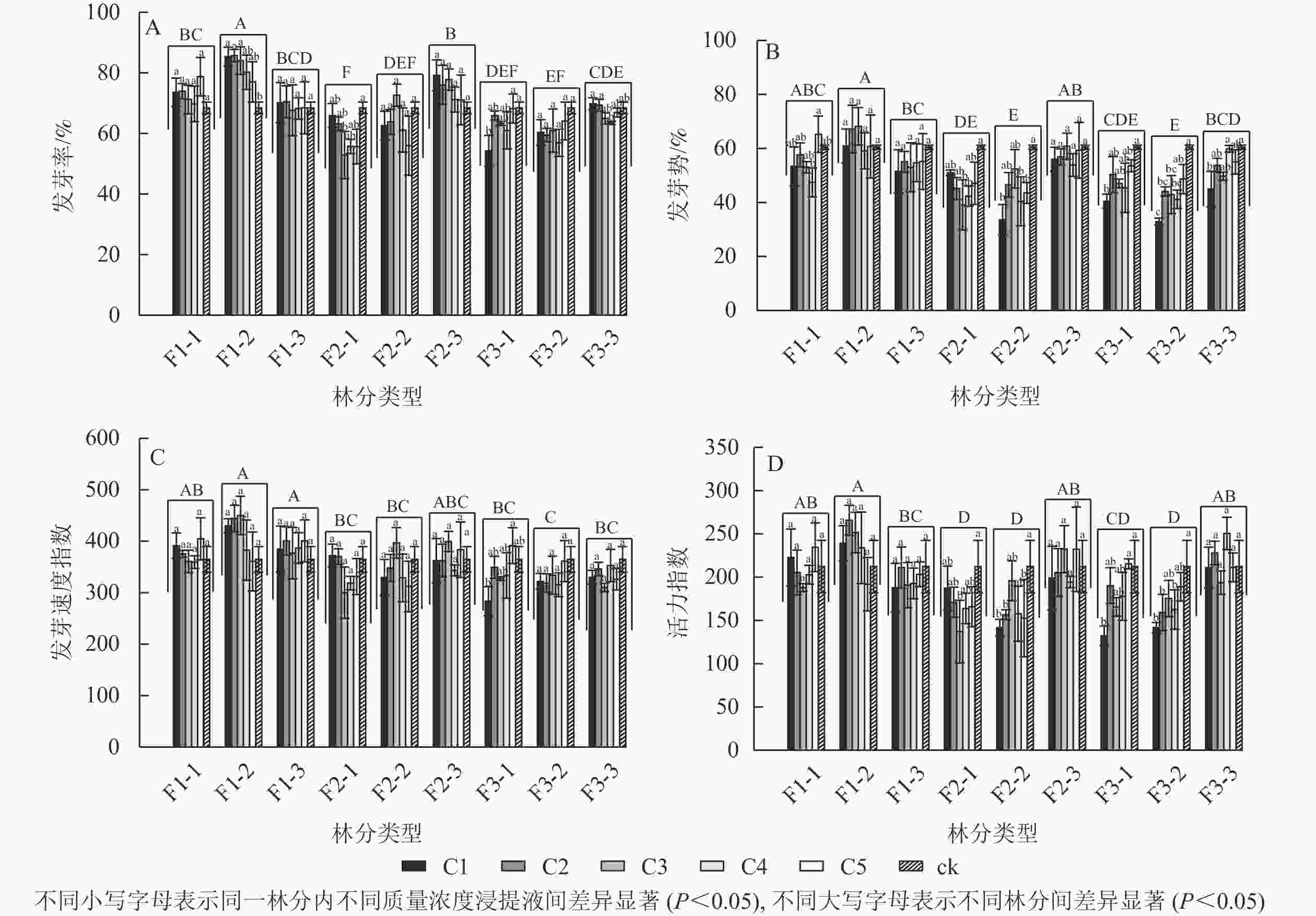
Figure 1. Effects of different forests and different concentrations of R. pseudoacacia litter extracts on their own seed germination
由图1B可见:不同林分凋落物浸提液处理下的刺槐种子发芽势具有显著差异(P<0.05),其中一代中龄林的凋落物浸提液处理下的刺槐种子发芽势最高,二代中龄林和三代中龄林其发芽势最低。随着凋落物质量浓度的变化,一代中龄林、一代成熟林、二代成熟林的凋落物浸提液对发芽势无显著差异。一代幼龄林中,与C5比较,C4处理的发芽势显著下降了27.30%(P<0.05)。二代幼龄林中,C3与对照组相比发芽势显著降低了35.27%(P<0.05),随着质量浓度的降低,其发芽势呈先下降后上升趋势。二代中龄林中,与对照组相比,C1、C4处理的发芽势分别下降了44.36%、33.17%(P<0.05)。三代幼龄林和三代中龄林下,其发芽势呈高质量浓度抑制作用,低质量浓度抑制作用减弱趋势,C1的发芽势与对照相比较均具有显著差异(P<0.05)。三代成熟林,与C4和对照相比,C1的发芽势分别显著下降了24.90%、25.64%(P<0.05),高质量浓度抑制其发芽势。
由图1C可见:不同林分凋落物浸提液处理下的刺槐种子发芽速度指数具有显著差异(P<0.05),其中一代中龄林和一代成熟林凋落物浸提液处理下的刺槐种子发芽速度指数最高,三代中龄林发芽速度指数最低。不同林分下的各质量浓度凋落物浸提液对刺槐种子发芽速度指数无显著差异,除了在三代幼龄林中的凋落物浸提液,与C5相比,C1的发芽速度指数显著下降了27.60%(P<0.05),呈高质量浓度抑制低质量浓度促进趋势。
由图1D可见:不同林分凋落物浸提液处理下的刺槐种子活力指数之间具有显著差异(P<0.05)。一代中龄林凋落物浸提液处理下的刺槐种子活力指数最高,二代幼龄林、二代中龄林、三代中龄林活力指数较低。部分林分下的各质量浓度凋落物浸提液对刺槐种子活力指数之间具有显著差异(P<0.05)。在二代幼龄林中,C3处理下的种子活力指数呈最低值,较对照组的活力指数显著下降了35.39%(P<0.05),随着质量浓度的降低,种子的活力指数呈先下降后上升趋势。在二代中龄林中,与对照组相比,C1、C2、C5处理下的活力指数分别显著下降了33.25%、26.18%、28.06%(P<0.05)。在三代幼龄林中,与对照和C5处理相比,C1处理下的种子活力指数分别下降37.60%、38.54%(P<0.05),高质量浓度抑制种子活力,低质量浓度促进种子活力。在三代中龄林中,与对照相比,C1、C2、C4处理下的种子活力分别显著下降了33.22%、24.96%、23.38%(P<0.05)。
-
由图2A可见:不同林分凋落物浸提液处理下的刺槐胚根长具有显著差异(P<0.05),三代成熟林凋落物浸提液处理下的刺槐胚根最长,二代中龄林胚根长达最低。除了三代成熟林,其他林分下各质量浓度凋落物浸提液的刺槐胚根长之间具有显著差异(P<0.05),对照胚根显著长于其他质量浓度处理下的胚根(P<0.05)。大部分林分下,高质量浓度对胚根长的抑制作用较强,低质量浓度对其抑制作用减弱。

Figure 2. Effect of different forestands and different concentrations of R. pseudoacacia litter extract on the growth of its own embryos
由图2B可见:不同林分凋落物浸提液处理下的刺槐胚芽长具有显著差异(P<0.05),三代成熟林凋落物浸提液处理下刺槐胚芽长值最高,一代成熟林胚芽长达最低。在一代成熟林中不同质量浓度处理下的胚芽长呈显著差异(P<0.05),与对照相比,C2、C3、C4、C5处理下的胚芽长分别显著下降了19.88%、24.06%、14.51%、21.07%(P<0.05)。在三代成熟林中,各质量浓度凋落物浸提液处理下的胚芽长显著高于对照的胚芽长(P<0.05)。其他林分下不同质量浓度之间处理下的胚芽长没有显著差异。
由图2C可见:不同林分凋落物浸提液处理下的刺槐胚根鲜质量具有显著差异(P<0.05),三代中龄林和三代成熟林凋落物浸提液处理下的胚根鲜质量较高,一代成熟林和二代成熟林胚根鲜质量较低。在所有的林分中,对照处理下的胚根鲜质量显著大于其他质量浓度处理(P<0.05),大部分呈现高质量浓度浸提液处理下对胚根鲜质量的抑制作用较强,而低质量浓度处理下对其抑制作用减弱。
由图2D可见:不同林分凋落物浸提液处理下刺槐胚芽鲜质量之间具有显著差异(P<0.05),一代中龄林凋落物浸提液处理下的胚芽鲜质量最高,二代成熟林胚芽鲜质量值最低。二代中龄林中,与对照相比,C4、C5处理下的胚芽鲜质量分别显著下降28.21%、28.39%(P<0.05);二代成熟林中,与对照相比,C1、C3处理下的胚芽鲜质量分别显著降低32.50%、37.23%(P<0.05);三代幼龄林中,C1、C3、C4处理下显著抑制胚芽鲜质量,其分别较对照降低了40.27%、30.78%、28.96%(P<0.05)。三代中龄林中,与对照相比,C4处理下的胚芽鲜质量显著降低了24.46%(P<0.05)。
由图2E可见:不同林分凋落物浸提液处理下的刺槐胚根干质量之间具有显著差异(P<0.05),二代成熟林凋落物浸提液处理下的胚根干质量最大,二代幼龄林、三代中龄林、三代成熟林胚根干质量较小。绝大多数林分下凋落物浸提液处理下刺槐胚根干质量之间没有显著差异,除了一代中龄林和三代幼龄林。在一代中龄林中,与C1相比,C4和对照的胚根干质量分别显著下降了32.31%、30.86%(P<0.05),高质量浓度显著促进胚根干质量。在三代幼龄林中,与C5相比,C1、C3、对照的胚根干质量分别显著下降了42.10%、26.82%、31.64%(P<0.05),低质量浓度显著促进胚根干质量。
由图2F可见:不同林分凋落物浸提液处理下的刺槐胚芽干质量之间具有显著差异(P<0.05),一代中龄林凋落物浸提液处理下的胚芽干质量最大,三代中龄林胚芽干质量最小。大部分林分下不同质量浓度凋落物浸提液处理下刺槐胚芽干质量之间没有显著差异,除了三代幼龄林和三代中龄林。在三代幼龄林中,与C5相比,C1处理下的胚芽干质量显著下降了35.62%(P<0.05),高质量浓度抑制胚芽干质量,低质量浓度促进胚芽干质量。在三代中龄林中,与C5和对照相比,C4处理下的胚芽干质量显著下降了26.79%、28.98% (P<0.05)。
-
由图3A可见:从4个种子萌发指标的化感综合效应指数来看,大部分林分凋落物浸提液处理下的种子萌发受到抑制,只有在一代幼龄林C5,一代中龄林C1、C2、C3、C4,二代成熟林C3、C5处理下,表现出对种子萌发的促进作用。综合所有林分平均化感综合效应指数来看,各林分的抑制作用从大到小依次为三代中龄林、二代幼龄林、二代中龄林、三代幼龄林、三代成熟林、一代成熟林、一代幼龄林、二代成熟林、一代中龄林,其中一代中龄林凋落物浸提液对刺槐种子萌发表现为促进作用,化感综合效应指数为0.063,而三代中龄林化感综合效应指数为−0.211。在二代和三代林下,随着刺槐年龄的增加,其凋落物浸提液对刺槐种子萌发的抑制作用呈降低趋势。一代林中,中龄林凋落物浸提液促进种子萌发,幼龄林和成熟林其处理抑制种子萌发。在同一龄级下,随着刺槐经营世代的增加其凋落物浸提液对种子萌发的抑制作用呈上升趋势。
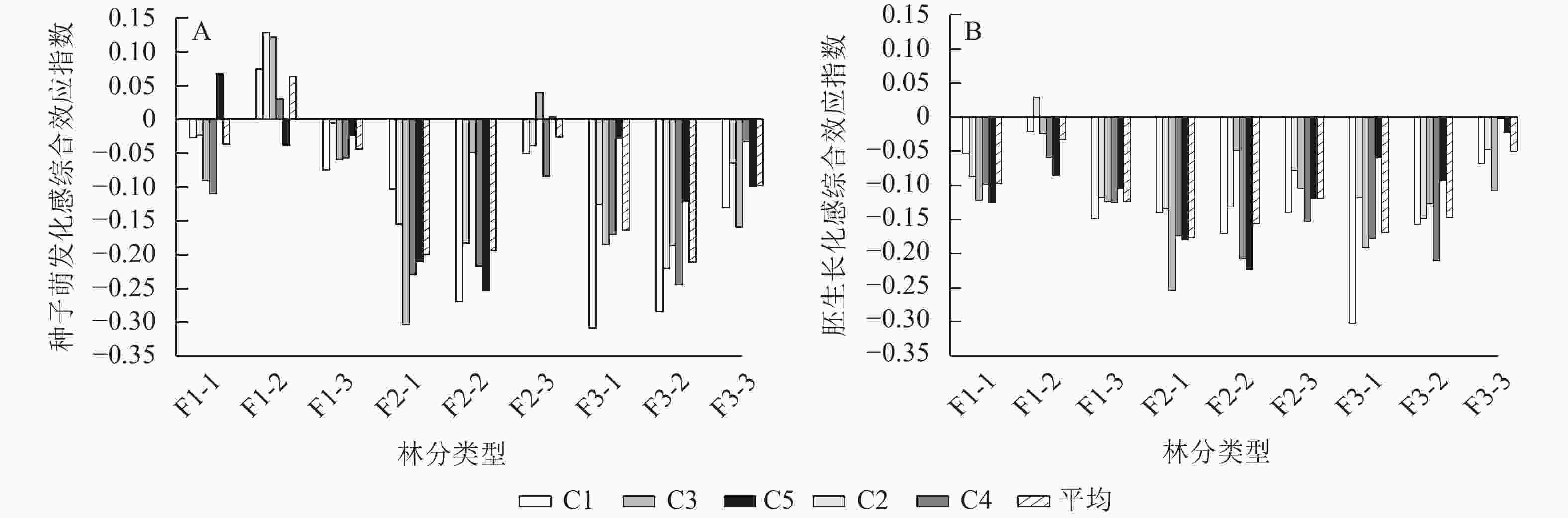
Figure 3. Allelopathy of different forest types and different concentrations of R. pseudoacacia litter extracts on seed germination and embryo growth
由图3B可见:从6个胚生长指标的化感综合效应指数来看,凋落物浸提液处理下的胚生长都被抑制生长。综合所有林分平均化感综合效应指数来看,各个林分的抑制作用从大到小依次为二代幼龄林、三代幼龄林、二代中龄林、三代中龄林、一代成熟林、二代成熟林、一代幼龄林、三代成熟林、一代中龄林,最强与最弱的抑制作用化感综合效应指数分别为−0.156和−0.032。在一代林下,凋落物浸提液的抑制作用从大到小依次为一代成熟林、一代幼龄林、一代中龄林。在二、三代林下,随着刺槐人工林年龄的增加,其林下凋落物浸提液对刺槐胚生长的抑制作用呈减弱趋势。在幼龄林和中龄林下,一代林刺槐凋落物浸提液处理下对胚生长的抑制作用最弱,二代林和三代林处理下对胚生长的抑制作用相同。在成熟林中,随着经营的增加,凋落物浸提液对刺槐胚生长的抑制作用呈现下降的趋势。
-
本研究发现:刺槐凋落物浸提液对刺槐自身种子萌发和胚生长表现出一定的抑制作用,与已有研究[15-16]结论一致。刺槐凋落物浸提液对种子萌发的抑制作用主要表现在降低种子发芽率、延长种子发芽时间、降低种子活力,刺槐凋落物浸提液对胚根的抑制作用强于对胚芽的抑制作用,胚根对浸提液的敏感性强于胚芽。这与前人的研究结果一致[22-23],这可能是胚根最先接触和吸收化感物质[24],而当胚根受到一定程度伤害时,水分和养分不能正常供给,胚芽才会表现出受害症状。已有研究[25-26]发现:化感物质对胚根生长的抑制作用导致植物根系表面积减小,吸水以及吸肥能力降低,因此导致胚根鲜质量较小。本研究也发现:对照的胚根鲜质量均大于刺槐凋落物浸提液处理的胚根鲜质量。刺槐凋落物浸提液对胚芽的抑制作用,也表现在鲜质量较小,这是由于根部受到损害,水分和养分不能正常供给时,地上部分表现出一定的症状[27]。刺槐凋落物浸提液对胚根和胚芽干质量的影响,可能由于影响叶绿素功能,造成胚的呼吸作用和光合作用的不平衡,导致生物量的积累或者消耗[28]。
同一物种在不同的生长时期会表现不同的化感潜力[29]。本研究表明:在二代林和三代林中,随着林龄的增加,刺槐凋落物浸提液对种子萌发和胚生长表现出自毒作用减弱趋势,对毛白杨Populus tomemtosa[26]、 对苜蓿Medicago sativa作用[30]的研究也得出一样的结论。陈淑芳[31]研究表明植物化感作用的强弱与植物自身的状况以及生存策略有关。刺槐为速生树种,当在幼龄林时期植物生长旺盛,其次植物种群之间的竞争也较为激烈,会分泌较多的化感物质,因而刺槐凋落物浸提液对刺槐自身化感抑制作用较强,而随着刺槐林龄的增加,个体生长趋于稳定,树木进入成熟期,为继续繁衍后代个体会产生较少的化感物质,其化感作用减弱。本研究发现:在一代林中,中龄林刺槐凋落物浸提液对种子萌发具有促进作用,对胚生长的抑制作用最小,这可能与环境因子有关[31-32]。环境因子会影响化感物质种类和数量的产生[33],在适宜的环境下,植物之间生存竞争小,向外界释放的化感物质较少,化感作用强度相对较弱[25]。本研究表明:在幼龄林和中龄林时,刺槐凋落物浸提液对自身种子萌发和胚生长的抑制作用随着经营世代的增加而增加;在成熟林时,随着经营世代的增加,刺槐凋落物浸提液对种子萌发的抑制作用呈上升趋势,对胚生长的抑制作用呈下降趋势,说明连栽刺槐会产生自毒作用。植物的化感物质浓度会随着种植密度的增加而增加[34]。前期调查发现随着刺槐林经营世代的增加,其密度呈增长趋势,因此刺槐的自毒作用增强。其次,随着经营世代增加,刺槐繁殖方式由实生繁殖转变为无性繁殖,刺槐产生的次生代谢物质的数量和种类发生一定的变化,并且部分代谢产物会通过各种方式累积下来。次生代谢产物不断增加,抑制作用也就加强[35]。此外,凋落物水淋溶的化感物质还受到微生物的作用,微生物影响化感物质的数量以及种类。有研究表明:二、三代刺槐人工林的微生物含量以及多样性大于一代林,微生物含量以及多样性增加会产生更多的化感物质[20],因此对植物生长的抑制作用增强。
本研究发现:刺槐人工林下凋落物浸提液的化感作用具有质量浓度效应,部分林分表现出随着质量浓度的增加,其抑制作用呈增加趋势,这种抑制作用的质量浓度效应与赵利等[36]的研究结果一致。这是由于化感物质影响植物自身对水分的吸收,质量浓度越高,植物水势就越低,水分胁迫就越严重,最终导致种子萌发延迟,胚生长也受到抑制[25]。其次,一些化感物质在高质量浓度会表现出强烈的抑制作用[27],高质量浓度下由于受到胁迫,植物细胞结构或者细胞功能遭到破坏[28],抑制种子萌发和胚生长。本研究发现:一些林分下种子萌发和胚生长的指标其抑制作用并没有随着浸提液稀释质量浓度的变化而变化,这可能是化感作用和资源限制之间相互抵消[18]。部分林分下种子萌发和胚生长指标在高质量浓度、中质量浓度表现促进作用,可能由于凋落物浸提液中含有植物生长需要的有机物质和营养元素[9],营养成分对植物生长的促进作用大于化感物质的抑制作用[37],也可能由于化感物质的复合效应[38],高质量浓度浸提液中同时含有对自身生长促进和抑制的化感物质,只是促进作用大于抑制作用。
Allelopathy of the litter extracts from Robinia pseudoacacia forest on its seed germination and embryo growth
doi: 10.11833/j.issn.2095-0756.20220247
- Received Date: 2022-03-16
- Accepted Date: 2022-10-11
- Rev Recd Date: 2022-09-26
- Available Online: 2023-01-18
- Publish Date: 2023-01-17
-
Key words:
- Robinia pseudoacacia /
- litter /
- seed germination /
- embryo growth /
- allelopathy
Abstract:
| Citation: | JING Rong, PENG Zuodeng, LI Yun, et al. Allelopathy of the litter extracts from Robinia pseudoacacia forest on its seed germination and embryo growth[J]. Journal of Zhejiang A&F University, 2023, 40(1): 97-106. DOI: 10.11833/j.issn.2095-0756.20220247 |










 DownLoad:
DownLoad:

