-
红花石蒜Lycoris radiata为石蒜科Amaryllidaceae石蒜属Lycoris特色球根花卉[1]。鳞茎中丰富的生物碱是制药的原材料,具有催吐、治水肿等功效[2]。红花石蒜花色艳丽、形态雅致,在中国民间被称为“魔术花”,在国外素有“中国郁金香”之美誉,观赏价值较高[3],市场需求量激增[4−5]。在自然条件下,由于红花石蒜存在结实率低、种子含水量高、不耐储藏以及随采随播成苗较少等问题[6],目前其种球仍然以无性繁殖为主[7]。已有研究表明:中国石蒜L. chinensis和长筒石蒜L. longituba种子均属于典型的顽拗型种子,具脱水敏感性[8]。当种子成熟脱落时含水量分别为70%和50%,失水后种子逐渐皱缩干瘪,当失水超过某一临界值时,种子活力大幅下降,失水严重时种子无法萌发[9−10]。硅胶脱水法是开展顽拗性种子脱水敏感性研究的有效方法之一[11]。随着脱水程度的加重,种子细胞膜受损[12],细胞内大量电解质外渗[13],超氧阴离子过度积累[14]造成种子发芽率和生活力均显著下降[15]。抗氧化酶系统能够有效清除过度积累的活性氧[16],进而保护细胞免受不可逆损伤。
目前,石蒜属植物种子的相关研究集中在原生鳞茎的形成机制[9]、萌发特性[17]和储藏方式[18]等方面,鲜见种子脱水敏感性及其机制研究,这不利于石蒜属植物种子种质资源的保存与利用。鉴于此,本研究对红花石蒜种子在脱水过程中的细胞形态及生理指标变化进行研究,以期为阐明石蒜属植物种子脱水敏感性生理机制提供科学依据。
-
供试材料红花石蒜源自江西九连山,定植于江西农业大学第五教学楼前。2022年10月31日,选择红花石蒜完全成熟脱落的果实带回实验室,将种子从种壳中剥出并清除杂质,随后放入自封袋中备用。
-
种子含水量采用烘干法测定[19]。随机选择饱满、大小均匀一致的种子,称取种子鲜质量(W1),随后将其置于(103±2) ℃恒温干燥箱烘干,每隔2 h取出称量,2次称量不超过0.003 g即为种子恒量(W2)。每个处理重复3次,每个重复10粒种子。种子含水量=(W1−W2)/W1×100%。
-
将1.2.1同批次新鲜的红花石蒜种子放入尼龙网袋称量(W1),转入装有1 kg变色硅胶的密闭干燥容器中进行脱水。每2 h称取种子质量(W2),计算t时间内种子的脱水速率。每个网袋10粒种子,重复3次。脱水速率=(W1−W2)/t×100%。
-
采用单因素实验设计和硅胶脱水法[20],以1.2.1同批次红花石蒜未脱水种子(含水量为70%)为对照(ck),将种子均匀埋入硅胶中,使种子含水量快速脱水至60% (T1)、50% (T2)、40% (T3)、30% (T4)、20% (T5)、10% (T6),共7个处理。每个处理400粒种子,重复3次。分别测定这7个处理的种子生活力、相对电导率及发芽率,并将用于测定抗氧化酶活性和活性氧的种子投入液氮速冻,置于−80 ℃超低温冰箱内保存。
-
种子生活力采用氮蓝四唑法(TTC法)测定[19]。随机挑选大小均匀一致的成熟种子,经蒸馏水冲洗后剥除种皮并沿种脐纵向切开。将种子置于培养皿中并倒入质量浓度为0.5%的TTC溶液,转入人工气候箱中进行观察,设置温度为25 ℃,无光照。每个处理3次重复,每个重复30粒种子,以沸水处理的种子作为对照。于8 h后统计染色情况,种胚染为红色视作有生活力种子。种子生活力=(有生活力种子数/供检种子总数)×100%。
-
萌发试验采用培养皿滤纸法[21]。随机挑选大小均匀一致的成熟种子,经质量浓度为1%的高锰酸钾溶液消毒5 min后再用蒸馏水清洗,置于培养皿内,移入人工气候培养箱进行萌发试验。人工气候培养箱设置温度为25 ℃,湿度为55%,光周期为光12 h/暗12 h。每个处理重复3次,每次重复30粒种子。当胚根伸长至1 mm时,视为萌发,统计时间持续1个月,1个月后未萌发的种子视为不能发芽。在萌发试验进行期间及时清理污染发霉的种子并更换滤纸,同时补充蒸馏水保持滤纸湿润。种子萌发率=(发芽种子粒数/供试种子粒数)×100%。
-
参照韩建国等[22]的研究方法。随机选择大小均匀一致的成熟种子,经蒸馏水清洗后放入烧杯中,并加入100 mL蒸馏水,于25 ℃条件下静置24 h后用雷磁电导仪(DDS-307)测定种子电导率(S1);随后将封口的烧杯转入沸水浴中30 min,取出冷却至室温,测定种子电导率(S2)。以不加种子的纯水作为空白对照(S0),每个处理3次重复,每次重复10粒。种子相对电导率=(S1−S0)/(S2−S0)×100%。
-
将7个处理的红花石蒜种子沿种脐纵切,转入体积分数为50%的FAA溶液中固定,FAA溶液为V(甲醛)∶V(冰醋酸)∶V(体积分数为50%乙醇)=1∶1∶18。随后采用常规石蜡切片法制作样品切片,切片厚度为8 μm,采用番红-固绿进行染色,将获得的永久切片在正置荧光显微镜(ZEISS-Axio Imager.A2)下观察并拍摄图片。
-
采用ImageJ软件对7个不同含水量红花石蒜种子的永久切片进行测量,测量每个处理组胚乳细胞壁至细胞质之间的距离(mm),胚轴部分亦进行相同操作。每个处理重复3次,每次重复30个视野。
-
①活性氧:过氧化氢(H2O2)、超氧阴离子(${\mathrm{O}}^{·-}_2 $)质量摩尔浓度分别采用硫酸钛比色法及α-萘胺比色法(苏州科铭生物技术有限公司试剂盒)进行测定。②抗氧化酶:超氧化物歧化酶(SOD)、过氧化物酶(POD)、过氧化氢酶(CAT)活性分别采用WST-8法、愈创木酚及钼酸铵比色法(苏州科铭生物技术有限公司试剂盒)进行测定。
-
利用Excel 2019进行数据整理,采用SPSS 22.0进行方差分析和多重比较,用Origin 2021绘图。
-
从图1可见:新鲜成熟脱落的红花石蒜种子含水量为70%,外部圆润饱满,内部结构(种皮、胚乳、胚轴)完整,种胚发育完全,胚乳与胚轴的间隙较小,胚乳细胞明显大于胚轴细胞且结构更为清晰、紧密。种子石蜡切片经番红-固绿染色后,种子的细胞壁(绿色)、细胞质、淀粉粒及细胞核(均为红色)等结构清晰可见。当种子含水量失水至60% (T1)时,种皮、胚轴与胚乳的细胞均无明显变化;当种子含水量失水至50% (T2)时,胚轴与胚乳的间隙开始变大;而当种子含水量失水至40%时,种皮会出现明显的破裂和皱缩堆叠现象,胚乳与胚轴细胞的间隙继续变宽,个别还会质壁分离。
当种子含水量降至30%时,种皮进一步皱缩且无明显分层,胚轴与胚乳之间的间隙变宽,质壁分离现象明显。随着种子含水量的进一步减小,胚细胞降解加剧,细胞质内的淀粉粒也由颗粒状逐步转为片状(种子含水量为20%),分布密度明显增大,种皮细胞进一步堆叠成带状(10%),质壁分离现象更为普遍。
-
由表1可以看出:脱水会导致红花石蒜种子不同部位(胚乳和胚轴)的细胞发生质壁分离,质壁分离随脱水程度的增加而加剧。新鲜未经脱水处理(ck)的种子胚轴及胚乳细胞质壁分离程度明显低于其他处理,至种子含水量降至10% (T6)时,胚乳及胚轴细胞质壁分离程度均达到最大值。此外,在相同的脱水条件下,胚乳细胞的质壁分离程度通常高于胚轴,这可能是因为胚乳细胞含有更高的含水量,且细胞壁结构更容易在脱水过程中发生变化,从而导致明显的质壁分离现象。这一结果说明红花石蒜种子不耐脱水,且胚乳细胞对脱水更为敏感,严重的质壁分离会导致细胞活性降低甚至细胞死亡,进而影响种子的萌发。
处理 细胞壁至细胞质之间的距离/mm 胚乳 胚轴 ck 14.79±0.49 f 20.19±0.64 e T1 16.44±0.50 f 20.89±0.63 e T2 21.40±0.59 e 21.59±0.64 e T3 36.88±0.95 d 28.26±0.81 d T4 45.44±1.26 c 31.85±0.90 c T5 50.99±1.40 b 34.10±0.71 b T6 60.24±1.86 a 36.47±0.79 a 说明:不同小写字母表示不同处理间差异显著(P<0.05)。处理ck、T1、T2、T3、T4、T5、T6分别表示种子含水量为70%、60%、50%、40%、30%、20%、10%。 Table 1. Effect of dehydration on the degree of cytoplasmic wall separation of L. radiata seed cells
-
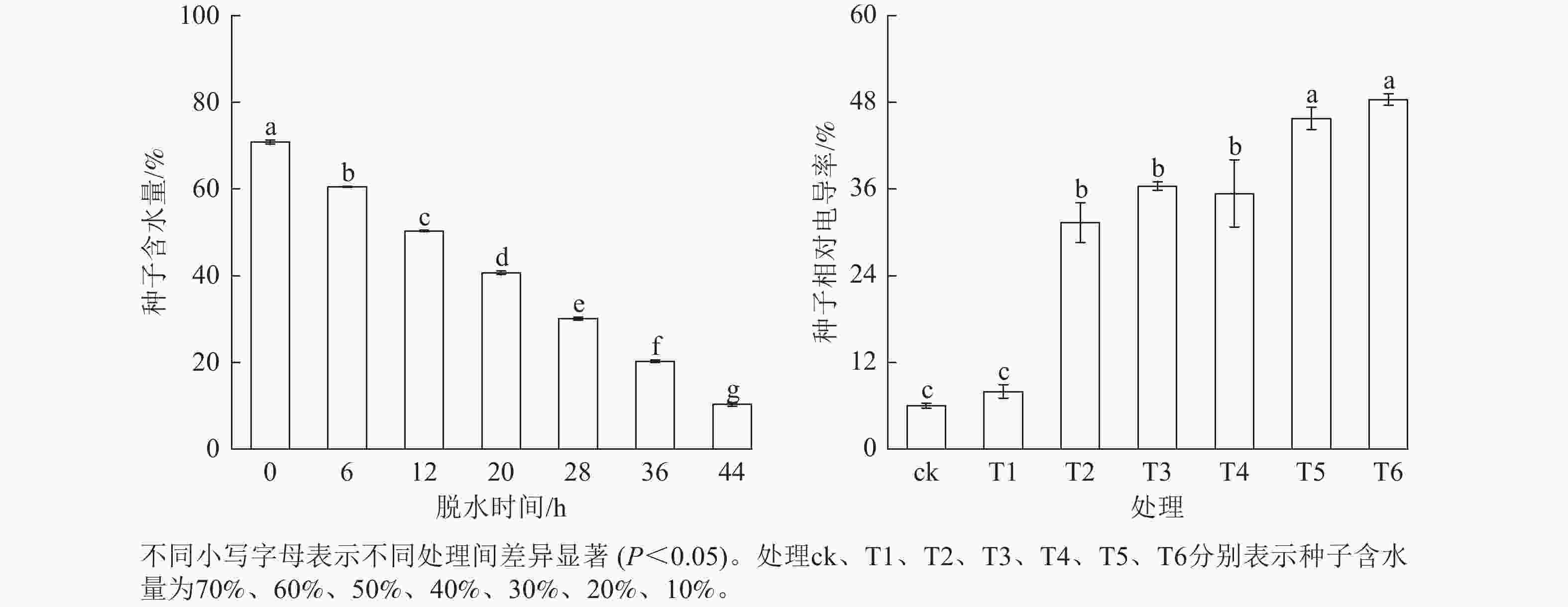
从图2可见:成熟脱落的红花石蒜种子平均含水量为70%,且会随着脱水时间的延长显著下降(P<0.05),而相对电导率则随着种子含水量的降低呈上升趋势。当种子含水量降至10% (T6)时,电导率升至最大;当含水量从30% (T4)降至20% (T5)时,电导率显著上升(P<0.05)。在整个脱水期间,相对电导率随含水量的下降显著上升(P<0.05)。可知,红花石蒜种子不耐脱水,脱水时间的延长使种子细胞膜结构受到破坏,细胞内的电解质大量外渗。
-
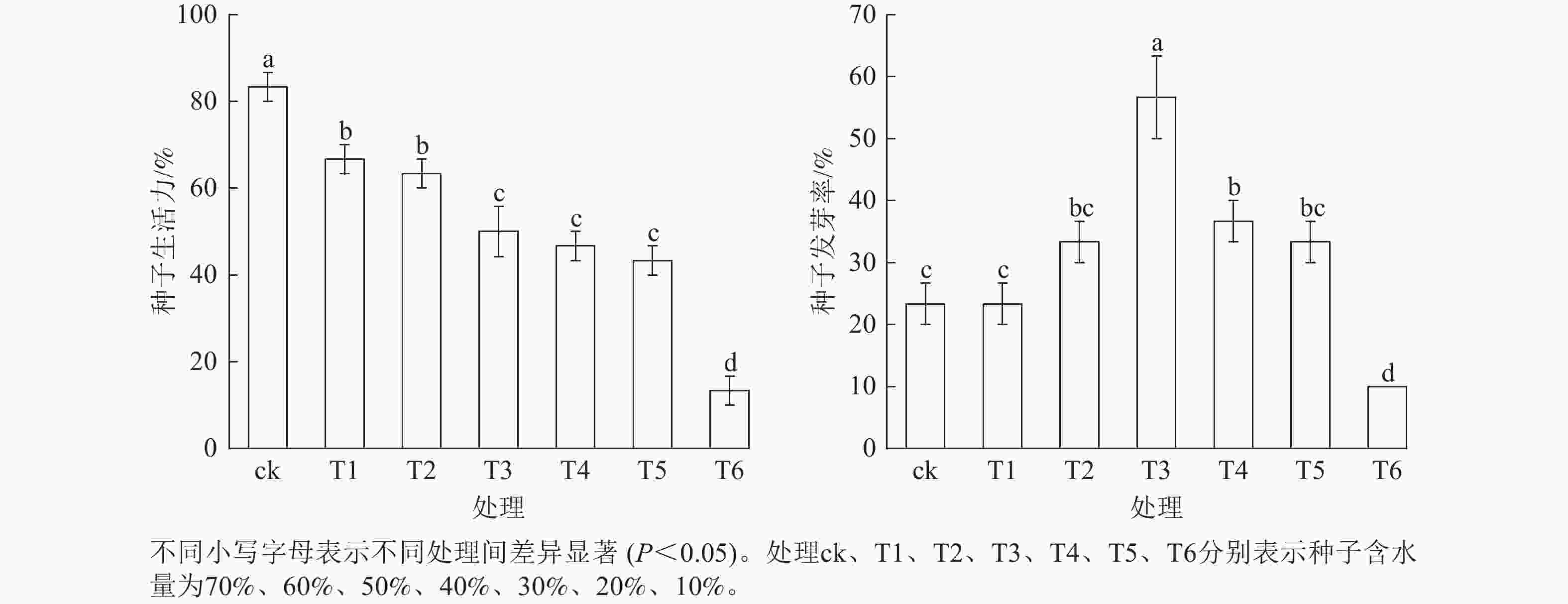
由图3可知:红花石蒜不同含水量种子的生活力及发芽率存在差异。随着种子含水量的下降,种子发芽率呈先升后降的变化趋势,种子生活力则呈下降趋势。其中,当种子含水量为40% (T3)时,种子发芽率最佳,与其他处理差异显著(P<0.05)。当种子含水量降至30%时,种子发芽率降至20%,与种子含水量为40%的处理相比,种子发芽率下降了20%,说明红花石蒜种子活力开始下降的临界含水量为30%~41%;当种子含水量降至10%时,种子发芽率降至10%,大部分种子失去萌发能力。
-
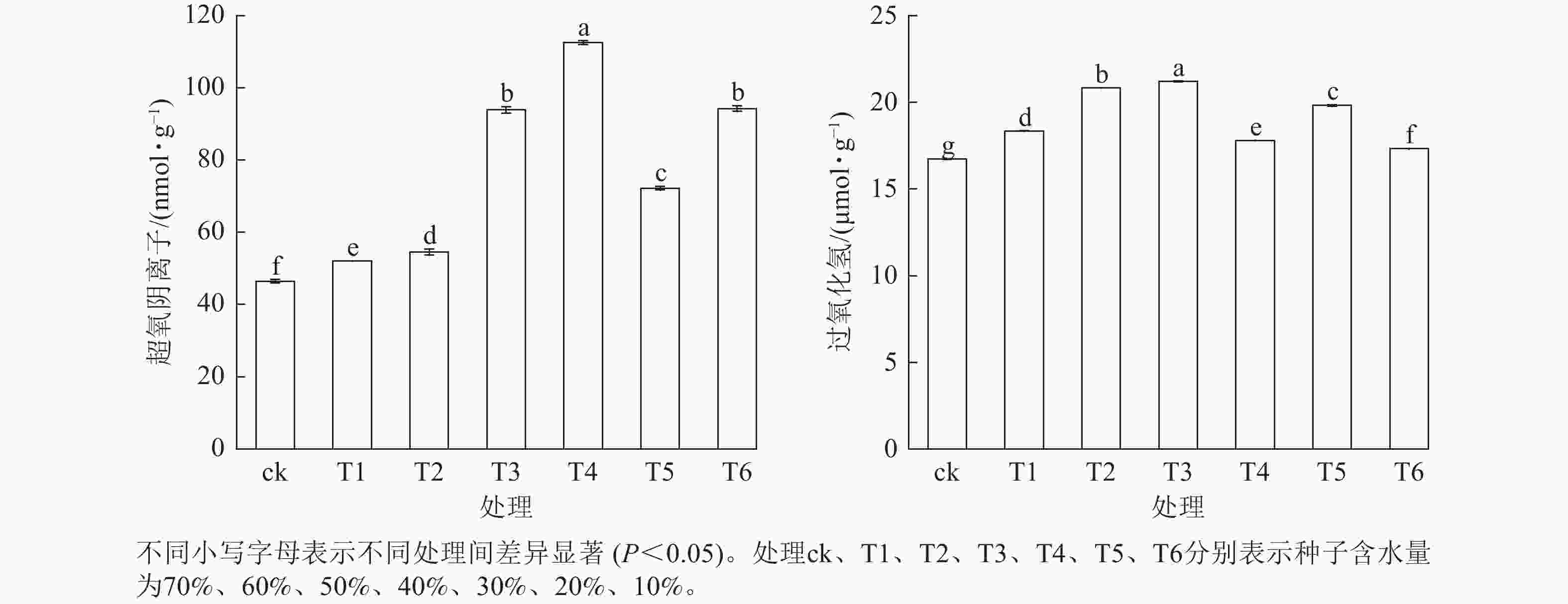
活性氧质量摩尔浓度在红花石蒜种子脱水过程中总体呈波动式上升趋势(图4)。当种子含水量从70% (T1)降至40% (T3)时,过氧化氢和超氧阴离子质量摩尔浓度均显著上升(P<0.05),变化趋势较为一致。而当种子含水量从40% (T3)降至10% (T6)时,过氧化氢和超氧阴离子质量摩尔浓度的变化规律恰好相反。当超氧阴离子质量摩尔浓度达到峰值时,过氧化氢质量摩尔浓度出现谷值,T5、T6处理变化趋势亦如此。
-
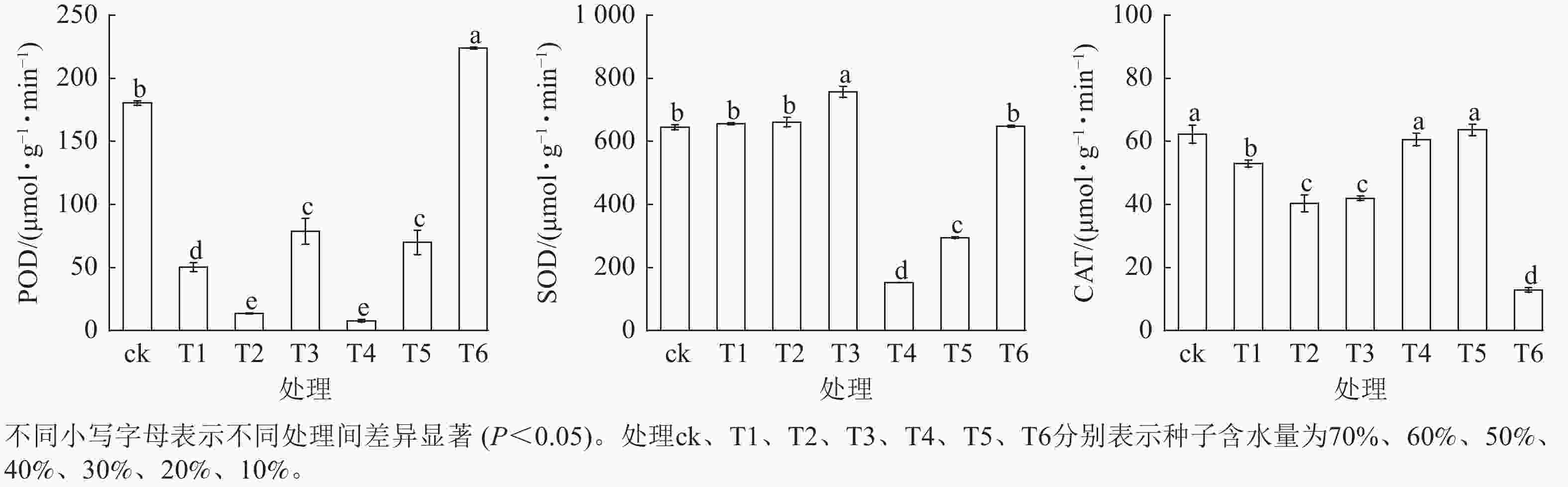
从图5可见:3种抗氧化酶活性在种子脱水过程中的变化较复杂。其中,POD和SOD的活性总体均呈上升趋势,而CAT活性则呈下降趋势。当种子含水量从70% (ck)降至40% (T3)时,SOD活性显著上升(P<0.05),而POD、CAT活性则显著下降(P<0.05);当种子含水量从30% (T4)降至10% (T6)时,SOD、POD变化趋势一致,均为先降后升的变化趋势,而CAT活性则呈先升后降的变化趋势。
-
为进一步探析红花石蒜种子脱水敏感性特征,将种子脱水过程中的含水量与种子生活力、萌发率、相对电导率、过氧化氢、超氧阴离子、POD、SOD、CAT等生理指标进行相关性分析。图6A表明:种子含水量与种子生活力呈极显著的正相关性(P<0.01),与CAT活性也呈显著正相关(P<0.05),而与相对电导率、过氧化氢质量摩尔浓度呈极显著负相关(P<0.01)。另外,种子发芽率与过氧化氢质量摩尔浓度呈极显著正相关(P<0.01),而与POD活性呈极显著负相关(P<0.01)。
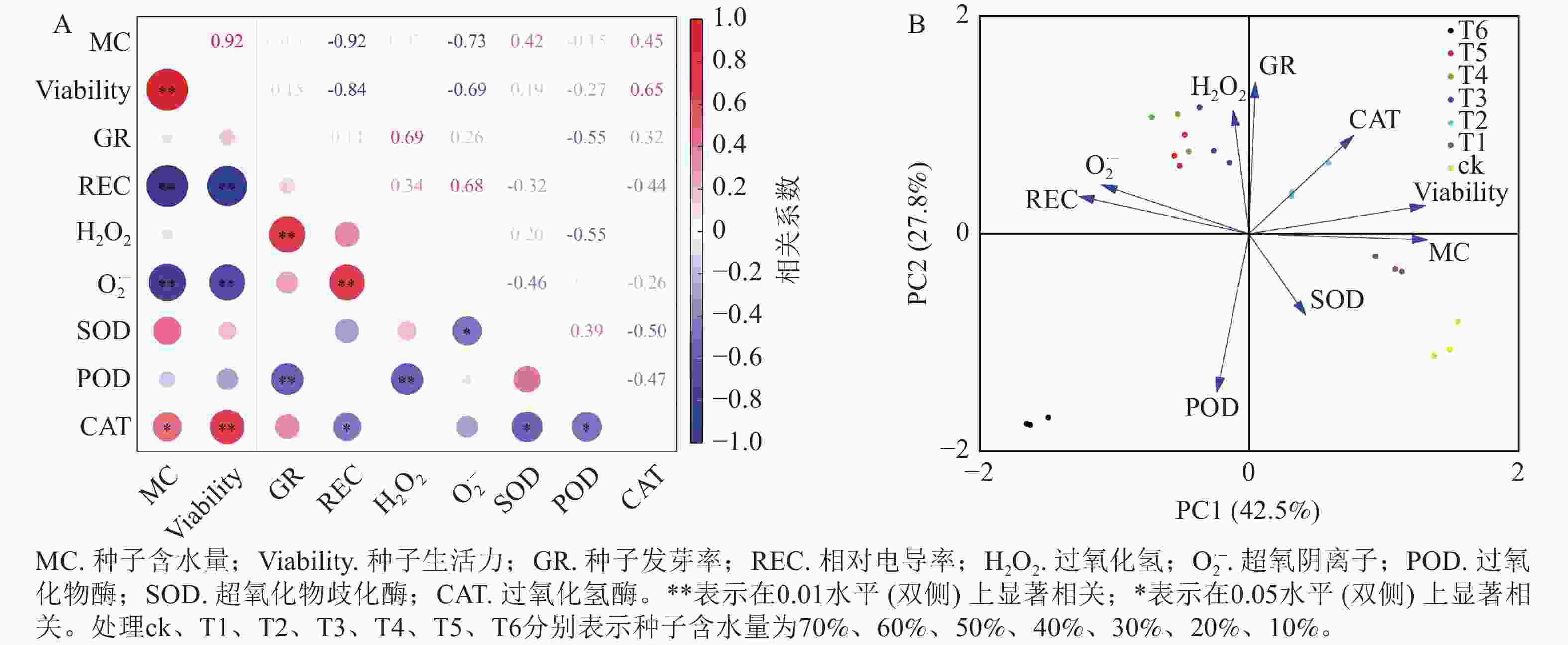
Figure 6. Correlation and principal component analysis of various indexes in the process of dehydration
主成分分析(图6B)表明:种子含水量对红花石蒜种子的脱水敏感性影响最大(即第1主成分,贡献率为42.5%,权重为0.496 4),其次为种子发芽率(即第2主成分,贡献率为27.8%,权重为0.522 0)。由此得知,种子的含水量和发芽率可作为衡量红花石蒜种子脱水敏感性的重要指标。
-
相对电导率是评价植物遭受逆境胁迫时电解质外渗率的重要指标,能够直接反映植物细胞组织受伤害的程度[23]。本研究表明:红花石蒜种子成熟脱落时含水量高达70%,在整个脱水期间含水量显著下降、电解质浓度显著上升,这与桑寄生Taxillus chinensis[12]和桢楠Phoebe zhennan[24]顽拗型种子对脱水胁迫的反应一致。说明红花石蒜种子在母株上并未经历成熟脱水阶段,在脱水进程中细胞膜的损伤程度较高,对脱水行为极为敏感。此外,当红花石蒜种子含水量降至40%~50%时发芽率显著增加,而含水量进一步降低至30%时,发芽率却显著下降,当含水量降至10%时,种子发芽率低至新鲜种子发芽率的一半,这与正常种子的现象截然不同,正常种子能够忍受脱水至5%~10%的含水量,且不影响发芽率。说明轻度脱水有助于提高红花石蒜种子的发芽率,但种子活力存在明显的临界含水量阈值,当该阈值低于40%时,种子活力会显著减弱。可见,含水量是调控红花石蒜种子萌发过程的关键因素,同时也是影响种子有效保存的重要限制条件之一。因此,为保持红花石蒜种子的最佳保存状态,种子含水量应维持在30%~40%,含水量降至10%时,种子活力已达到半致死状态。
-
顽拗型种子在脱水过程中活性氧自由基过度积累,对种子造成脱水损伤[25−26],抗氧化酶系统能够有效保护种子的结构和功能完整性。其中,SOD的主要作用是将有害的超氧阴离子歧化为过氧化氢,从而保护植物免受氧化应激的损害,但过氧化氢水平亦取决于POD和CAT的分解程度[27]。在本研究中,SOD活性在种子脱水过程中呈先升后降的变化趋势。这与见血封喉Antiaris toxicaria[28]和南洋杉Araucaria bidwillii[29]种子脱水过程中SOD活性变化的趋势一致。当红花石蒜种子含水量降至30%时,SOD和POD活性均降至最低值,超氧阴离子质量摩尔浓度达到峰值,细胞结构表现出明显的受损特征。这说明种胚在遭遇脱水胁迫时CAT活性虽有显著上升,但依靠单一抗氧化酶并不能有效抵御活性氧的氧化伤害作用,细胞仍会出现质壁分离等不可逆损伤,进而影响其细胞功能。在红花石蒜种子含水量降至10%~20%时,细胞间隙逐渐变宽,质壁分离现象加剧,细胞质内的淀粉粒由颗粒状转为片状,细胞中的红色面积明显变大。这与板栗Castanea mollissima种子经历重度失水后出现细胞降解,最终导致种胚活力完全丧失的研究结果一致[30]。由此可知,红花石蒜种子过度脱水使抗氧化酶系统和细胞的调控力下降,最终导致种子失去活力。
-
红花石蒜的种子在脱落时,胚已基本发育成熟,种子含水量和生活力均较高。在密闭条件下,采用硅胶脱水法将种子的含水量从70%降至10%仅需44 h,其临界含水量和半致死含水量分别是40%和10%。在失水过程中,种子外部形态呈逐渐皱缩、干瘪状态,失水严重时种子细胞内还会发生明显的质壁分离现象,胞内淀粉粒也由颗粒状逐渐转为片状,还会伴有大量的细胞降解现象,种子内的CAT活性、含水量和生活力均显著下降,活性氧质量摩尔浓度、POD和SOD活性则显著上升。红花石蒜的种子含水量与发芽率显著相关,轻度脱水有利于其种子萌发,但过度失水会使种子生活力很快丧失。为保证种子发芽率,在种子储藏时应将含水量控制在30%~40%。综上,红花石蒜种子在形态与生理方面,均表现出明显的脱水敏感性特征。
Morphological and physiological changes during the dehydration process of Lycoris radiata seeds
doi: 10.11833/j.issn.2095-0756.20240480
- Received Date: 2024-08-06
- Accepted Date: 2025-02-20
- Rev Recd Date: 2025-01-23
- Available Online: 2025-05-30
- Publish Date: 2025-05-30
-
Key words:
- Lycoris radiata /
- dehydration sensitivity /
- recalcitrant seeds /
- viability /
- germination rate /
- plasmolysis
Abstract:
| Citation: | SA Rina, CAI Junhuo, WEI Xuying, et al. Morphological and physiological changes during the dehydration process of Lycoris radiata seeds[J]. Journal of Zhejiang A&F University, 2025, 42(3): 572−580 doi: 10.11833/j.issn.2095-0756.20240480 |











 DownLoad:
DownLoad:




