-
随着农村劳动力结构和农民观念的变化,设施园艺作物生产对机械化的需求愈发迫切[1−2]。园艺作物标准化栽培是机械化发展的前提。无土栽培作为一种集约、高效、标准化的栽培模式,可以摆脱土壤束缚[3],通过精准的营养供应和环境调控,显著提高作物产量和品质,节约水肥资源,是实现农业现代化生产和标准化种植的重要途径。
然而,传统无土栽培使用泥炭、椰糠等散体基质,存在基质装填与更换操作繁琐、标准化程度不及营养液无土栽培等问题,难以满足机械化作业要求。成型栽培基质具有水气比可控、质量轻、外形美观、可塑性强、使用简单方便等优点[4],是提高设施园艺生产机械化和自动化水平的有效途径。目前,成型园艺种植泡沫以岩棉种植块及合成树脂聚氨酯泡沫为主,其中岩棉生产能耗高、体积大运输成本高、难降解且回收成本高。聚氨酯泡沫成本较低,但以石油基聚合物为原料,具有不可再生性、难降解、环保性差等缺陷。为此,研究人员常用泥炭或其他可降解原料与聚氨酯复合制备具有一定降解能力的成型栽培基质[5−6],但仍以石油基原料为主。
植物纤维泡沫材料因其原料可再生、来源广泛、易于制备等优势受到广泛关注,并应用于建筑、包装、运输等领域[7]。然而,这种具有良好孔隙结构的泡沫材料在无土栽培基质领域的相关研究报道十分有限。由于疏松通气、水肥传导性强、可再生等优势,近年来木纤维已成为重要的无土栽培基质原料。木纤维存在水肥吸持性能差等缺陷,使用木纤维制备的泡沫,因配方和成型工艺研究不足,常伴随着孔隙粗大、结合力弱、结构均匀性较差等问题[8]。为此,本研究以木纤维为骨架,复合壳聚糖凝胶,通过机械发泡协同冻融定型-介质解冻工艺,制备一种孔隙结构均匀且有良好水肥吸持性能的木纤维-壳聚糖全生物质泡沫。本研究通过成型工艺研究明确木纤维-壳聚糖泡沫的最佳成型方法及配方比例,引入柠檬酸交联体系改善泡沫水肥吸持性能,并以萝卜Raphanus sativus进行栽培试验,探讨泡沫的栽培应用性能。
-
杂木纤维,景谷永盛林业综合开发有限公司提供。冰乙酸(AA)分析纯,天津市风船化学试剂科技有限公司;壳聚糖(CS,分子量为30 000),上海麦克林生化科技股份有限公司;柠檬酸 (CA)分析纯,成都市科隆化学品有限公司;质量分数为98%的曲拉通(TX-100),北京索莱宝科技有限公司;氢氧化钠分析纯,成都市科隆化学品有限公司;无水乙醇分析纯,成都市科隆化学品有限公司;氯化钠分析纯,国药集团化学试剂有限公司;曙红分析纯,天津市科密欧化学试剂有限公司。
JE3002电子天平,上海浦春计量仪器有限公司;DF-101S集热式恒温加热磁力搅拌器,巩义市创元仪器制造有限公司;BCD-407WSPZM(E)电冰箱,合肥美的电冰箱有限公司;101型电热鼓风干燥箱,北京市永光明医疗仪器有限公司;SUST
5569 万能力学试验机,珠海市三思泰捷电气设备有限公司;TESCAN MIRA4扫描电子显微镜,泰思肯(中国)有限公司;Thermo Nicolet iS5傅里叶红外光谱仪,赛默飞世尔科技公司;DDQ-B02L1打蛋器,小熊电器股份有限公司;Nexsa X射线光电子能谱仪,赛默飞世尔科技公司。 -
准确称取一定量的壳聚糖溶解于质量浓度为1%的冰乙酸溶液中,充分溶解后,得到壳聚糖水凝胶预聚体。加入一定量的柠檬酸 40 ℃反应1 h后混合纤维。然后,加入一定比例的表面活性剂TX-100后搅打发泡。研究不同质量分数的表面活性剂对成型工艺的影响。分别采用直接烘干的方式和冻融定型-介质解冻后烘干的方式对成型工艺进行探讨。
-
准确称取一定量的壳聚糖置于质量浓度为1% 100 mL的冰乙酸溶液中充分溶解,形成透明均相溶液后,加入一定比例的柠檬酸,在40 ℃下反应1 h,得到壳聚糖水凝胶预聚体。随后加入过60目筛的杂木纤维,经过打蛋器搅拌2~3 min,静置30 min,使壳聚糖水凝胶预聚体与木纤维充分混合。加入一定比例的表面活性剂TX-100,利用打蛋器搅拌2~3 min发泡。经机械发泡后的泡沫放入冰箱中,在−20 ℃下冷冻12 h,随后于室温中解冻得到初步成型的泡沫。随后分别在水、乙醇、氢氧化钠溶液中洗涤3次,去除未反应的试剂。最后将泡沫置于80 ℃的烘箱中进一步反应干燥24 h,得到木纤维-壳聚糖泡沫,其中未添加柠檬酸的泡沫为壳聚糖-木纤维泡沫(W-CS),添加柠檬酸的泡沫为柠檬酸交联壳聚糖木纤维泡沫(W-CS-CA)。
-
利用乙醇作为置换溶液,首先将泡沫浸入定量体积(V1)的无水乙醇溶液中,记录此时泡沫和无水乙醇的总体积(V2),5 min后取出样品,记录此时无水乙醇体积为(V3)。孔隙率(P,%)通过公式(1)计算:
-
用数显游标卡尺测量出泡沫的长、宽、高,并计算泡沫样品的体积(V),用电子分析天平称出样品的质量(m),样品的表观密度(ρ)通过公式(2)计算:
-
将干燥的样品置于分析天平上称量并记录,随后分别浸入装有蒸馏水和质量分数0.9% 氯化钠溶液的烧杯中24 h,取出后放滤筛上静置1 min后称量。吸水(盐)率(Q)通过公式(3)计算:
式(3)中:M1为吸液前样品的质量,M2为吸液后样品的质量。
-
根据文献报道[9],将干燥的样品放入足量水中静置,待样品吸饱水后,放入40 ℃的鼓风干燥箱中,每隔1 h进行1次称量与记录,连续记录12 h。失水率(W,%)通过公式(4)计算:
式(4)中:m1为充分吸水后的样品质量;m2为失水后的样品质量。
-
参照GB/T1041—1992《塑料压缩性能试验方法》,采用三思万能力学试验机(珠海市三思泰捷电气设备有限公司)对样品的压缩性能进行测试,压缩速率为2 mm·min−1。
-
将干燥的样品放置于装有质量分数为0.2%的曙红溶液的培养皿中,记录样品30 min内吸附情况。样品吸附程度(C,%)通过公式(5)计算:
式(5)中:mt1为原有的曙红溶液质量,mt2为剩余的曙红溶液质量。
-
干燥后的实验原料及样品通过傅里叶红外光谱仪进行结构表征,采用溴化钾压片法测试泡沫材料的结构特征,扫描范围为4 000~500 cm−1,扫描次数为32次,分辨率为4 cm−1。利用X射线光电子能谱分别测量凝胶的碳(C)、氢(H)、氮(N)谱。
-
样品的表面微观形貌特征使用TESCAN MIRA4型扫描电镜进行观察。用导电胶带将样品固定在观察台上,用离子溅射仪进行喷金处理,扫描加速电压为15 kV,放大倍数根据需要调整。
-
选取商用聚氨酯泡沫作为对照,分别在每种样品上种植9颗萝卜种子,每种样品重复3组。栽培12 d,测定植株的根长、茎长以及茎粗形态学。
-
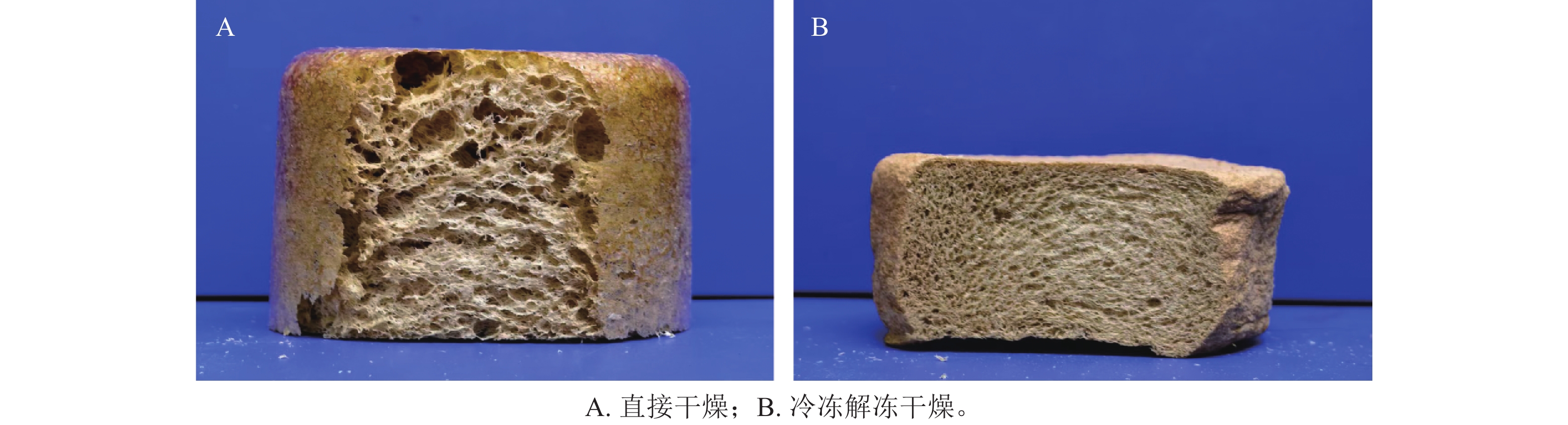
通过前期预实验发现:木纤维-壳聚糖泡沫通过机械发泡直接干燥成型,所得泡沫的孔隙结构极不均匀(图1A)。当前,冻融定型-介质解冻这一处理过程被广泛应用于制备壳聚糖物理水凝胶,利用低温冷冻,并在室温下解冻的方式可以使壳聚糖分子链部分单元重新排列,其分子链上的氨基可与交联剂相互作用形成交联点[10]。在介质解冻的过程中壳聚糖溶解度降低从而致使壳聚糖重新凝固,理论上可通过与柠檬酸进行一定程度的交联,有助于构建更加稳定的交联网络结构,应用于木纤维-壳聚糖泡沫制备,同时稳定泡沫孔隙结构。本研究在机械发泡后,经冷冻稳定泡沫形态,室温解冻,进行干燥定型后,得到的泡沫材料如图1B所示。可见增加冻融定型-介质解冻制备的泡沫,切面呈现出均匀的孔隙结构,因此,后续试验中泡沫制备工艺均采用冷冻-解冻后干燥成型方法。
-
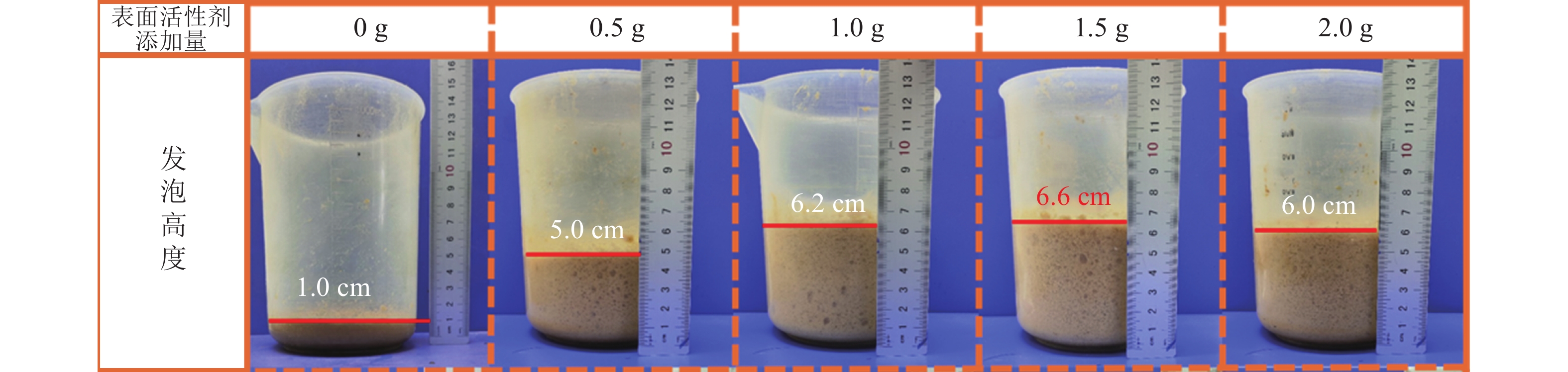
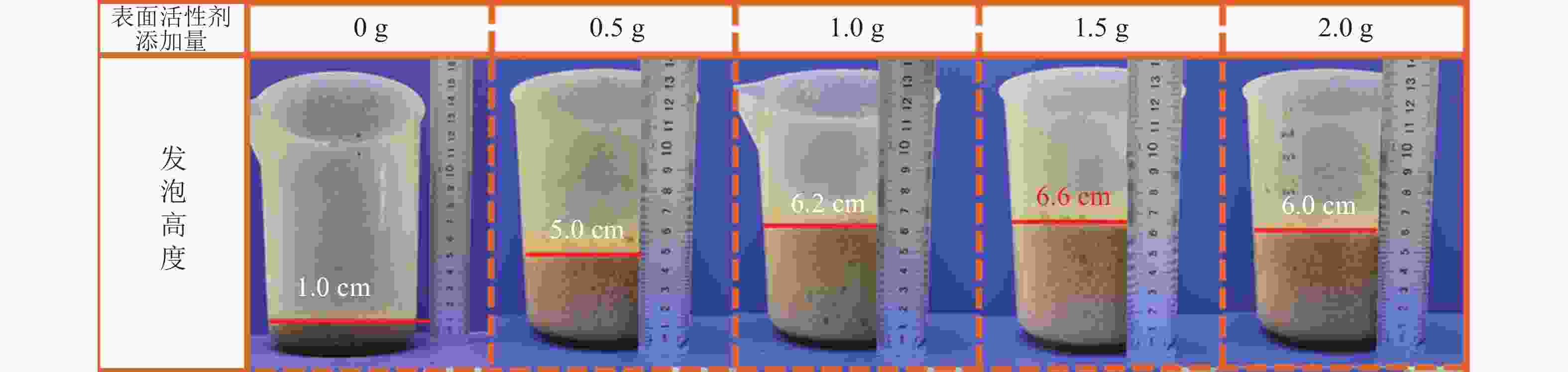
本研究采用机械搅打物理发泡的方式,因此表面活性剂使用量对泡沫的孔隙度和孔隙结构有显著影响,进而影响木纤维-壳聚糖泡沫的综合性能。图2为不同TX-100添加量经机械搅打后泡沫体积变化情况。在不添加表面活性剂的情况下,机械搅打难以形成气泡网络,最终成型的材料结构致密。添加TX-100后,随着使用量的增加,发泡体积也呈现上升趋势,当TX-100添加大于1.0 g时,发泡高度没有明显的变化,均保持在6~7 cm,干燥后的发泡材料也维持在4~5 cm的高度。这是因为虽然表面活性剂的用量决定了发泡材料的体积以及内部孔隙的分布情况,但两者之间不存在绝对的线性关系。许多研究表明:随着表面活性剂添加量增加,发泡体积逐渐上升,但当表面活性剂添加量超过临界胶束浓度(CMC)时表面张力不再降低,溶液的起泡性不会继续增大[11],表现为泡沫的体积也不再变化。
-
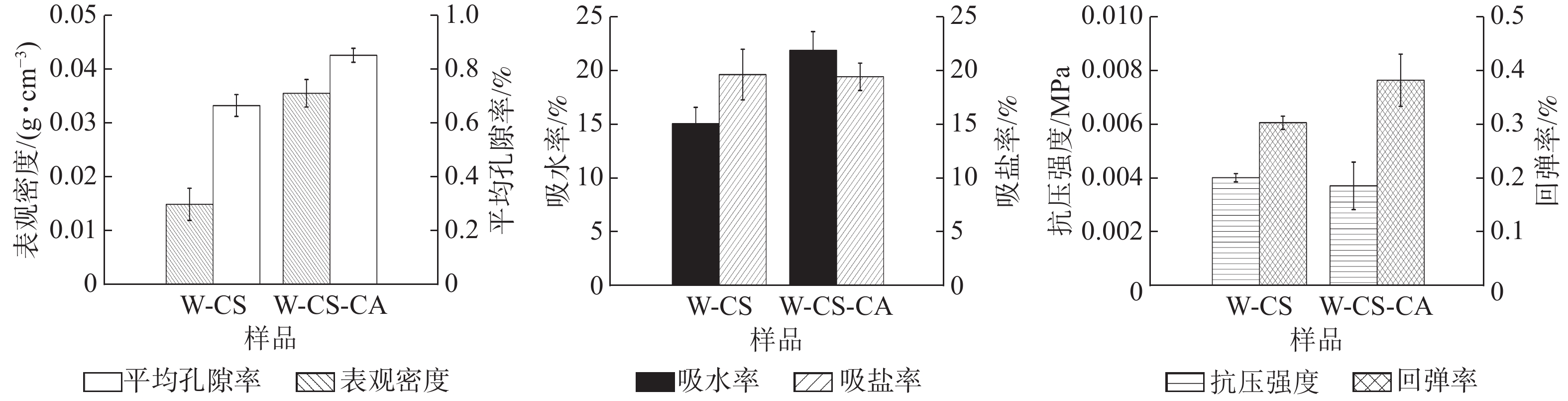
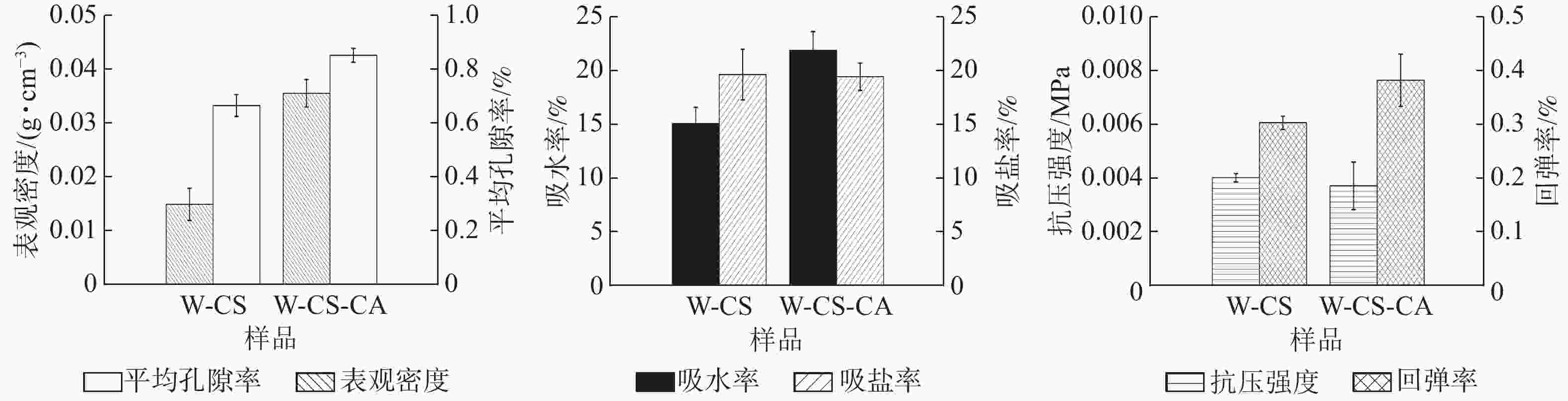
由图3可知:W-CS-CA的表观密度、孔隙率、吸水度和回弹率相对于W-CS均呈上升趋势。这是由于泡沫交联度的增加使泡沫的整体结构稳定性增强[12]。柠檬酸的加入并没有明显改变泡沫的抗压强度。考虑到W-CS-CA泡沫的表观密度较高,所以可能存在柠檬酸破坏壳聚糖在木纤维表面的加固作用[13],致使壳聚糖凝胶分散在孔隙内。在泡沫的吸盐率方面,壳聚糖氨基与柠檬酸羧基间发生的反应致使壳聚糖消耗部分具有吸盐能力的氨基官能团[14],但由于添加量较小,致使其吸盐能力降低并不明显。
-
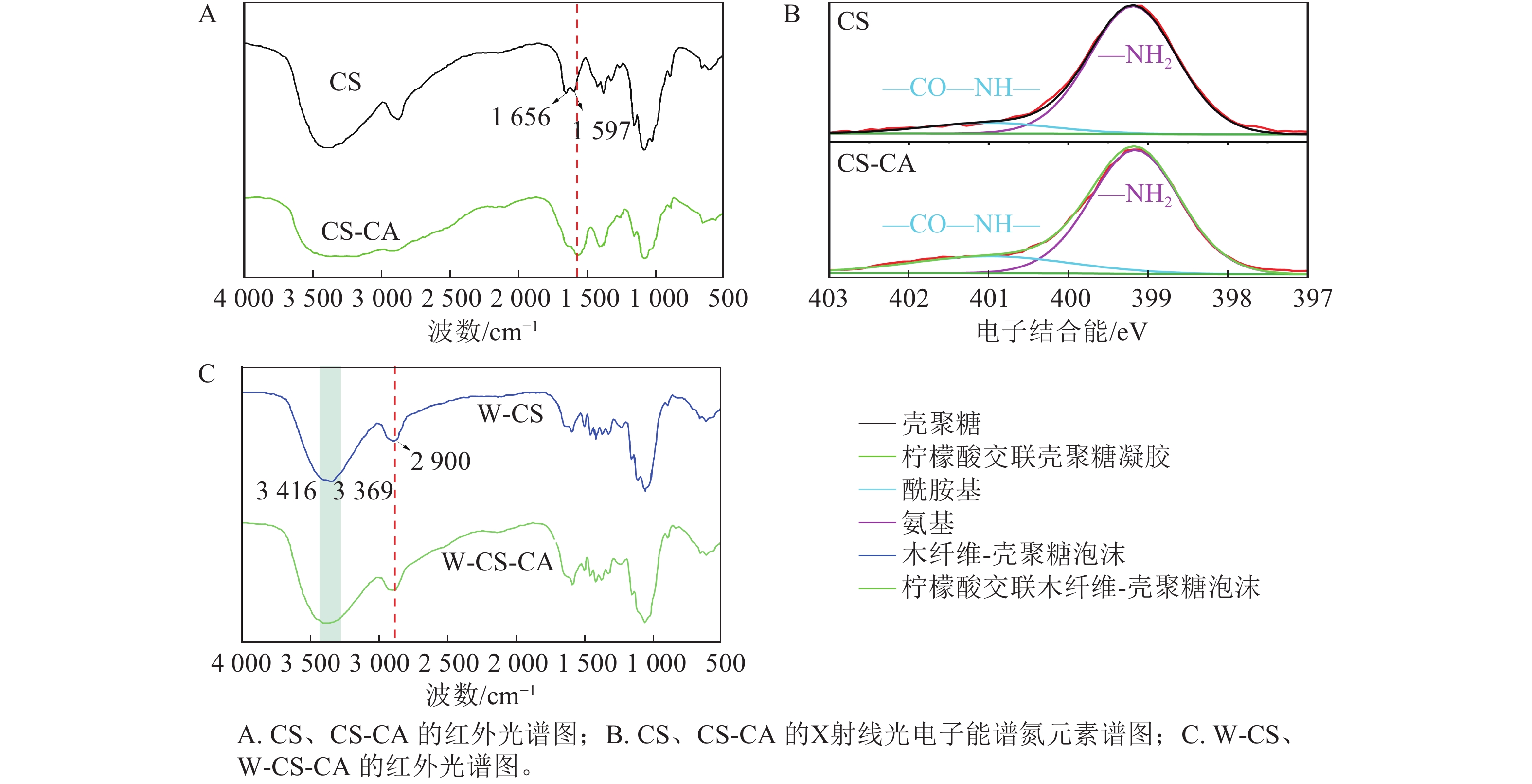
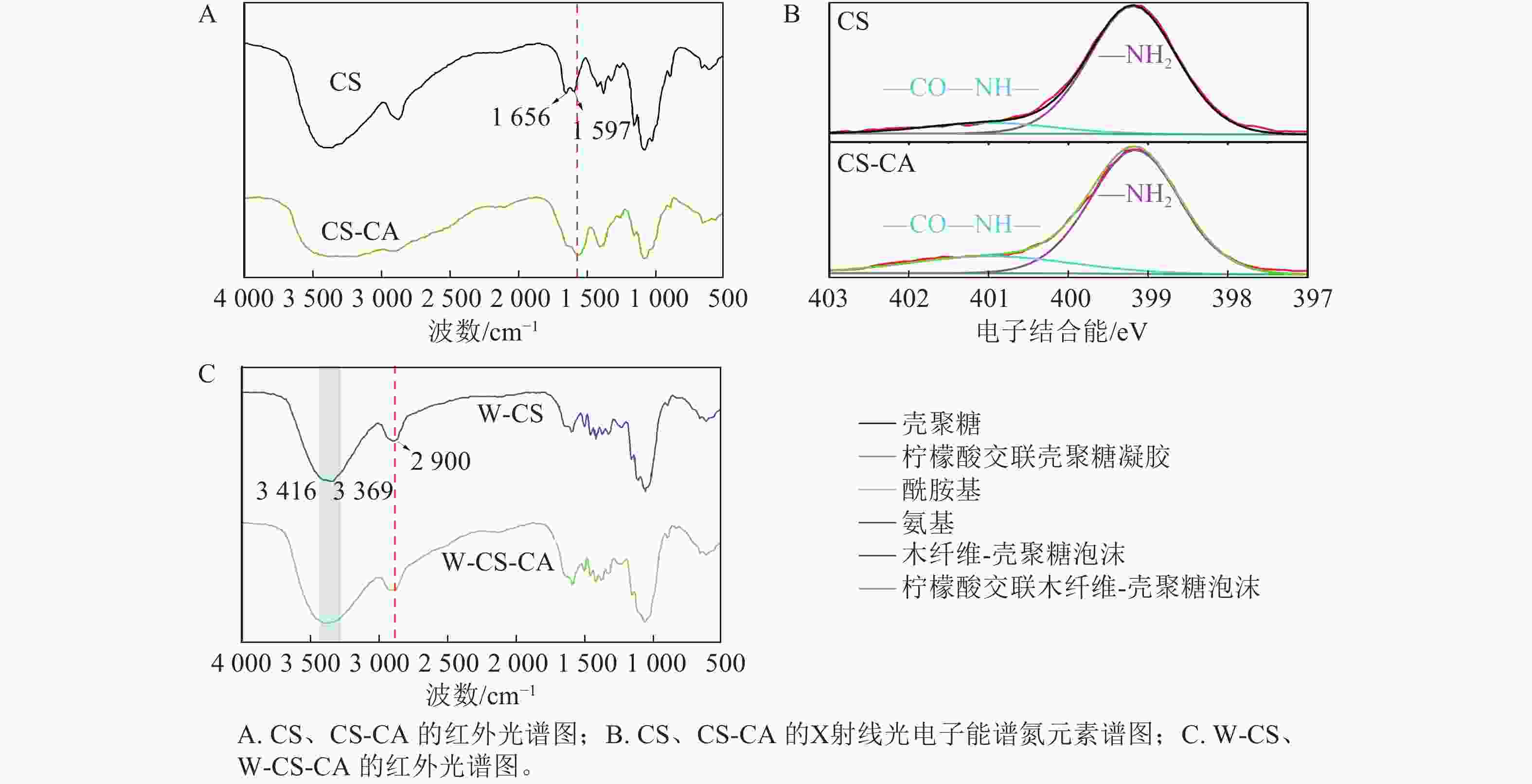
为探究W-CS-CA中主要交联组分壳聚糖的化学结构变化,对未添加木纤维的CS、CS-CA进行红外光谱分析。图4A可以明显观察到壳聚糖中位于
1656 和1597 cm−1的酰胺Ⅰ的C=O伸缩振动峰和酰胺Ⅱ的N—H弯曲震动峰在交联后峰面积明显增大[15],证明该条件下壳聚糖和柠檬酸之间成功发生酰胺化反应,从而形成了更多的酰胺键。对未添加木纤维的CS、CS-CA进行N谱的X射线光电子能谱技术(XPS)分析(图4B),可见添加柠檬酸后,酰胺键的强度发生轻微的增强而氨基发生轻微的减弱。这是由于壳聚糖和柠檬酸之间的酰胺化反应[16],并且由于柠檬酸的总量较少致使反应起峰值变化不够明显。为进一步解释纤维对该泡沫化学变化的影响,对W-CS、W-CS-CA进行红外光谱分析(图4C),观察到当木纤维添加到柠檬酸壳聚糖溶液后,W-CS-CA在2 900 cm−1 出现了新峰,来自纤维素、半纤维素中—CH2和—CH3的吸收振动峰之和[17],该峰在W-CS也有观察到。同时,在3 416~3 369 cm−1都存在的羟基官能团,从峰的大小上可以观察到W-CS-CA拥有更多的羟基官能团,并且从峰的强度可以观察到W-CS-CA中含有更多的氢键[18]。这与泡沫的吸水度观察到的现象一致。
-
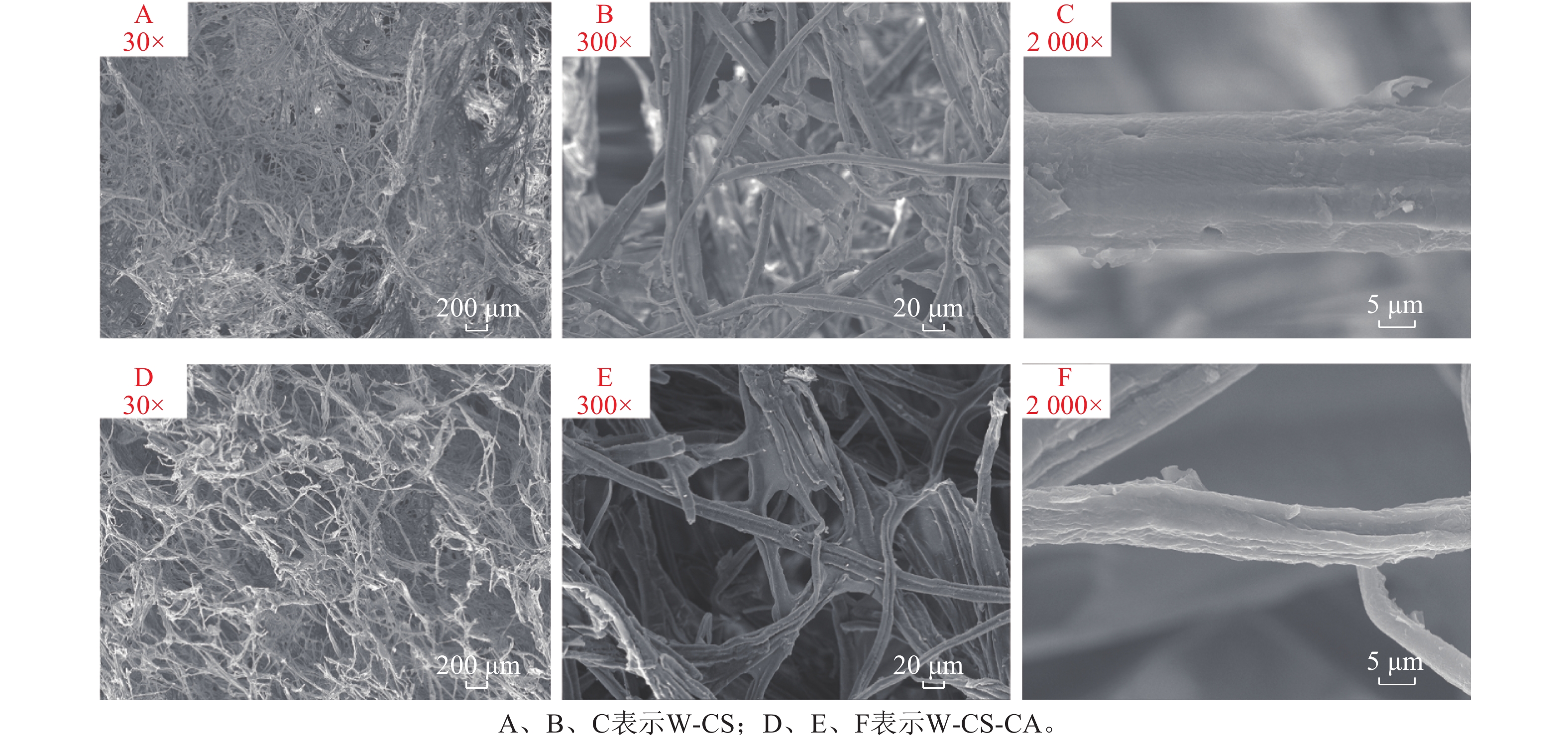
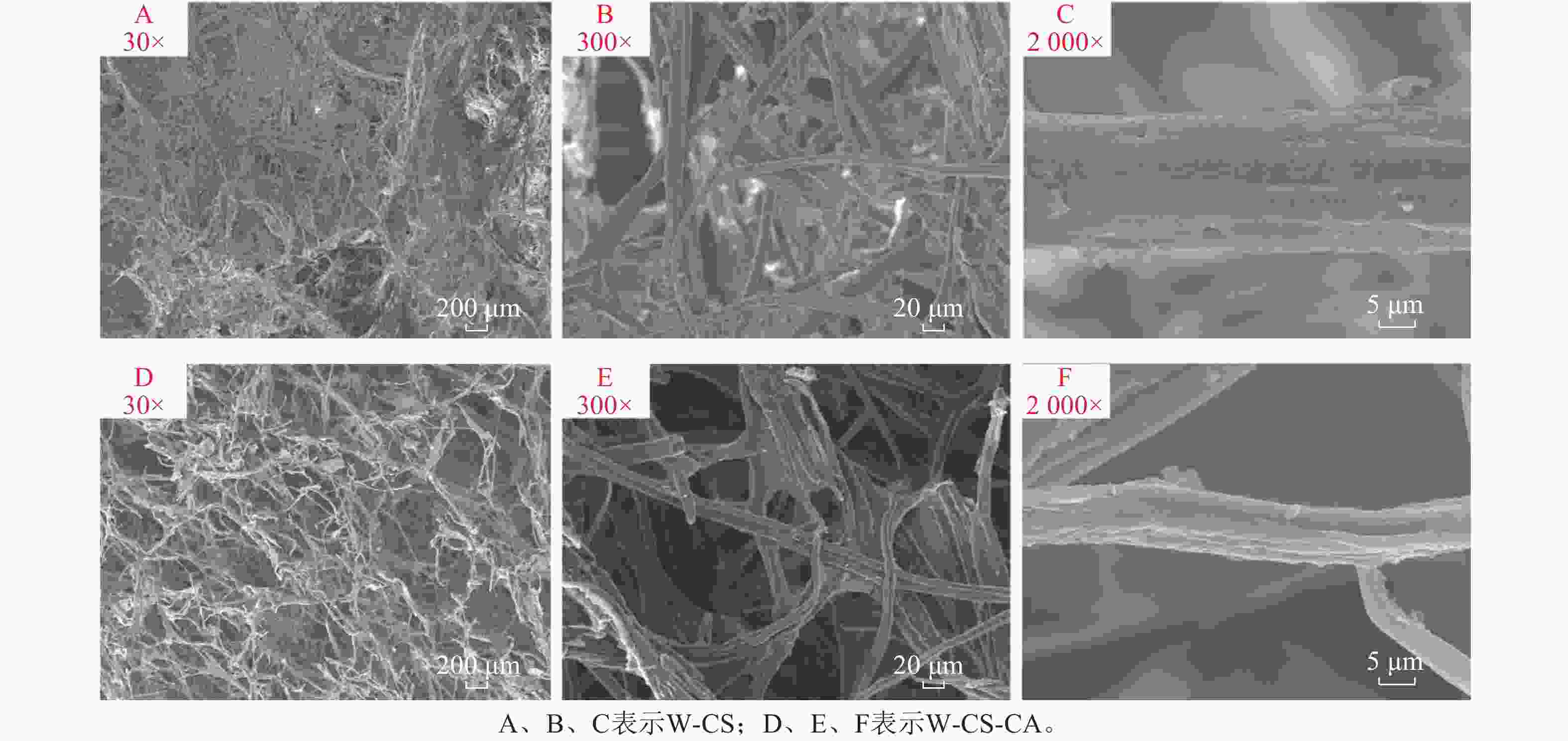
图5为不同放大倍数的木纤维-壳聚糖泡沫的扫描电镜图。放大倍数为30倍的扫描电镜图下,可以观察到W-CS的孔隙结构并不明显,并且纤丝之间的交织成大面积的网状结构。W-CS-CA纤维的孔隙结构更为明显,并且纤丝之间明显分散从而增加了泡沫的孔隙率。此种纤维的交织结构也与LUJAN等[19]的研究结果相似。放大300倍后可以明显观察到纤丝间主要依靠壳聚糖凝胶相互连接。同时从图5可以观察到随着交联度的上升,单根纤丝表面从较为光滑变为粗糙。这是由于柠檬酸的添加致使包裹在纤丝上的壳聚糖之间形成了更强的相互作用,并且由于交联度的上升,粗糙的纤丝表面有效增加了水分子与凝胶的氢键结合面积,有利于泡沫保水能力的上升。
-
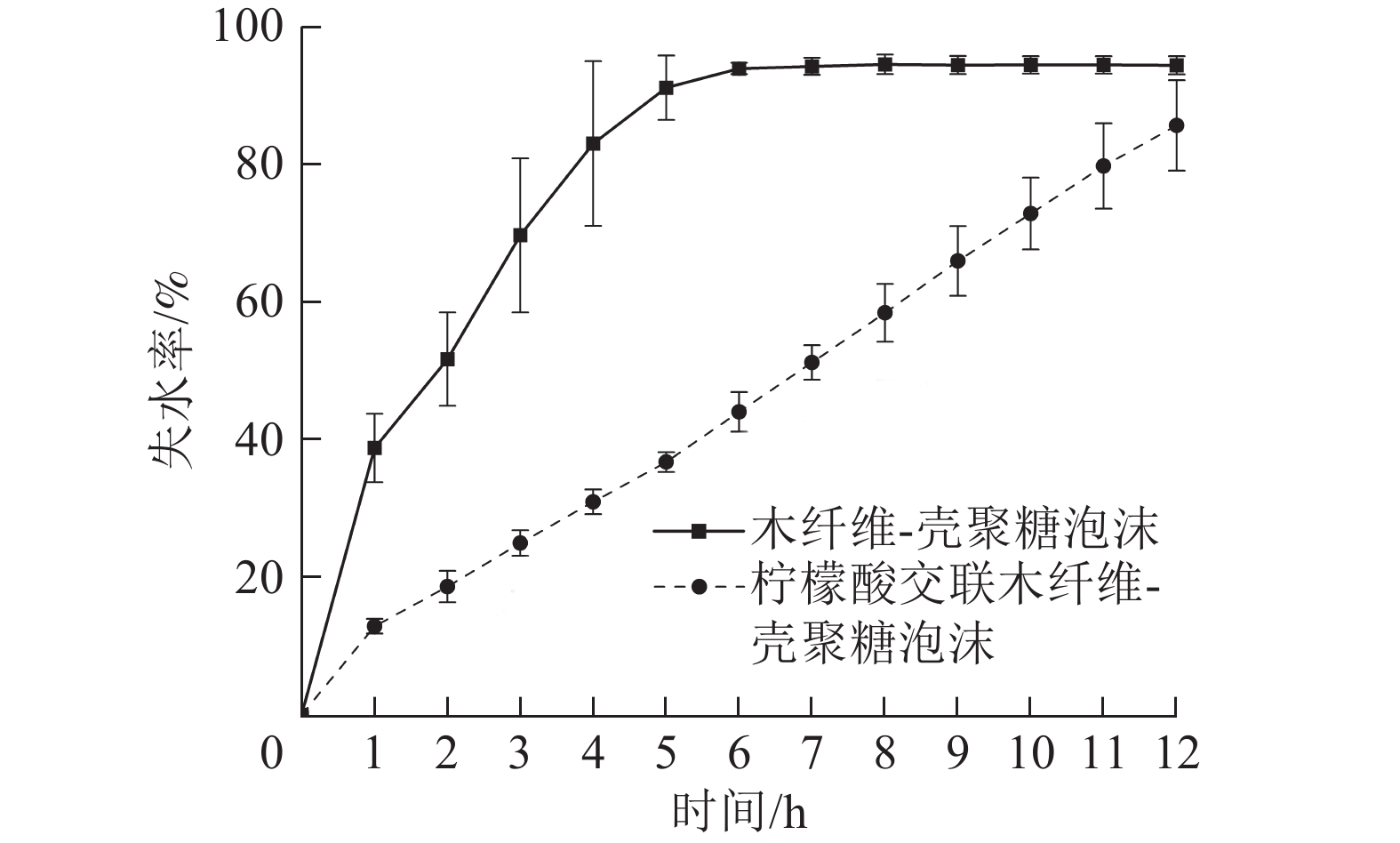
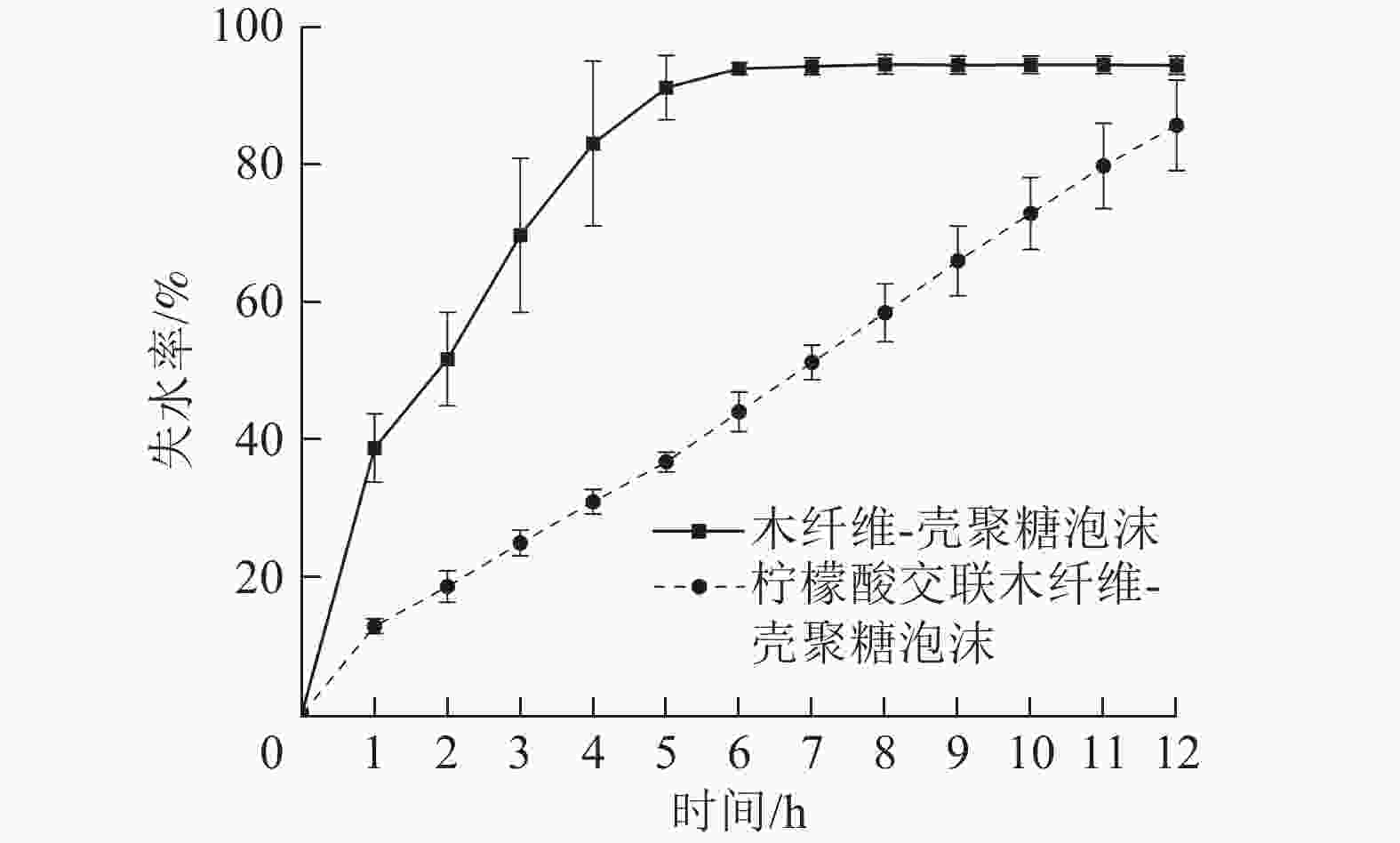
保水性能体现了栽培基质泡沫材料保持水分的能力,保水性能差的栽培基质材料易干燥,不利于水分和养分储存,需要频繁提供水肥,增加栽培管理难度。由图6可知:在40 ℃下,W-CS泡沫5 h时失水率已达91.35%,6 h已趋近完全失水状态。由于柠檬酸的加入,W-CS-CA泡沫具有更高的孔隙率,比表面积有所提升,在5 h时失水率仅为36.81%。
-
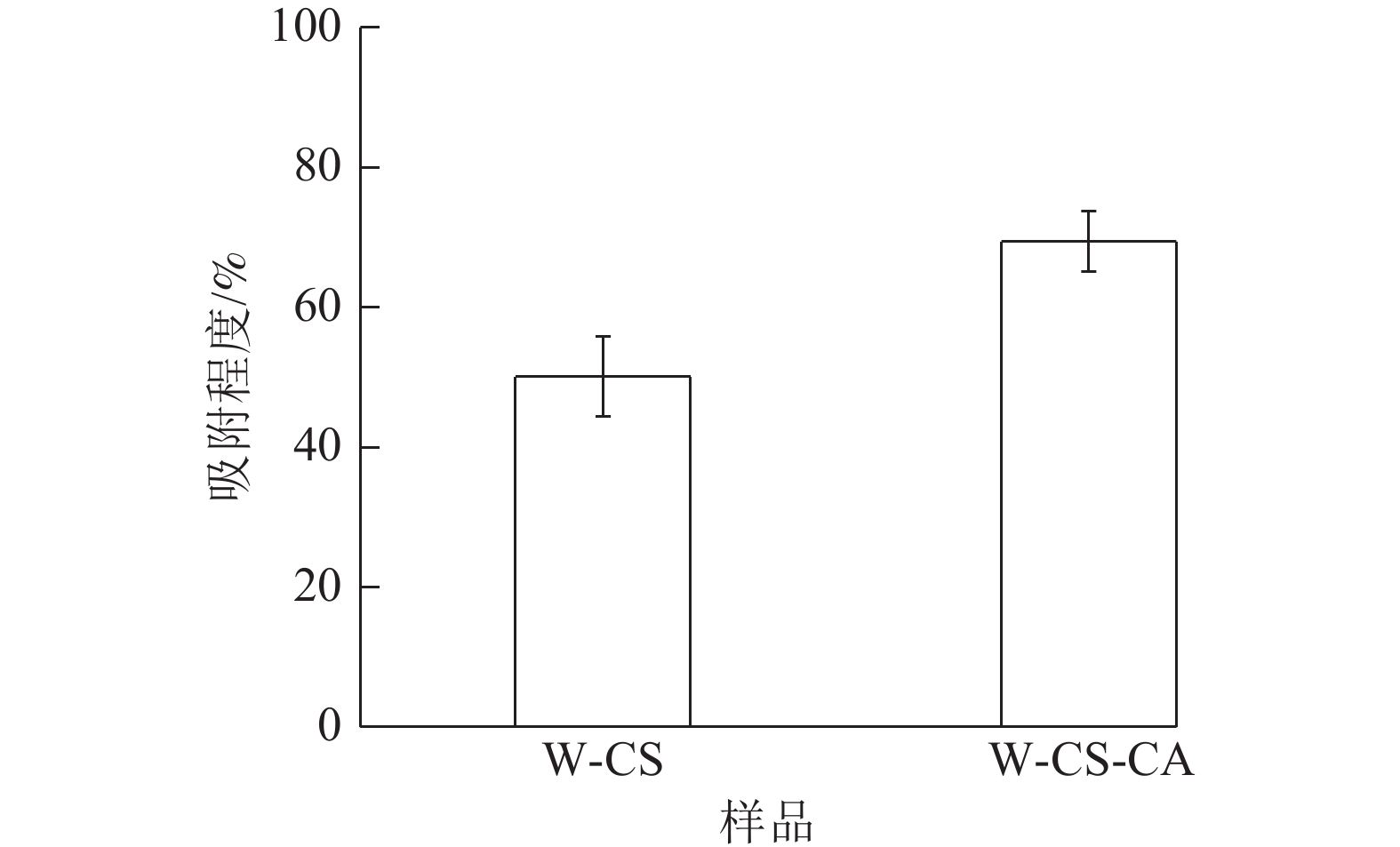
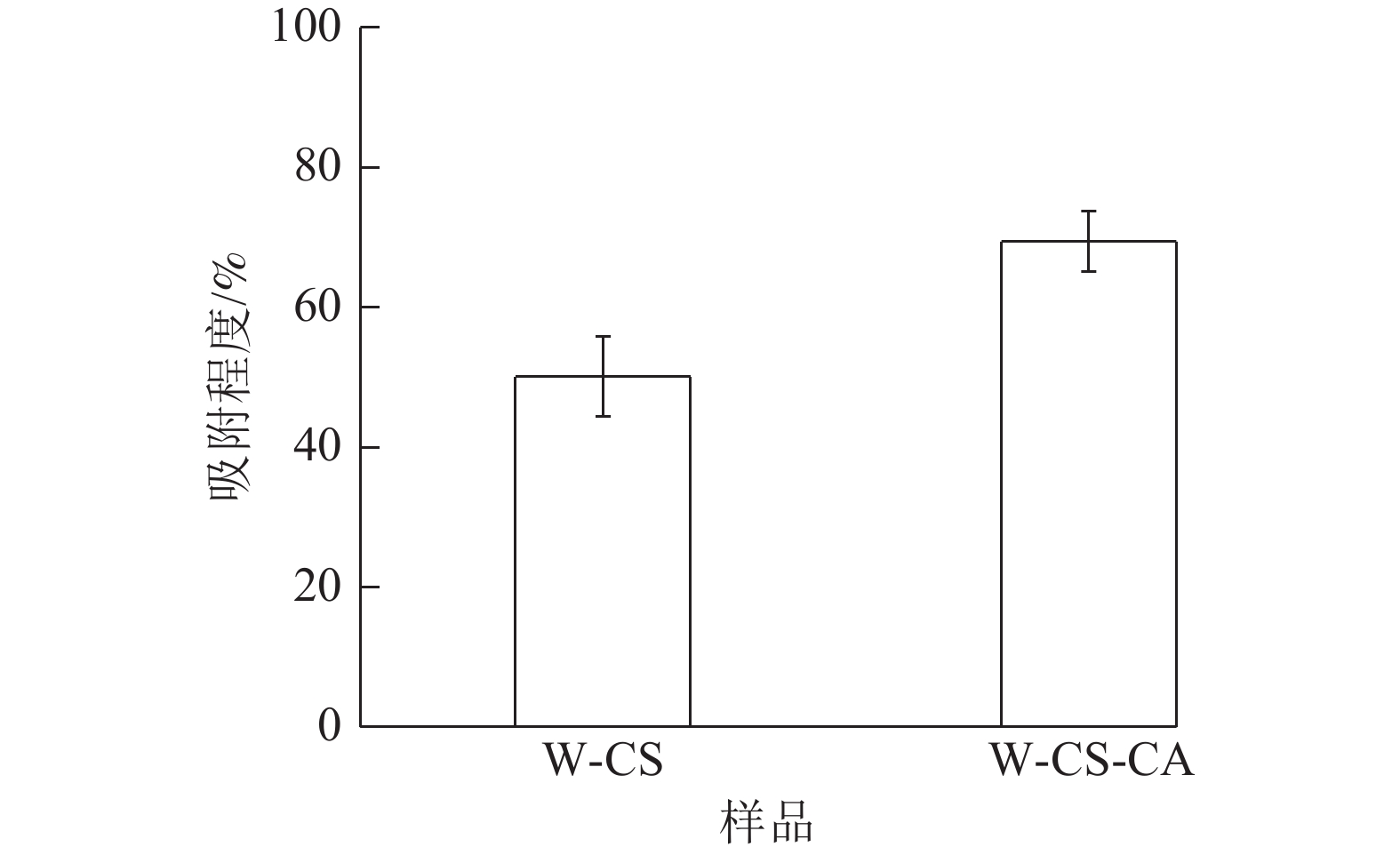
水吸力以基质在一定时间内所吸收的水分、水分所能达到的最大高度以及吸附速率作为评价依据[20],是评价基质导水能力强弱的方法,一定程度上表现了基质作为栽培基质的可能性。由图7可知:W-CS泡沫吸附程度为50.06%。W-CS-CA吸附程度达69.41%。W-CS-CA泡沫纤丝更为分散从而形成更多的毛细管结构,具有更高的吸附程度。
-
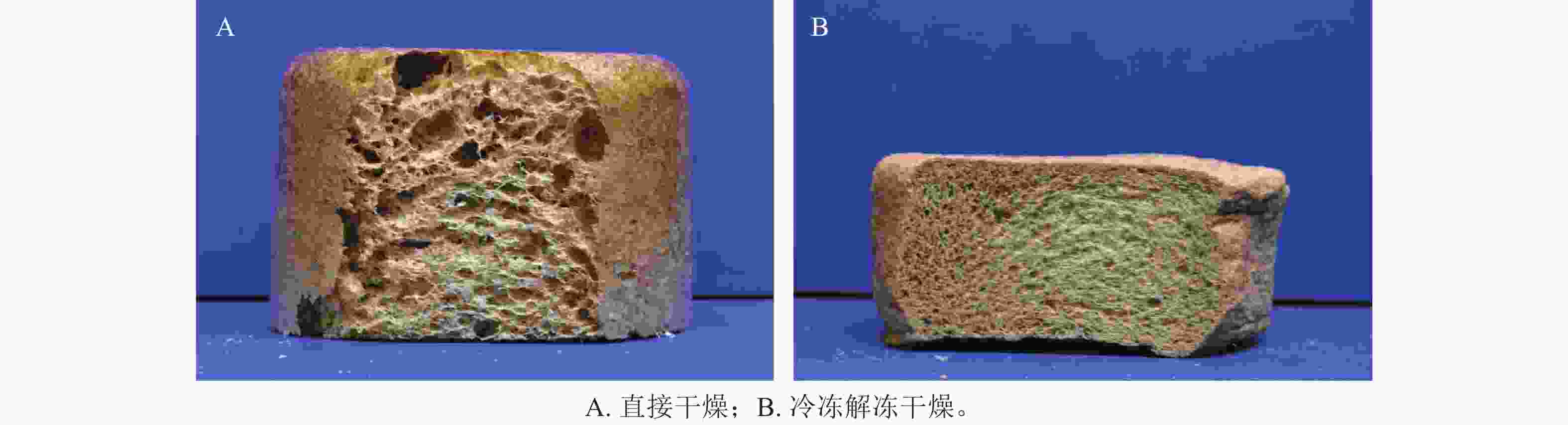
在W-CS、W-CS-CA中萝卜种子均可发芽生长。由表1可知:栽培在W-CS-CA中的萝卜株高、根长、茎粗均优于栽培在W-CS中。一方面是由于柠檬酸增加了泡沫的交联度从而产生了更多的持水孔隙,另一方面归因于交联形成的壳聚糖凝胶,改善了泡沫的持水持肥能力[21]。整体而言,萝卜苗在制备泡沫中的形态学指标均低于对照组,可能与泡沫组分中植株生长抑制成分浓度过高有关[22]。但本研究制备工艺条件下,木纤维-壳聚糖泡沫在栽培过程中可保持良好泡沫形态,说明在无土栽培基质领域有良好应用前景。
编号 株高/cm 根长/cm 茎粗/mm 对照组 5.11±1.26 3.70±1.00 1.30±0.09 W-CS 1.94±0.91 0.86±0.72 0.71±0.67 W-CS-CA 2.46±0.69 3.00±2.12 1.10±0.49 说明:对照组为商用聚氨酯泡沫。W-CS表示利用木纤维-壳聚糖泡沫所栽培的植株;W-CS-CA表示利用壳聚糖交联木纤维-壳聚糖泡沫所栽培的植株。 Table 1. Physiological indicators of plants in control group, W-CS and W-CS-CA
-
木纤维-壳聚糖泡沫的制备工艺包含机械发泡、冷冻定型以及介质解冻。成型成分主要分为发泡液(壳聚糖溶液)、表面活性剂(曲拉通)以及解冻介质(氢氧化钠溶液、水、乙醇)。发泡液是由一定质量分数的壳聚糖溶液组成,壳聚糖质量分数直接关系到溶液的黏度,从而影响泡沫在相同发泡条件下的泡沫密度。当壳聚糖的质量分数过高,机械发泡后的发泡体积明显降低,形成较为密实的泡沫。反之,当壳聚糖质量分数过低,难以形成大孔隙结构从而影响植物根系的呼吸。在未添加表面活性剂的情况下,通过单一的机械搅打,难以形成均匀的孔隙,并且由于发泡液的水分较高,在干燥过程中大量水分从泡沫内部蒸发,体积膨胀,破坏孔隙结构,导致泡沫内部孔隙较大,难以维持机械发泡时形成的稳定孔隙形态,无法保证泡沫的整体性能。本研究采用表面活性剂TX-100是典型的非离子表面活性剂,是聚乙二醇的衍生物,可以有效降低气体分子从空气相向水相的扩散速率[23],同时还拥有良好的乳化性能,可以将发泡体系中的固相与液相均匀分散在一起,形成乳液或悬浊液,有助于稳定混合物的状态,使纤维分散更加均匀,从而增加泡沫的稳定性。结合机械发泡法,利用高速搅拌的方式带入大量空气形成庞大的气泡网络结构,同样也有助于木纤维的均匀分散。工艺的最后采用冷冻定型的工艺可以使湿泡沫保存机械发泡工艺中形成的大量气泡,并且壳聚糖逐渐凝胶化,从而附着在木纤维表面加固结构。冷冻后的泡沫在介质解冻的过程中,由于壳聚糖柠檬酸凝胶的溶解速度相对较慢,与介质中的水溶液充分接触稀释了泡沫中的酸性物质,同时在碱性介质中进一步提高了泡沫体系的pH,最终阻碍了壳聚糖的继续溶解[21]。
-
在不添加柠檬酸的条件下,由于壳聚糖分子链没有交联剂的限制,与木纤维的复合体系在机械搅打过程中,气泡会部分合并,从而无法形成均匀的孔隙。并且,由于木纤维的目数大小不同,壳聚糖更容易附着在小目数的木纤维上进一步造成W-CS的孔隙不均[24],最终致使泡沫的孔隙率下降,并伴随着表观密度的上升以及吸水度、吸盐度的下降。在柠檬酸交联条件下,柠檬酸与壳聚糖之间形成酰胺键,使壳聚糖凝胶形成更加牢固的相互作用力,凝胶形态更为规整,在发泡的过程中形成的气泡可以均匀地存在于溶液中,从而获得更高的孔隙率,致使柠檬酸交联壳聚糖-木纤维泡沫具有更加优异的孔隙率,从而获得更高的吸水度。
-
未添加柠檬酸的条件下,部分种子难以在泡沫中生长,或者生长出的植株根系小,难以支撑植株生长以及营养的吸附,其子叶黄化过快。这是由于W-CS形成的大片网状结构,造成部分种子根系难以进入泡沫中,减少了植株对于水分的吸收。柠檬酸交联条件下,泡沫的孔隙结构更加均匀,根系更加容易进入泡沫中,所以柠檬酸交联条件下的植株具有一定的根系以及茎长,但是可以明显观察到柠檬酸交联条件下的植株侧根明显发达。一般情况下植株的侧根生长发达可能是由于水分和磷元素缺乏造成的[25]。本研究在育苗实验中主要以蒸馏水作为浇灌用水,在植株的实际生长过程中会由于缺乏营养元素而生长更多的侧根。
-
本研究以木纤维为骨架,复合壳聚糖凝胶,在表面活性剂作用下,通过机械发泡协同冻融定型-介质解冻工艺,能有效构建泡沫材料的孔隙结构,改善直接烘干法气泡不均匀以及孔隙率较低的问题。此外,柠檬酸交联能够有效提升木纤维-壳聚糖泡沫的吸水性能以及孔隙度,表现出较好的保水能力以及水吸力。同时,在育苗试验中萝卜长势仅次于聚氨酯泡沫栽培基质,表明该方法制备的泡沫具备一定的应用潜力。
Preparation of wood fiber-chitosan biomass foam and its application in soilless cultivation
doi: 10.11833/j.issn.2095-0756.20250349
- Received Date: 2025-06-22
- Accepted Date: 2025-11-28
- Rev Recd Date: 2025-11-17
-
Key words:
- agricultural and forestry residue /
- wood fiber /
- chitosan /
- molding substrate /
- soilless cultivation
Abstract:
| Citation: | TANG Zhi, CHEN Xinyi, ZHOU Xiaojian, et al. Preparation of wood fiber-chitosan biomass foam and its application in soilless cultivation[J]. Journal of Zhejiang A&F University, 2025, 43(X): 1−9 doi: 10.11833/j.issn.2095-0756.20250349 |









 DownLoad:
DownLoad: