-
土地盐碱化增大了土壤渗透压,导致植物吸水困难,对植物造成了生理干旱[1]。过多钠离子(Na+)、氯离子(Cl−)的积累,导致膜结构破坏,对植物造成渗透胁迫[2−3]。盐胁迫还导致植物内源活性氧(ROS)增加,引起细胞膜损伤甚至细胞死亡,抑制植株生长发育[4]。ROS作为响应盐胁迫的关键因子,在低水平下,诱导增强抗氧化酶活性,抵御盐胁迫;在高水平下,过量积累造成氧化胁迫,导致生物大分子产生不可逆的损伤,改变细胞形态结构,抑制植株生长发育[5]。为缓解ROS积累引起的氧化胁迫,植物通过增强抗氧化酶系统相关酶活性来降低体内的ROS,从而提高抗逆性[6]。植物的抗氧化酶系统主要包括超氧化物歧化酶(SOD)、过氧化氢酶(CAT)、抗坏血酸过氧化物酶(APX)、过氧化物酶(PRX)等[7]。
在小麦Triticum aestivum中过表达TaPRX-2A,植株抗氧化能力增强,ROS下降,耐盐性增加[8]。拟南芥Arabidopsis thaliana AtPRX19参与胁迫(盐害、干旱、病虫害等)后的氧化应激反应,使得ROS增加,对植物造成氧化胁迫。盐胁迫下,拟南芥AtPRX19表达量上调,ROS减少,抵御胁迫能力增强[9]。在胡萝卜Daucus carota中异源表达OsPRX114同样降低过氧化氢(H2O2)水平,提高植株的耐盐性[10]。玉米Zea mays的PRX家族成员ZmPRX26、ZmPRX42、ZmPRX71、ZmPRX75和ZmPRX78参与了对包括盐胁迫在内多种非生物胁迫的响应[11]。部分PRX家族成员通过协调水杨酸(SA)、茉莉酸(JA)和乙烯(ET)等激素水平发挥作用[12]。
研究林木对盐胁迫的响应,揭示耐盐性相关机理,对培育耐盐性更强林木品种具有重要意义。为研究杨树Populus PRX家族对林木耐盐性的影响,本研究以银腺杨‘84K’ Populus alba × P. glandulosa ‘84K’ (84K杨)为材料,通过构建PagPRX19的过表达转基因株系,改变杨树H2O2水平,并分析了杨树耐盐相关生理指标,以期揭示PagPRX19参与调控杨树盐胁迫响应的机制,为杨树的分子育种提供理论依据。
-
研究材料为84K杨,过表达PagPRX19株系为本研究获得。
-
Phytozome v13数据库中获取毛果杨Populus trichocarpa和拟南芥的PRX家族的蛋白序列、蛋白编码区(CDS)序列、启动子序列。利用P1ant CARE 在线软件对PRX家族启动子顺式作用元件预测。使用MEGA v7软件,构建拟南芥和杨树的PRX家族的系统发育树,分析杨树和拟南芥PRX基因家族成员在进化关系上的同源性。利用MEGE v5.4.1 在线软件分析PRX家族基因保守结构域。
-
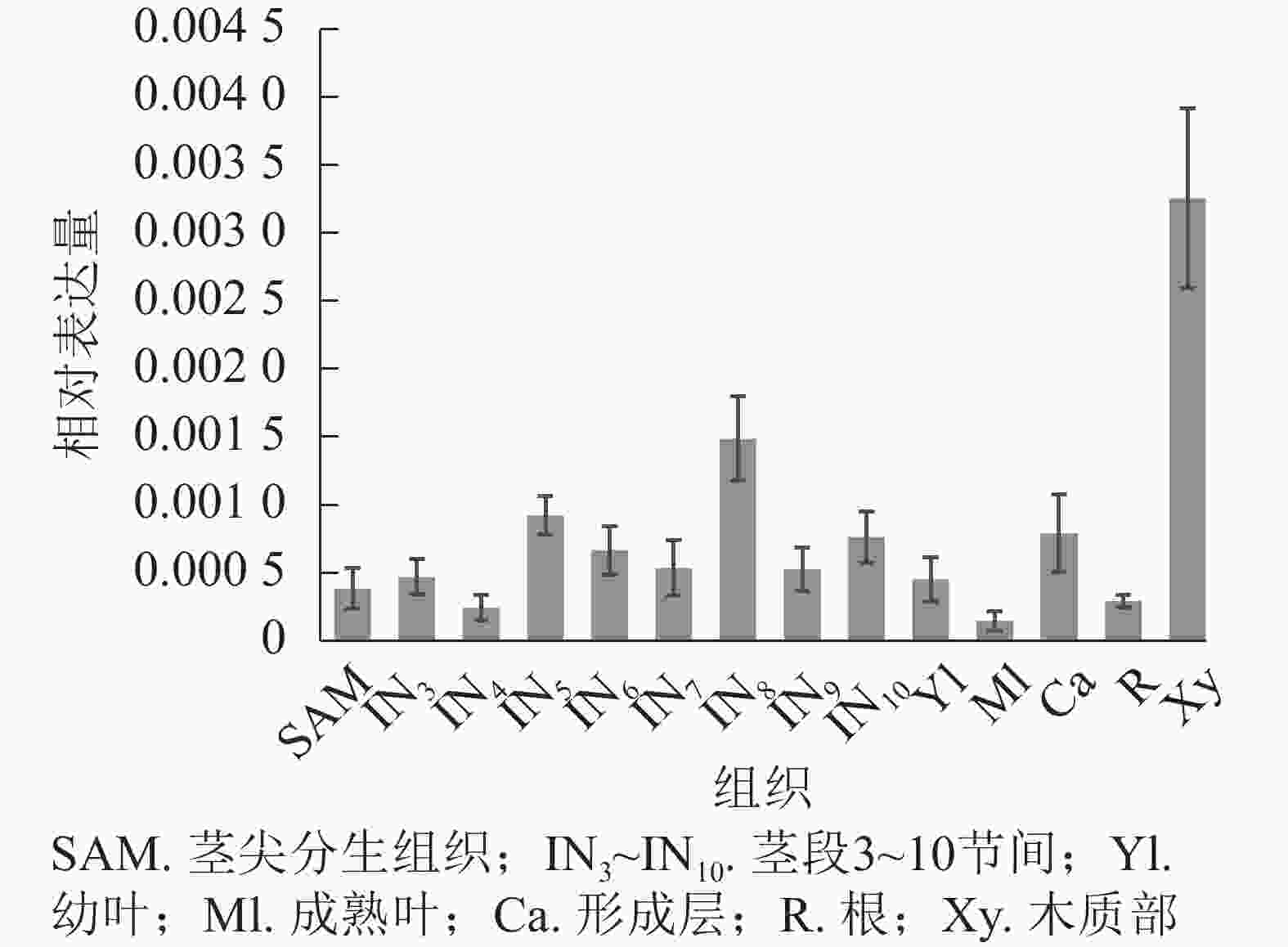
选用生长一致的84K杨树苗,分别从茎尖(SAM)、茎段3~10节间(IN3~IN10)、形成层(Ca)、幼叶(YL)、成熟叶(ML)、根(R)、木质部(Xy)取样,所有材料取3个生物学重复。采用实时荧光定量PCR (RT-qPCR)检测该基因在不同组织内的表达模式。
-
在84K杨基因组数据库中,通过Blast获取毛果杨PRX19同源基因PagPRX19,设计引物扩增获得目的基因(F端引物:ATGTATACAACAATCATGCCT,R端引物:TTATGTATGCATACTGCAAAC),使用Gateway技术构建过表达载体35S::PagPRX19,利用叶盘转化法转化84K杨获得过表达PagPRX19转基因苗[13]。
-
①组培盐胁迫处理。以组培培养45 d的过表达株系为材料,以非转基因植株为对照。盐胁迫组接顶芽于100 mmol·L−1氯化钠(NaCl)生根培养基,对照组接顶芽于0 mmol·L−1NaCl生根培养基。每组处理12瓶,每瓶接2株顶芽。培养温度为23~25 ℃,光周期为8 h/16 h(黑暗/光照)。处理25 d,观察植株在长期盐胁迫下耐盐能力。②土培盐胁迫处理。以土培生长2个月的过表达株系为实验材料,以非转基因植株为对照,每组处理5株。盐胁迫组隔2 d浇灌1次100 mmol·L−1的NaCl溶液,对照组隔2 d浇灌1次0 mmol·L−1的NaCl溶液。培养温度为23~25 ℃,光周期为8 h/16 h(黑暗/光照)。处理7 d,取样测定各项生理指标,处理12 d后拍照[14]。
-
①脯氨酸质量分数测定。采用脯氨酸测试盒(南京建成,A107-1-1)测定脯氨酸质量分数。②相对含水量测定。取新鲜叶片记录鲜质量(WF),超纯水(ddH2O)浸泡24 h记录质量(WT),65 ℃烘箱干燥3 d,记录干质量(WD)。叶片相对含水量=(WF−WD)/(WT−WD)×100%[15]。③丙二醛(MDA)质量摩尔浓度测定。称取植物叶片0.2 g,加入4 mL质量浓度为10%的三氯乙酸(TCA)溶液和石英砂研磨至匀浆,4 000 r·min−1离心10 min,取上清液2 mL,加入2 mL硫代巴比妥酸(TAB)溶液,对照为2 mL ddH2O。摇匀后沸水浴15 min,−20 ℃迅速冷却后4 000 r·min−1离心2 min,取上清液,在532和600 nm波长下测量吸光度。MDA浓度CMDA(μmol·L−1)=[D(532)−D(600)]/155×1 000,MDA质量摩尔浓度(nmol·g−1)=(CMDA×V提取液体积)/F样品质量[16]。④电解质渗透率测定。采用五点取样法取样,加入6 mL ddH2O,28 ℃摇床震荡1 h。取出测第1次电导值(G1)。沸水浴30 min,冷却至室温,摇匀测量第2次电导值(G2)。电解质渗透率=第1次电导值/第2次电导值×100%[17]。⑤ROS水平定性测量。DAB染色剂试剂盒(索莱宝,DA1010)染色检测H2O2;氯化硝基四氮唑蓝(NBT)粉末(索莱宝,N8140)染色检测超氧阴离子(O2 .−)。
-
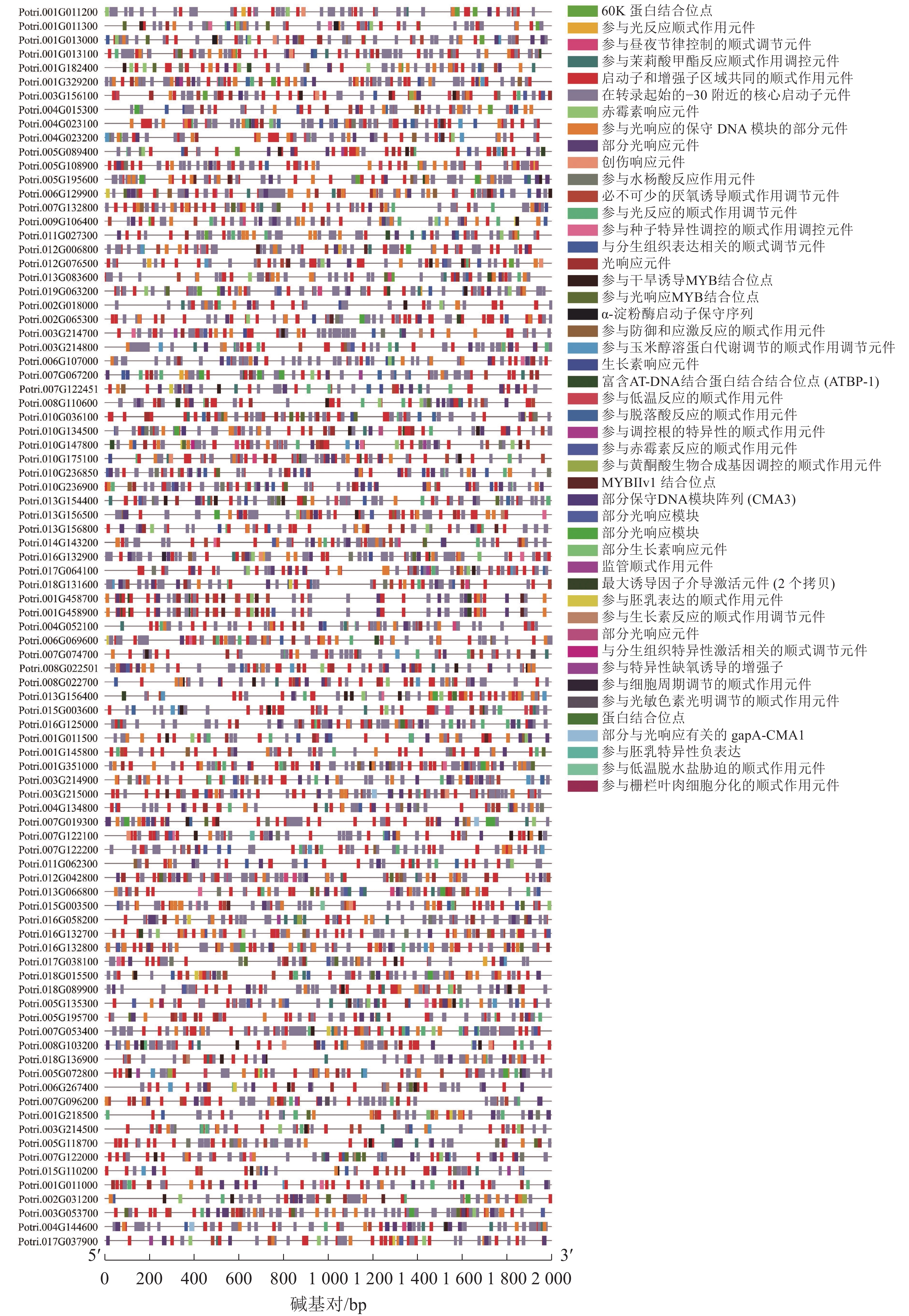
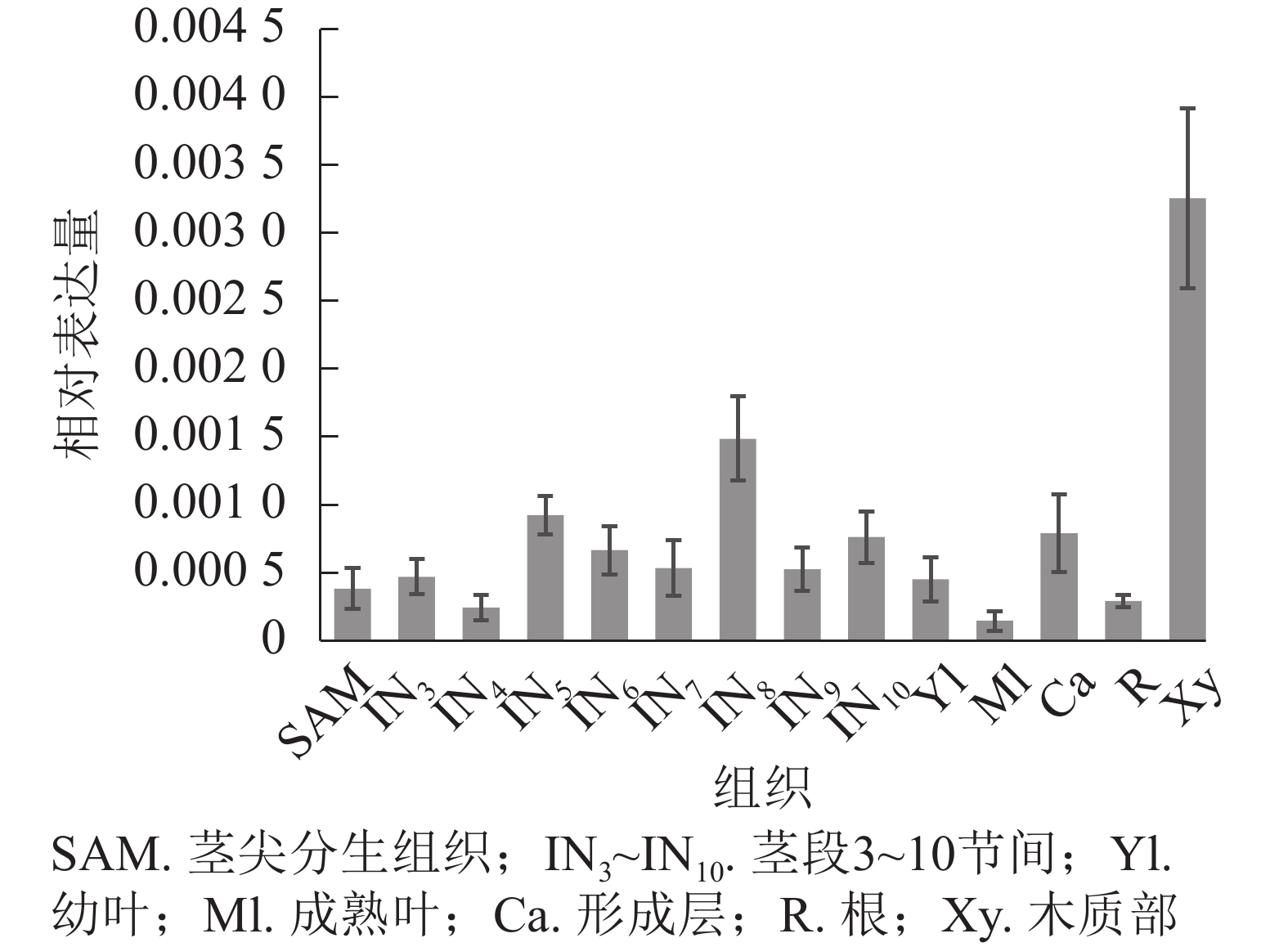
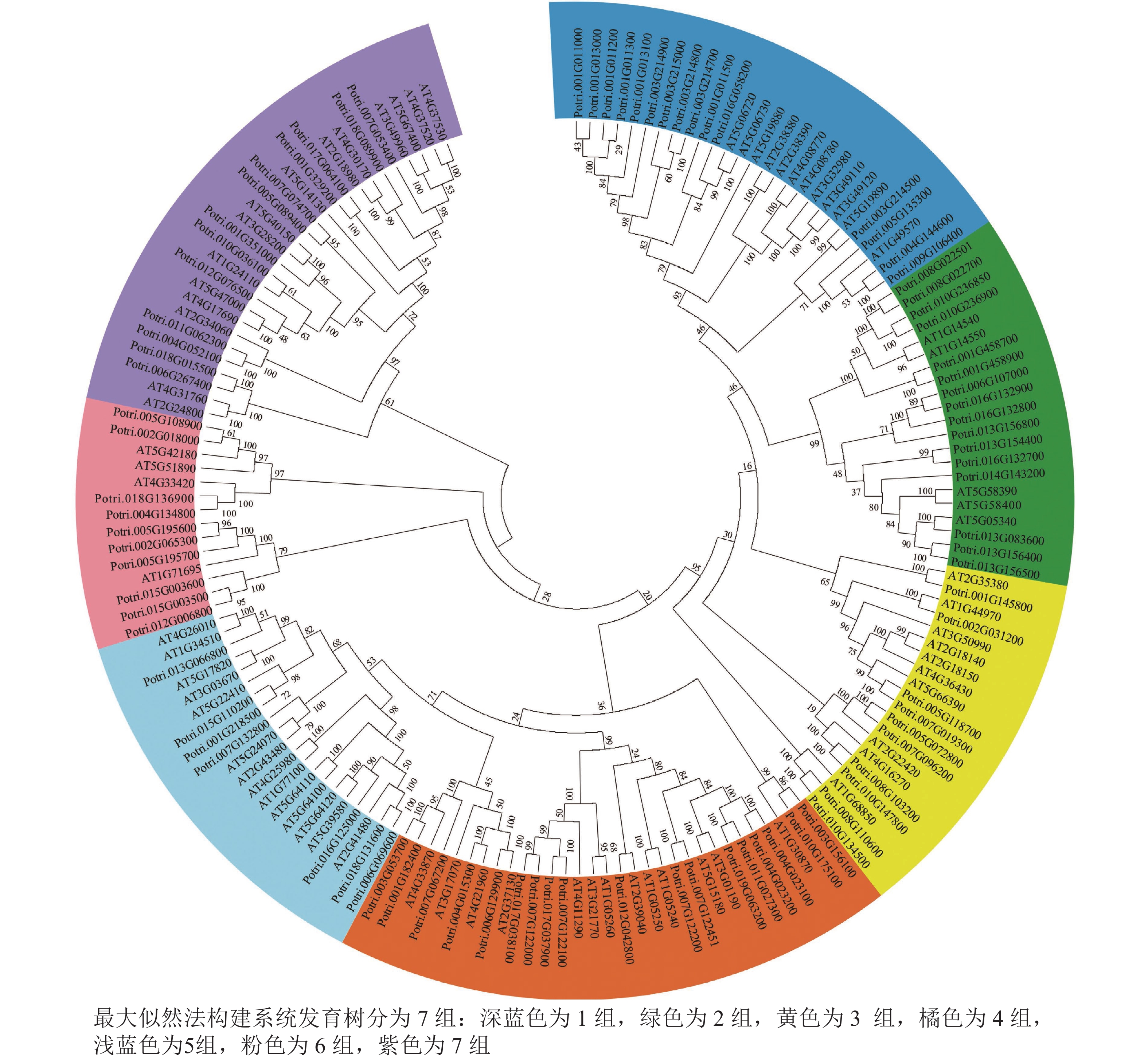
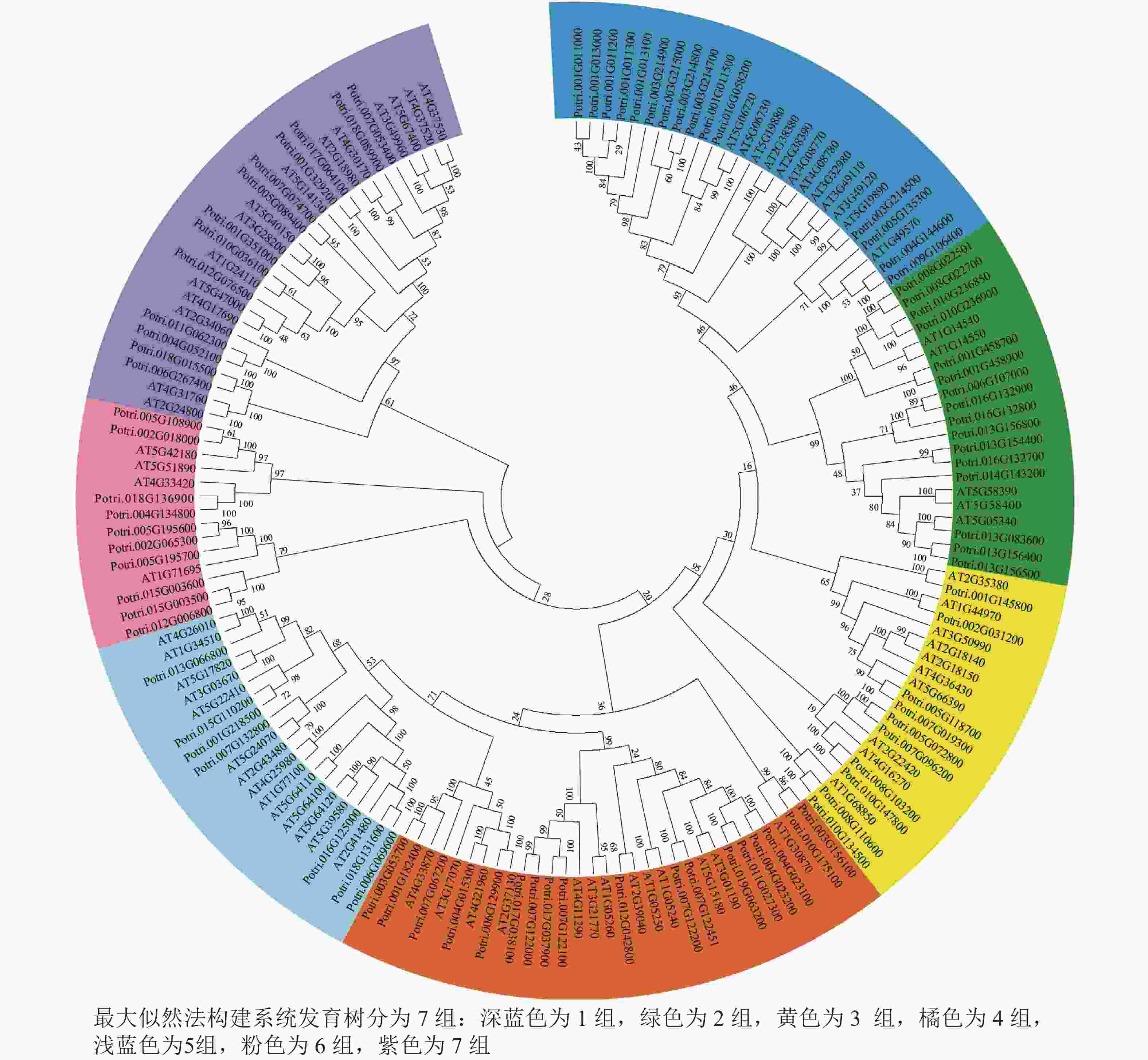
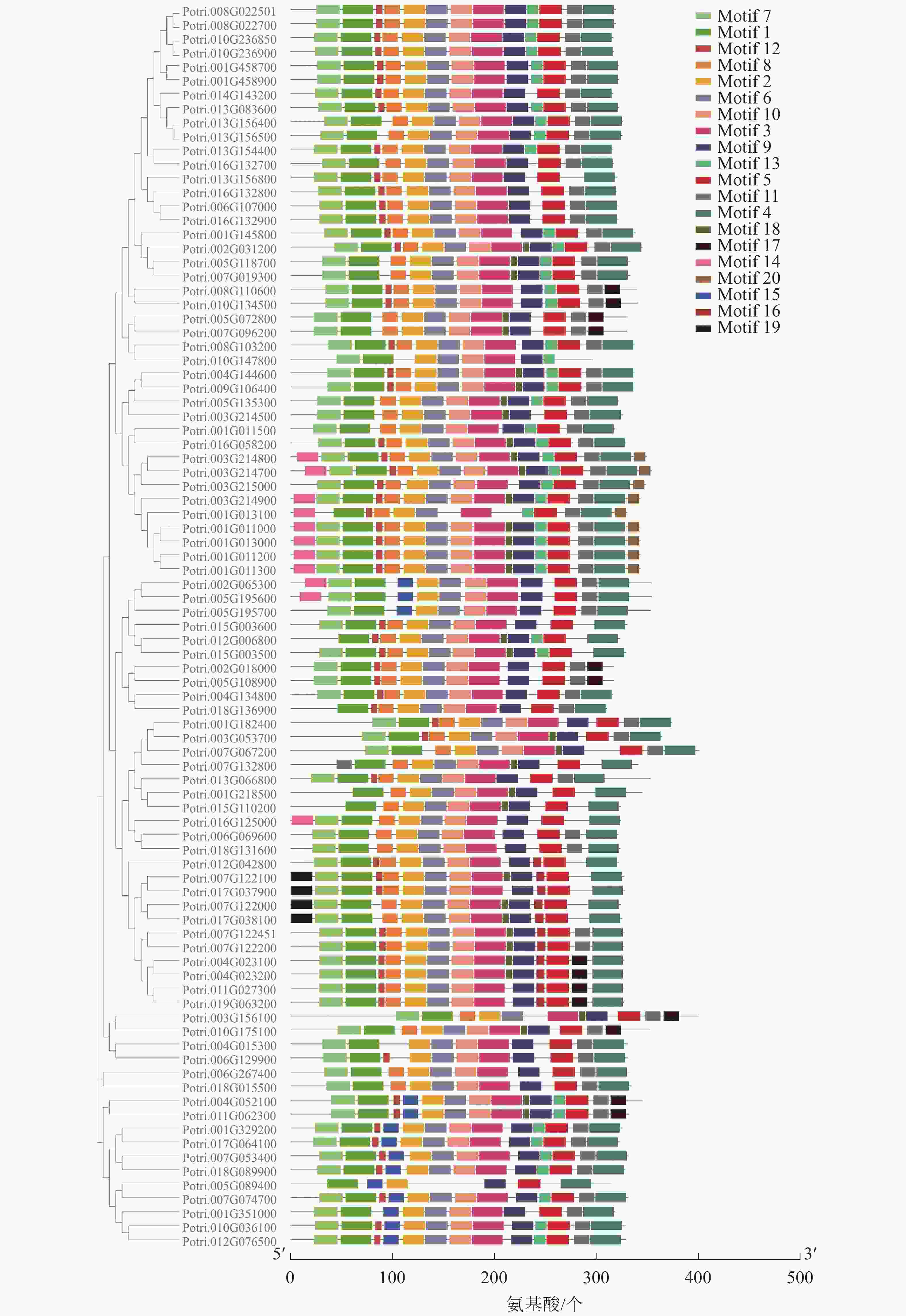
使用拟南芥和毛果杨PRX家族的同源蛋白序列,构建系统发育树(图1)并进行保守结构域分析(图2)发现:该家族基因高度保守。对PRX基因家族启动子进行顺式作用元件分析(图3)发现:该基因家族成员含有响应生长素(IAA)、脱落酸(ABA)、茉莉酸甲酯(MeJA)等激素的功能元件以及响应低温、干旱、盐害等非生物胁迫的功能元件。因此,PRX家族为植物生长发育和参与抵御环境胁迫中的重要家族。毛果杨Potri.004G052100 (84K杨同源基因命名为PagPRX19)与拟南芥AtPRX19 (At2G34060)的同源关系较近。研究表明:AtPRX19参与调控拟南芥的抗逆性[9, 16],推测与其同源的杨树基因PRX19也具有此功能。此外,PRX19启动子区域含有与盐胁迫密切相关的IAA、ABA和MeJA等激素相关顺式作用元件,参与植物耐盐调控。选取PagPRX19基因分析其在84K杨中的表达模式(图4),结果显示:PagPRX19基因在各个组织均有表达,在木质部表达量最高。
-
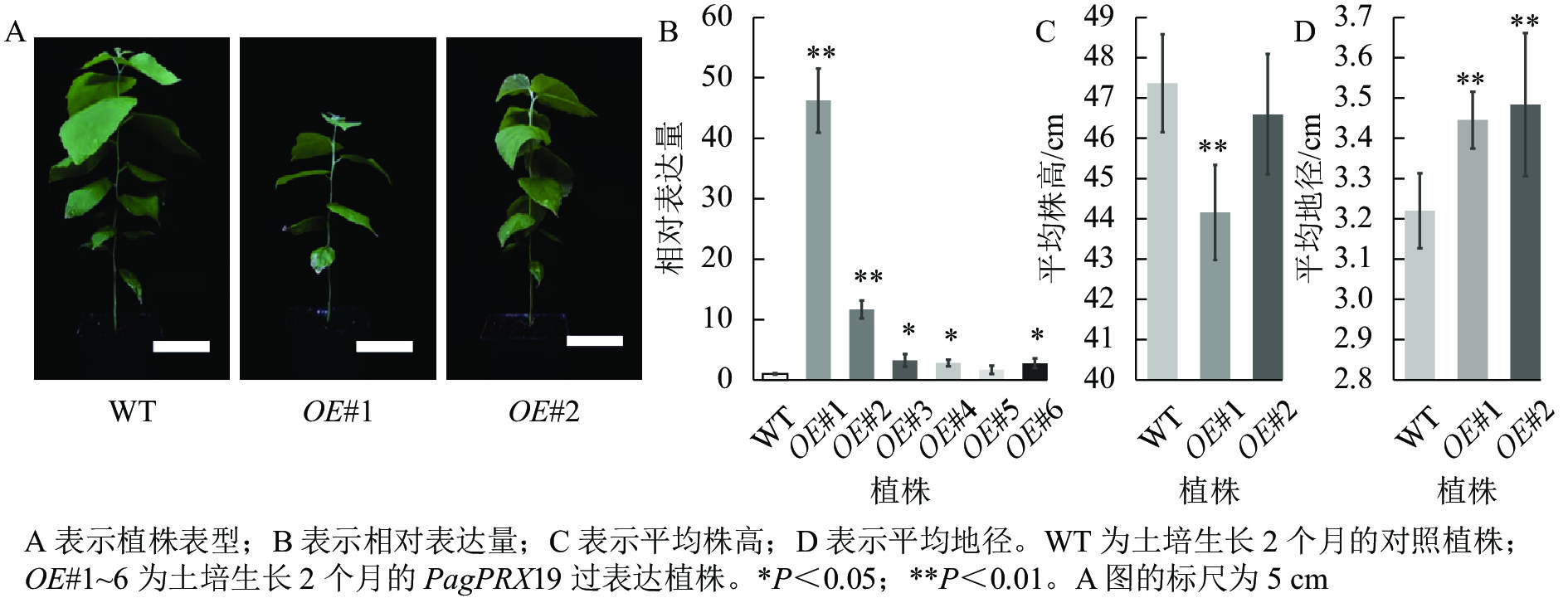
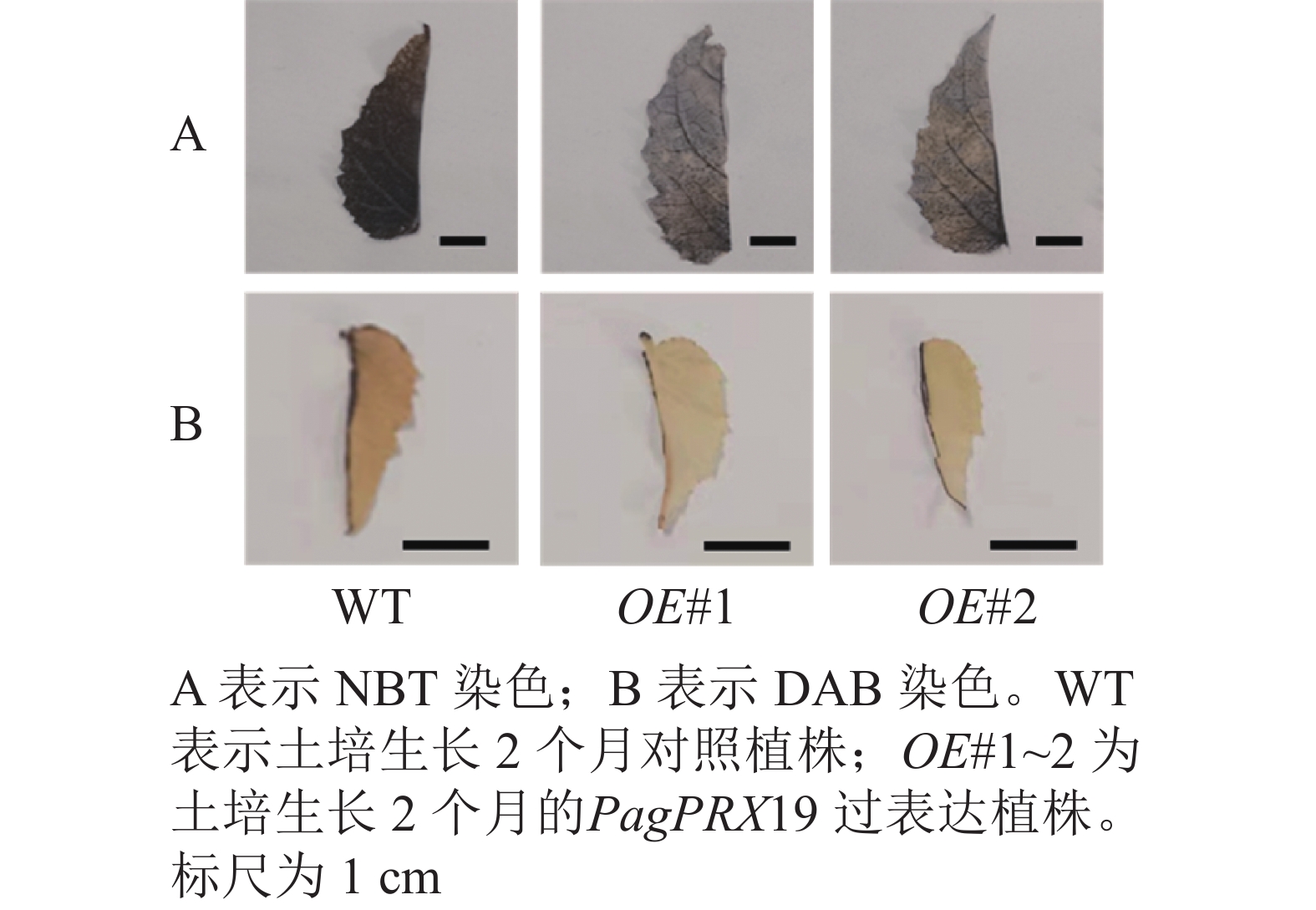
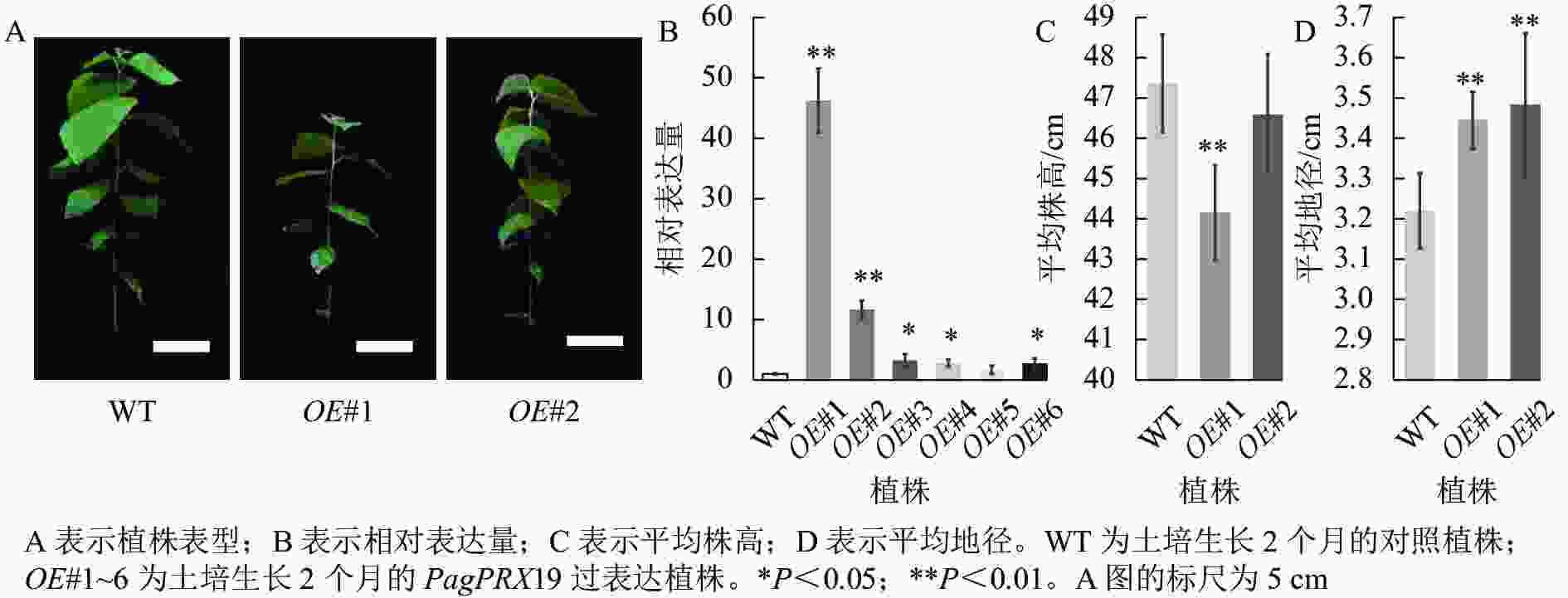
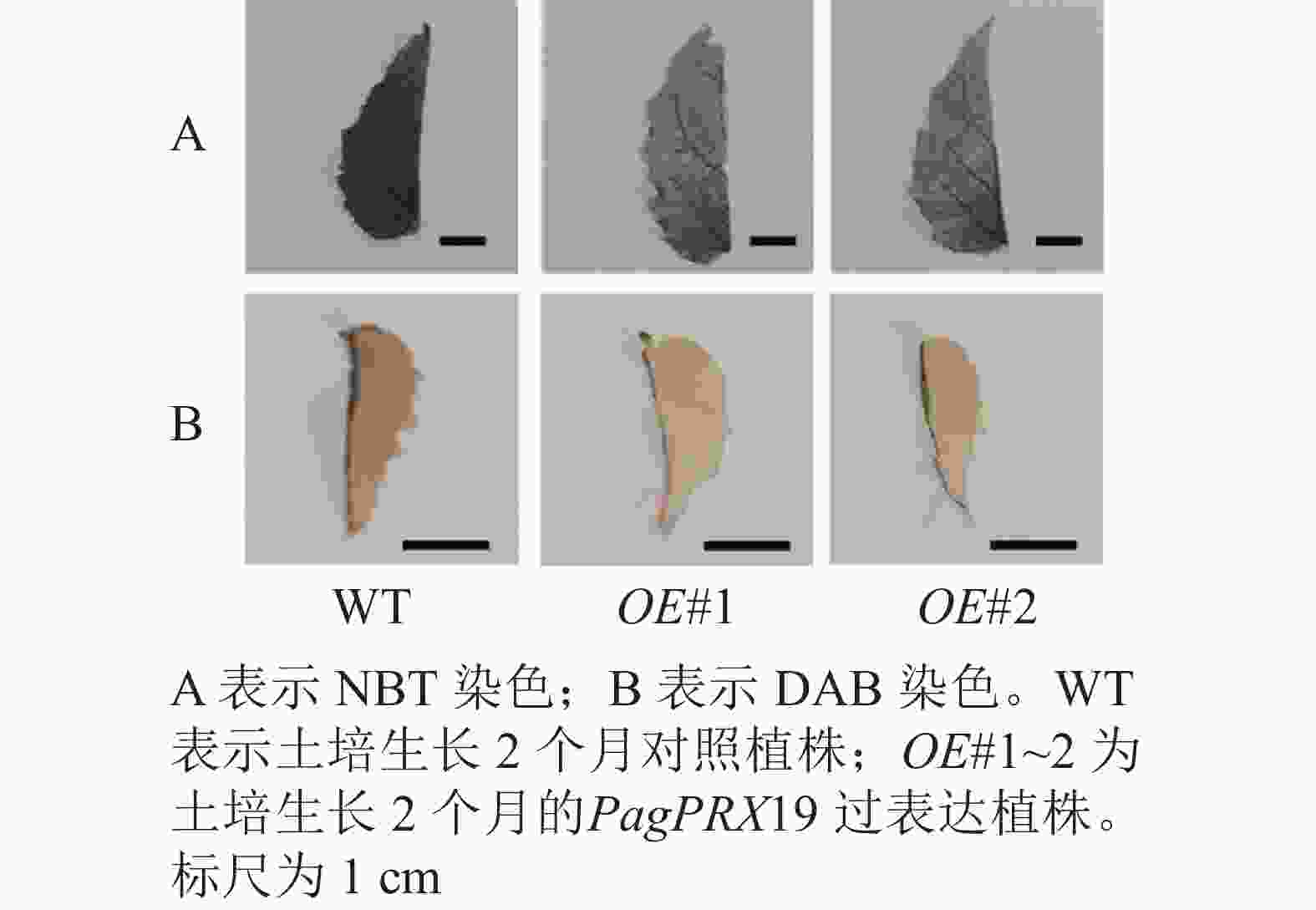
对转基因技术获得的6株植株进行阳性苗鉴定,通过RT-qPCR分析不同阳性株系PagPRX19的表达量,选取了相对表达量分别为对照46和12倍的2个株系OE#1、OE#2进行表型分析(图5)。分别选取6株生长2个月的土培苗统计株高和地径发现:株系OE#1的株高(44.15 cm)与对照(47.36 cm)相比极显著下降(P<0.01),而株系OE#2的株高(46.58 cm)与对照相比无显著差异。株系OE#1的地径(3.42 cm)和OE#2的地径(3.49 cm)与对照(3.22 cm)相比极显著增加(P<0.01)。取植株第3片叶进行DAB和NBT化学染色(图6)发现:与对照相比,PagPRX19过表达植株叶片的ROS水平显著减少。对植株第7节间茎段组织切片进行DAB和NBT化学染色(图7)发现:与对照相比,过表达植株茎段的ROS水平降低,说明过表达PagPRX19导致植株ROS降低。
-
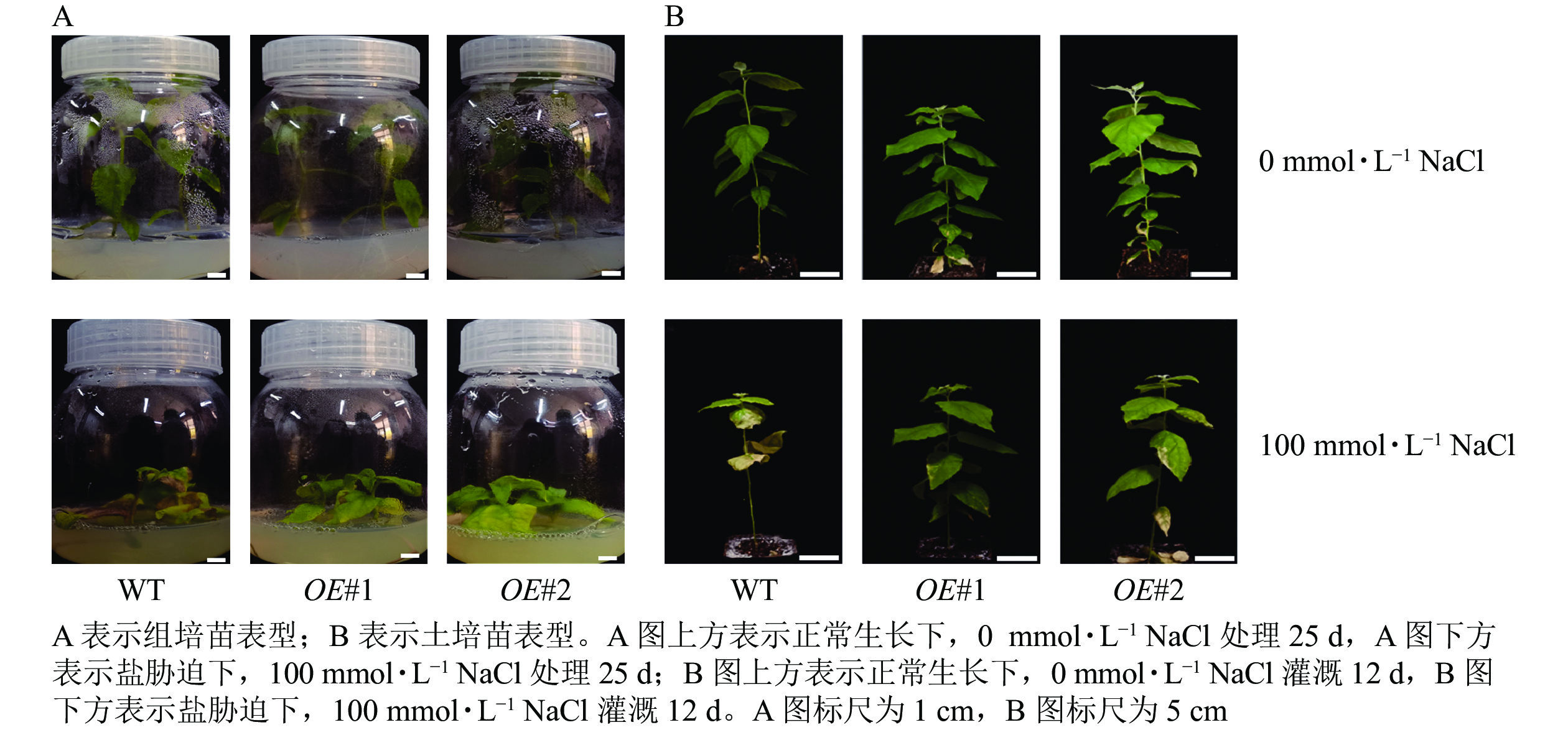
在100 mmol·L−1NaCl生根培养基培养条件下(图8),对照植株组培苗叶片大面积脱落,植株呈黄褐色,干枯严重甚至死亡。而过表达叶片失绿和皱缩情况与对照相比较轻,且无叶片脱落,说明过表达植株较对照具有更高的耐盐性。在100 mmol·L−1NaCl溶液灌溉处理下,对照植株叶片大面积脱落,皱缩明显,严重失绿。过表达株系OE#2叶片轻度失绿,叶缘处皱缩不明显。株系OE#1仅叶缘处轻微皱缩,与正常生长的处理差异不明显,与对照相比,过表达株系有更高的耐盐性,其中表达量较高的株系OE#1较OE#2耐盐性更强。
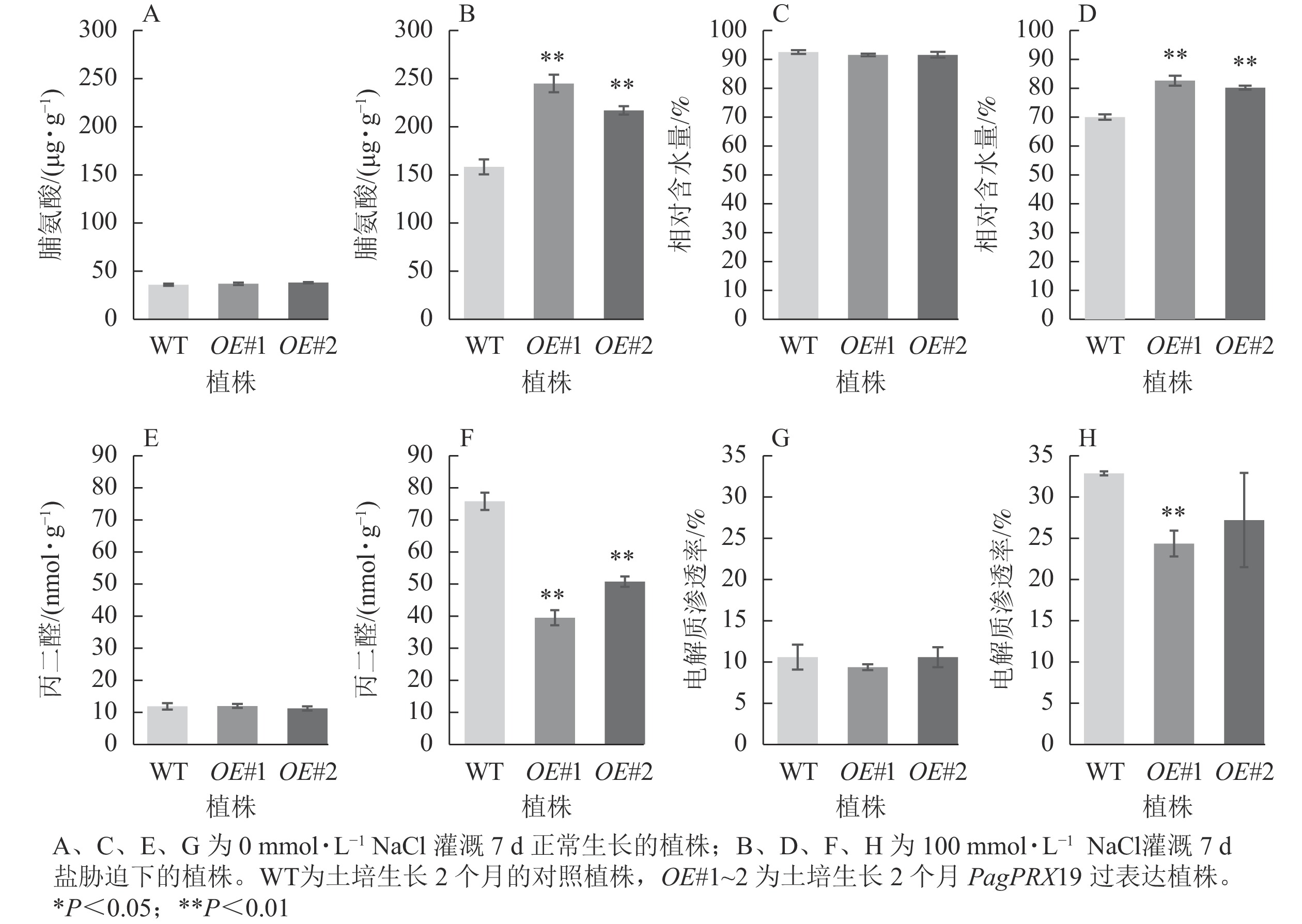
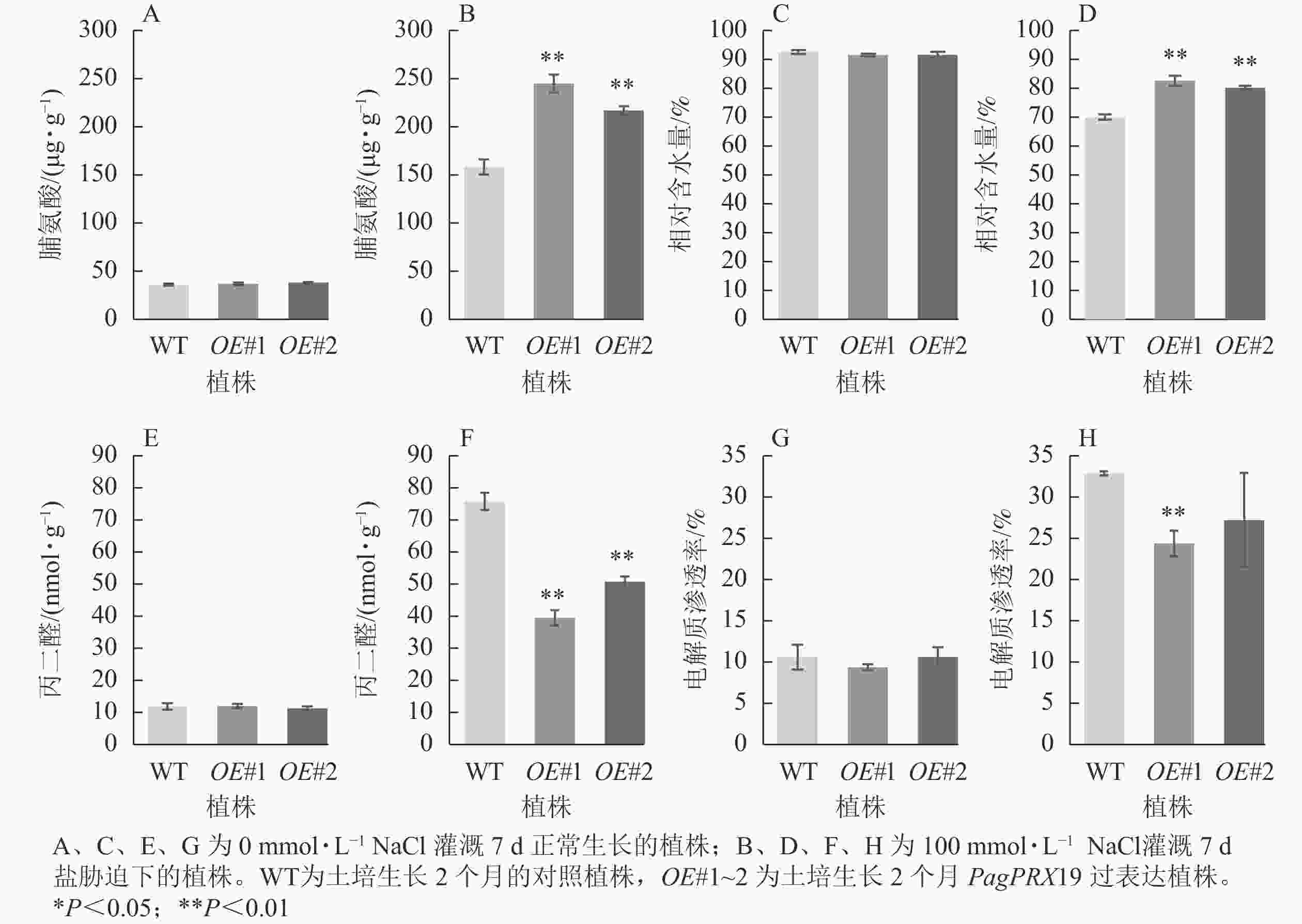
从图9可见:正常生长时,对照与过表达植株脯氨酸质量分数、相对含水量、丙二醛质量摩尔浓度、电解质渗透率无显著差异。盐胁迫下,相较于对照植株的脯氨酸质量分数(158.26 μg·g−1),过表达植株脯氨酸质量分数极显著增加(OE#1为244.94 μg·g−1,OE#2为217.02 μg·g−1)(P<0.01)。与对照叶片相对含水量(70.05%)相比,过表达植株叶片相对含水量极显著高于对照(OE#1为82.68%,OE#2为80.25%)(P< 0.01)。与对照丙二醛质量摩尔浓度(75.76 nmol·g−1)相比,过表达植株丙二醛质量摩尔浓度(OE#1为39.44 nmol·g−1,OE#2为50.76 nmol·g−1)较低且差异极显著(P<0.01)。与对照电解质渗透率(32.75%)相比,过表达植株电解质渗透率(OE#1为24.24%,OE#2为27.09%)较低,且株系OE#1与对照差异极显著(P<0.01)。
-
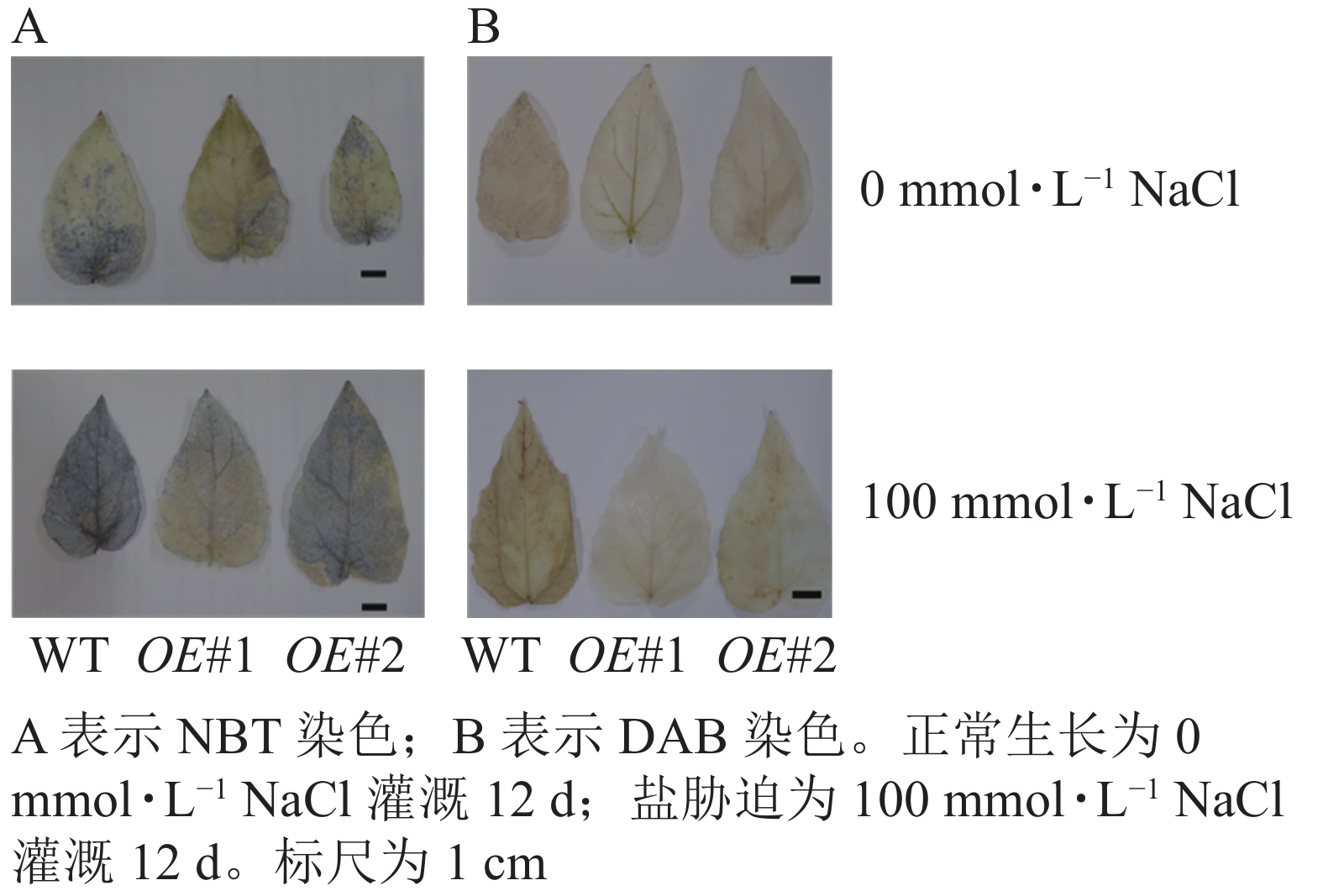
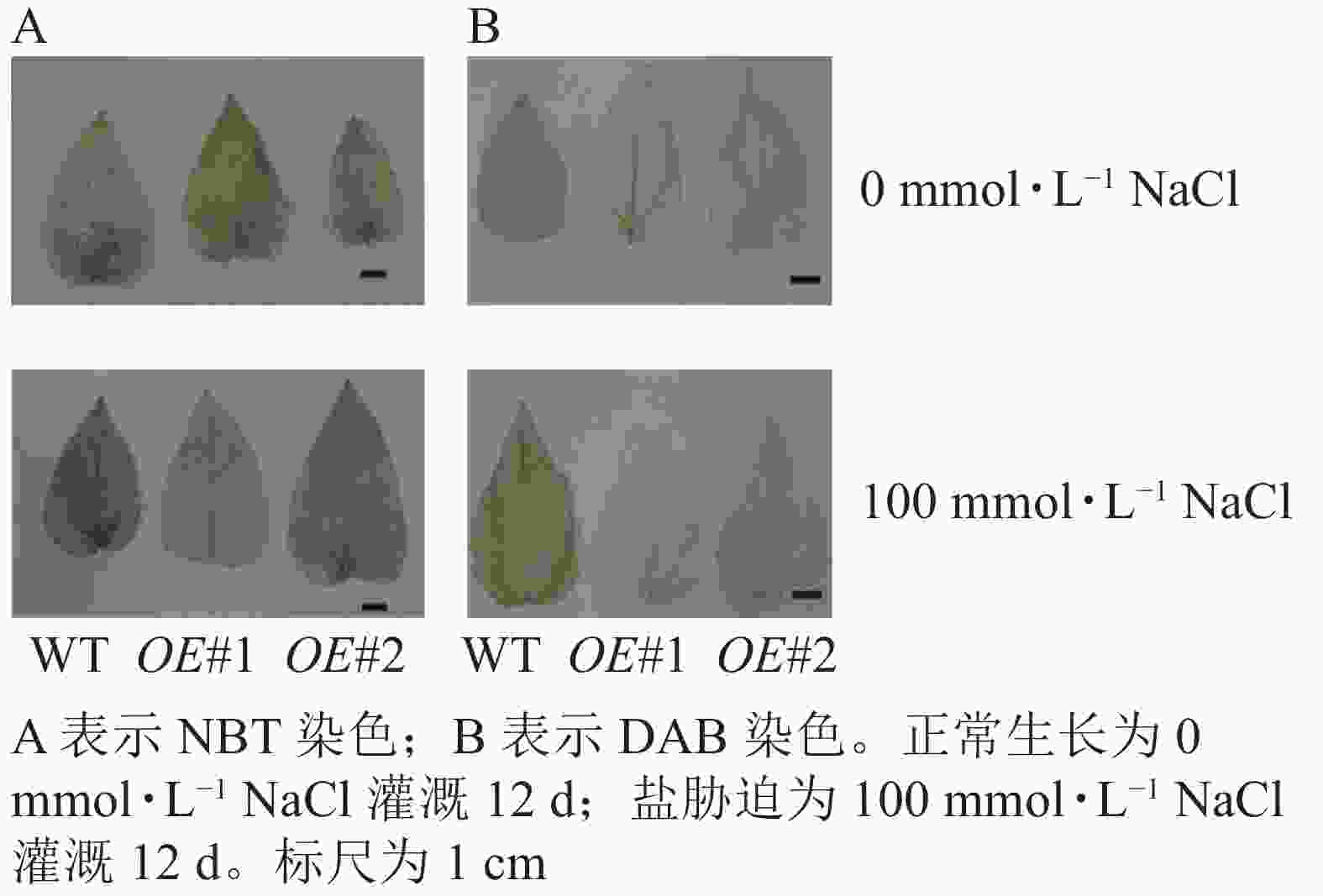
对盐胁迫处理后的土培苗叶片进行NBT和DAB染色(图10)发现:与对照相比,过表达植株叶片的ROS信号少。对盐胁迫处理后的组培苗根部进行DAB和NBT染色(图11)后发现:与对照相比,PagPRX19过表达植株根部的ROS信号少。说明在盐胁迫下,由于过表达PagPRX19导致植株ROS水平下降。
-
过氧化酶PRX可催化H2O2,降低ROS水平,减轻胁迫对细胞的伤害[3]。因此,通过抗氧化酶清除盐胁迫产生的ROS,以抵御胁迫带来的细胞损伤,提高林木的抗性,可作为抗逆分子育种手段。本研究通过过表达过氧化酶基因,改变84K杨内源ROS水平,从而探究ROS水平对杨树耐盐性的影响。
生物信息学分析发现:PagPRX19存在多个与IAA、ABA、或MeJA相关的顺式作用元件,这些激素与盐胁迫密切相关,参与植物耐盐调控。基因表达模式分析发现:PagPRX19在植株各个组织都有表达。因此选取该基因作为研究对象,创制过表达植株。与对照植株相比,过表达植株叶片和茎段ROS水平降低。过基因植株H2O2降低,O2 .−也随之降低。可能是过表达加速了H2O2的清除,植物自身调控SOD酶加速分解O2 .−产生H2O2,从而导致了O2 .−减少[9]。对获得的转基因植株进行盐胁迫处理,在100 mmol·L−1NaCl处理下,对照植株出现了胁迫症状,但过表达植株症状轻或没有出现症状。过表达植株可能通过降低盐胁迫下植株氧化胁迫程度,增强耐盐性。ROS水平检测结果验证了这一推论,盐胁迫下,过表达植株与对照相比,ROS水平仍保持较低水平。表明过表达PagPRX19可降低盐胁迫下ROS的伤害,从而增强了转基因植株的抗性。
研究表明:盐胁迫下脯氨酸质量分数增加[17],葡萄Vitis vinifera砧木在一定程度内的耐盐性越强,脯氨酸等有机渗透物质就越多[18]。本研究表明:盐胁迫下,过表达PagPRX19植株脯氨酸质量分数高于对照植株,使得植株的耐盐能力增强。此外,盐胁迫造成植物叶片含水量下降,植物生理活动会受到影响[19]。丙二醛和电解质渗透率反映了细胞膜损坏程度[20]。盐胁迫下,植株细胞膜被破坏,细胞膜内物质外渗,电解质渗透率升高,丙二醛增加[21−22],而耐盐品种一般表现为叶片相对含水量高、电解质渗透率和丙二醛低的特点[19, 23]。本研究中,盐胁迫下PagPRX19过表达植株叶片相对含水量比对照高,丙二醛、电解质渗透率降低,表明转基因植株细胞膜完整性较好,耐盐性增强。
-
过表达过氧化物酶基因PagPRX19可降低84K杨植株内源ROS水平,且在盐胁迫下过表达植株ROS仍保持低水平,细胞膜的破坏程度降低、叶片的持水能力增强。表明在杨树中过表达PagPRX19,能促进盐胁迫下植株内源ROS的清除,从而减缓盐胁迫造成的氧化胁迫,提高植株的耐盐性。
Effects of peroxidase gene PagPRX19 on salt tolerance of poplar ‘84K’
doi: 10.11833/j.issn.2095-0756.20220387
- Received Date: 2022-06-09
- Accepted Date: 2022-09-19
- Rev Recd Date: 2022-09-14
- Available Online: 2022-11-21
- Publish Date: 2022-12-20
-
Key words:
- Populus alba × P. glandulosa ‘84K’ /
- peroxidase /
- reactive oxygen species (ROS) /
- salt stress /
- salt tolerance
Abstract:
| Citation: | HUANG Qingchen, LAI Jianxin, HUANG Lichao, et al. Effects of peroxidase gene PagPRX19 on salt tolerance of poplar ‘84K’[J]. Journal of Zhejiang A&F University, 2022, 39(6): 1163-1172. DOI: 10.11833/j.issn.2095-0756.20220387 |













 DownLoad:
DownLoad: