-
花色是植物观赏价值的重要性状之一,呈色主要取决于细胞内含有的色素[1]。山茶Camellia的花色主要有红色、粉色和复色[2],其丰富的花色主要由花瓣中花青苷决定。目前山茶中已鉴定出25种花青苷[3]。LI等[4−5]利用核磁共振、质谱和紫外可见光谱等技术在滇山茶Camellia reticulata中鉴定出5种含2-O-木糖基及相应不含2-O-木糖基的花青苷,在滇山茶‘大理茶’C. reticulata ‘Dalicha’中鉴定出10种含2-O-木糖基的花青苷,在香港红山茶C. hongkongensis中鉴定出8种矢车菊素和4种飞燕草素花青苷。李辛雷等[6−7]在山茶品种中鉴定出7种矢车菊素花青苷,发现随着山茶品种花色加深,花瓣中主要花青苷的含量也在增加。山茶花青苷的研究为其花色形成机制及花色育种提供了科学依据。
杜鹃红山茶C. azalea和越南抱茎茶C. amplexicaulis花色艳丽,花期较长,具有较高的观赏价值,是四季茶花新品种培育的优良亲本材料[8]。肇庆棕榈谷花园有限公司以杜鹃红山茶为母本,以越南抱茎茶为父本进行杂交,获得杂交后代进而培育出四季茶花新品种[9]。目前,有关杜鹃红山茶的研究集中于生理特性、栽培、繁殖等方面[10−11],而越南抱茎茶主要是对其光合特性[12]、无性繁殖等[13]进行了研究。花青苷研究方面,李辛雷等[14]通过特征颜色反应证明了杜鹃红山茶花瓣的红色源于花色素及其苷;杨美英等[15]明确了杜鹃红山茶与山茶品种‘媚丽’C. japonica ‘Meili’杂交后代花青苷的变化。本研究利用超高效液相色谱-质谱联用技术,对杜鹃红山茶、越南抱茎茶及其18个杂交后代花朵中花青苷的成分与质量分数进行分析,研究其花色与花青苷的关系,明确其花青苷遗传变异特征,为山茶种质创新及高效利用提供科学依据。
-
试验材料为母本杜鹃红山茶(D)、父本越南抱茎茶(Y)及其18个杂交后代(DY),均来源于肇庆棕榈谷花园有限公司。选取生长环境相同、长势一致的植株各5株,每株随机采集盛开期花朵3朵,用于花色测定及花青苷定性定量分析。
-
从每份新鲜花朵中随机选取5朵,应用NF555色差计对其外轮花瓣的上表皮中央部分进行花色测定。按照国际照明委员会(CIE)制定的CIE L* a* b*表色系法,利用色差计对花瓣的明度(L*)、色相(a*与b*)、彩度(C*)和色调角(h)等指标进行数字化测定。L*表示明暗变化程度;a*表示红绿色变化程度,a*为正值,数值越大,花色越红;b*表示黄蓝色变化程度,b*由负到正,蓝色减退黄色增强;C*描述色彩的鲜艳程度,C*越大,颜色越深。h表示颜色色调,红色区域分布在0°附近,逆时针旋转经过橙色到90°,其附近为黄色区域。样品测定重复5次,取平均值[16−17]。
-
称取2 g新鲜花瓣,液氮研磨成粉末,加入V(三氟醋酸)∶V(甲酸)∶V(水)∶V(甲醇)=1∶2∶27∶70的提取液5 mL,摇匀,封口,常温下浸提24 h。使用0.22 μm的滤膜对浸提液进行过滤,滤液转入2 mL棕色样品瓶中密封,−20℃冰箱中保存备用[18]。
利用高效液相色谱-光电二极管阵列检测(HPLC-DAD)和超高效液相色谱-四级杆-飞行时间质谱联用技术(UPLC-Q-TOF-MS)对花瓣中花青苷成分进行定性与定量分析,参照杨美英等[15]的方法进行流动相配制及梯度洗脱。
-
利用HPLC-DAD方法,在525 nm处检测花瓣中花青苷。标准品矢车菊素-3-O-β-葡萄糖苷(Cy3G)和矢车菊素-3-O-β-半乳糖苷(Cy3Ga),采用标准品半定量法计算各样品中花青苷质量分数[19],重复3次。应用SPSS 17.0进行多元逐步回归分析。
-
如表1所示:杜鹃红山茶、越南抱茎茶的L*分别为44.65、50.17,9个杂交后代L*大于越南抱茎茶,其余均在44.65和50.17之间。除DY35外,杂交后代a*均在双亲的47.92和57.81之间。13个杂交后代b*在双亲的8.48和19.78之间。16个杂交后代C*在双亲的48.67和61.10之间。18个杂交后代h均小于杜鹃红山茶,分布在红色区域的0°和20°之间。说明杂交后代花色变异总体上介于母本杜鹃红山茶与父本越南抱茎茶之间。
样品 L* a* b* C* h D 44.65±1.53 57.81±0.35 19.78±0.76 61.10±0.56 18.88±0.57 Y 50.17±0.42 47.92±0.62 8.48±0.99 48.67±0.44 10.05±1.26 DY9 46.63±0.41 54.49±0.87 14.51±1.01 56.40±1.06 14.90±0.83 DY12 46.63±0.79 56.26±0.31 16.26±0.88 58.57±0.45 16.12±0.80 DY31 56.21±1.33 48.14±0.77 4.62±0.57 48.37±0.74 5.49±0.73 DY34 47.13±1.06 56.17±1.55 17.30±0.42 58.78±1.47 17.13±0.62 DY35 56.32±1.48 47.86±1.24 3.73±0.93 48.01±1.30 4.43±1.01 DY41 46.14±2.86 53.87±16.6 12.24±5.21 55.36±16.98 12.58±5.01 DY43 50.83±1.77 51.57±1.21 8.40±1.08 52.26±1.31 9.23±1.05 DY44 45.77±1.89 50.57±1.87 14.60±0.50 52.64±1.91 16.11±0.41 DY45 45.12±1.38 51.56±1.64 14.27±0.78 53.50±1.79 15.46±0.34 DY46 47.14±1.64 53.75±0.37 15.27±0.07 55.88±0.36 15.85±0.05 DY47 50.97±1.70 54.72±0.74 8.55±1.65 55.40±0.92 8.86±1.61 DY49 57.07±0.41 50.57±0.49 9.07±0.23 51.38±0.50 10.17±0.21 DY51 54.13±1.36 48.62±1.08 4.03±0.65 48.79±1.03 4.75±0.87 DY52 49.45±2.29 51.65±0.70 8.78±0.17 52.39±0.71 9.65±0.08 DY53 55.64±0.35 51.97±1.43 9.82±0.42 52.89±1.48 10.7±0.16 DY56 54.04±0.82 52.46±1.02 6.26±0.88 52.84±1.10 6.79±0.85 DY63 52.86±2.04 52.67±1.03 10.69±1.24 53.76±1.25 11.45±1.09 DY64 50.17±1.26 55.01±1.12 9.07±1.24 55.76±1.27 9.34±1.11 说明:数值为平均值±标准误。D. 杜鹃红山茶,Y. 越南抱茎茶,DY. 二者的杂交后代。 Table 1. Data of flower colors in C. azalea, C. amplexicaulis and their hybrids
-
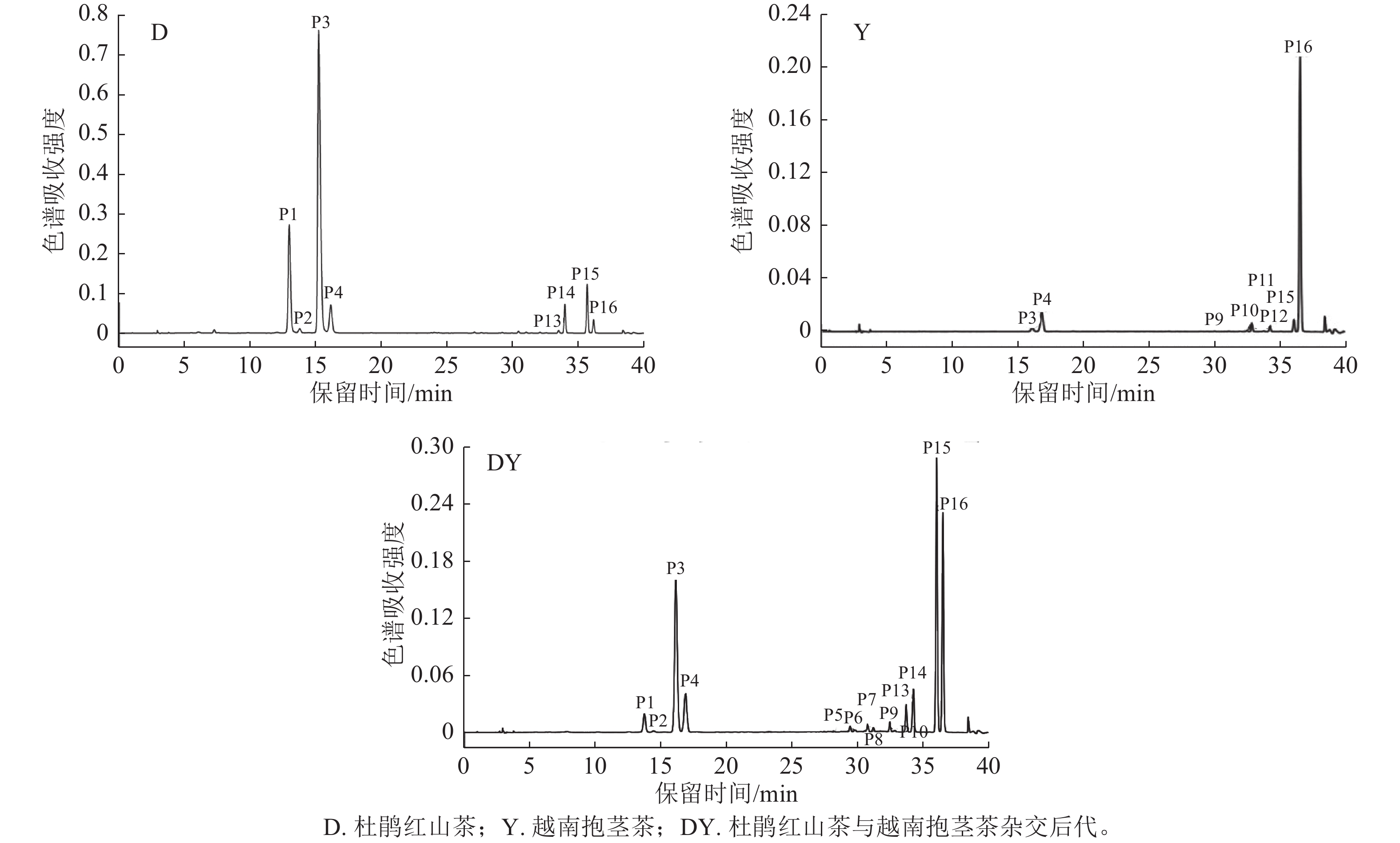
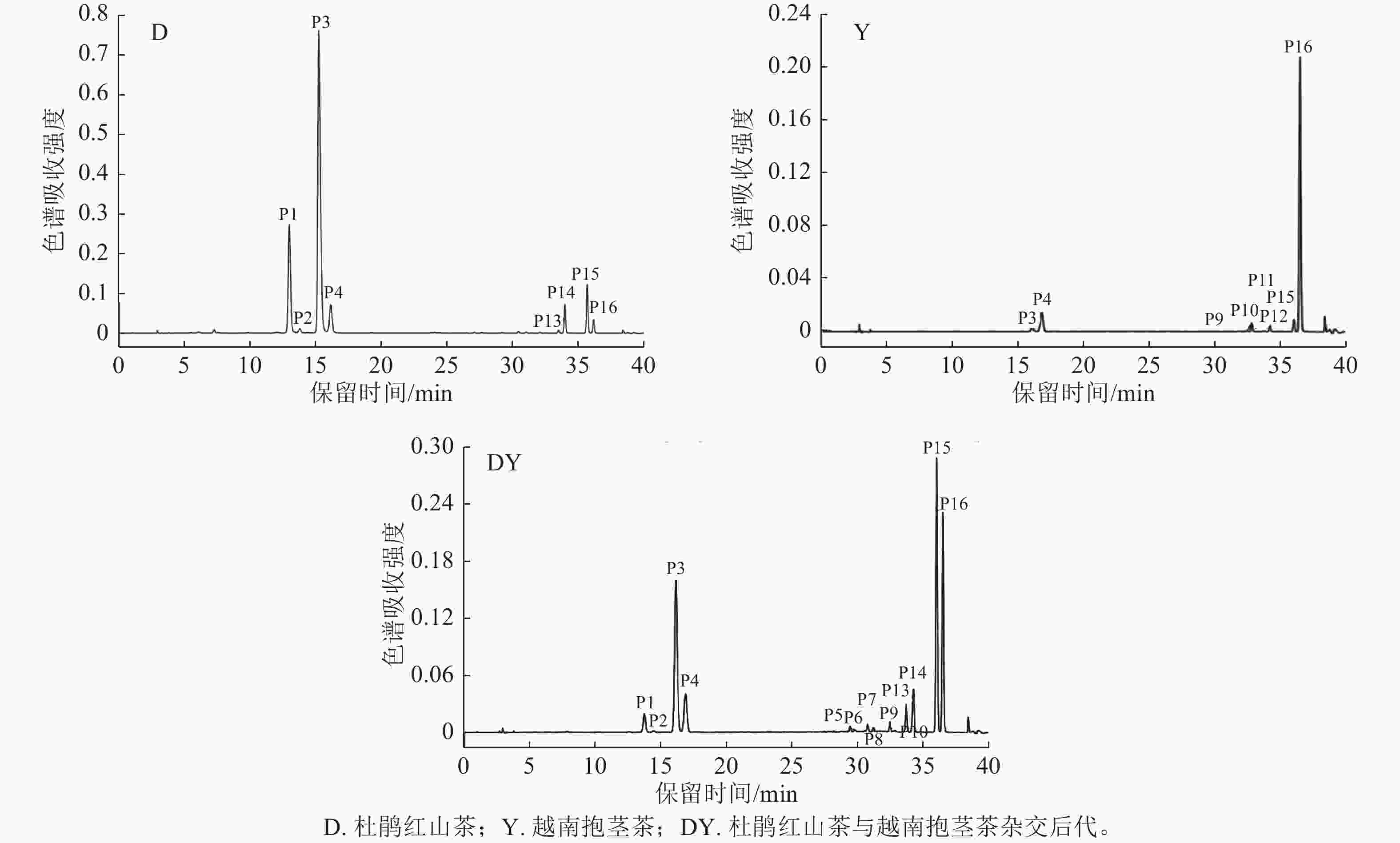
根据UPLC-Q-TOF-MS图谱及紫外-可见光谱共检测到杜鹃红山茶、越南抱茎茶及其杂交后代花瓣中16种花青苷成分(图1~2和表2)。根据矢车菊素(Cy)糖苷在513~520 nm有特征吸收峰及碎片离子m/z 287,可推测16种花青苷均为Cy型花青苷[20];由于花青苷在440 nm与可见光最大吸收波长(λvis, max)2处的吸光度之比为31%~34%,因此确定这16种花青苷均为3-O-糖苷类型[19, 21]。
色谱峰 保留时间/min 吸收波长(λ)/nm D(440)/D(λvis, max)/% 分子离子m/z 碎片离子m/z 化合物 P1 13.72 281,516 32 581 449,287 Cy3GaX P2 14.41 282,516 31 449 287 Cy3Ga P3 16.12 282,516 33 581 449,287 Cy3GX P4 16.85 282,514 32 449 287 Cy3G P5 39.41 281,315,516 33 611 449,287 Cy3GaECaf P6 29.78 282,315,517 33 743 611,449,287 Cy3GaECafX P7 30.74 284,311,515 34 727 595,449,287 Gy3GaZpCX P8 31.17 285,310,516 34 595 449,287 Gy3GaZpC P9 32.43 283,315,515 33 743 611,449,287 Cy3GECafX P10 32.82 283,316,515 33 611 449,287 Cy3GECaf P11 33.73 283,314,516 34 727 595,449,287 Cy3GZpCX P12 34.21 283,315,516 34 595 449,287 Cy3GZpC P13 33.68 283,313,516 34 595 449,287 Cy3GaEpC P14 34.22 282,312,516 33 727 595,449,287 Cy3GaEpCX P15 35.99 284,314,514 34 727 595,449,287 Cy3GEpCX P16 36.47 283,313,515 34 595 449,287 Cy3GEpC Table 2. Chromatographic and spectral data of anthocyanins in hybrids between C. azalea and C. amplexicaulis
峰P1和P3质谱数据为分子离子m/z 581,碎片离子m/z 449、287,m/z 581到m/z 449裂解释放132 u,m/z 449到m/z 287裂解释放162 u,根据山茶相关文献[4−5]推定峰P1为矢车菊素-3-O-(2-O-木糖基)-半乳糖苷(Cy3GaX),峰P3为矢车菊素-3-O-(2-O-木糖基)-葡萄糖苷(Cy3GX)。峰P2和P4的质谱数据相同,均含有分子离子m/z 449和碎片离子m/z 287,根据峰P2和峰P4与标准品Cy3Ga和Cy3G的共洗脱特性,以及花青素半乳糖苷洗脱时间小于花青素葡萄糖苷的特性[22],可判定峰P2为Cy3Ga,峰P4为Cy3G[23−24]。
峰P5和P10质谱数据为分子离子m/z 611,碎片离子m/z 449、287,m/z 611到m/z 449裂解释放162u,m/z 449到m/z 287裂解释放162 u,它们与山茶‘赤丹’C. japonica‘Chidan’等品种中特定花青苷的裂解特征相同[6−7],因此推测峰P5为矢车菊素-3-O-[6-O-(E)-咖啡酰]-半乳糖苷(Cy3GaECaf),峰P10为矢车菊素-3-O-[6-O-(E)-咖啡酰]-葡萄糖苷(Cy3GECaf)。峰P6和P9质谱数据为分子离子m/z 743,碎片离子m/z 611、449、287,m/z 743到m/z 611裂解释放132 u,m/z 611到m/z 449裂解释放162 u,m/z 449到m/z 287裂解释放162 u,参考LI等[4−5]的研究,峰P6为矢车菊素-3-O-[2-O-木糖基-6-O-(E)-咖啡酰]-半乳糖苷(Cy3GaECafX),峰P9为矢车菊素-3-O-[2-O-木糖基-6-O-(E)-咖啡酰]-葡萄糖苷(Cy3GECafX)。
峰P7、P11、P14和P15质谱数据为分子离子m/z 727和碎片离子m/z 595、449、287,m/z 727到m/z 595裂解释放132 u,m/z 595到m/z 449裂解释放146 u,m/z 449到m/z 287裂解释放162 u,参考LI等[4−5]的研究,推定峰P7为矢车菊素-3-O-[2-O-木糖基-6-O-(Z)-p-香豆酰]-半乳糖苷(Cy3GaZpCX),峰P11为矢车菊素-3-O-[2-O-木糖基-6-O-(Z)-咖啡酰]-葡萄糖苷(Cy3GZpCX),峰P14为矢车菊素-3-O-[2-O-木糖基-6-O-(E)-p-香豆酰]-半乳糖苷(Cy3GaEpCX),峰P15为矢车菊素-3-O-[2-O-木糖基-6-O-(E)-p-香豆酰]-葡萄糖苷(Cy3GEpCX)。
峰P8、P12、P13和P16质谱数据为分子离子m/z 595和碎片离子m/z 449、287,m/z 595到m/z 449裂解释放146 u,m/z 449到m/z 287裂解释放162 u;结合紫外吸光度D(440)/D(λvis, max)为34%及在310、313和315 nm波长下肩峰的出现,推测其为Cy-3-O-芳香酸酰化型糖苷[25];由于顺式花青苷洗脱时间小于反式花青苷及花青素半乳糖苷洗脱时间小于花青素葡萄糖苷的特性[26−27],推测峰P8为矢车菊素-3-O-[6-O-(Z)-p-香豆酰]-半乳糖苷(Cy3GaZpC),峰P12为矢车菊素-3-O-[6-O-(Z)-p-香豆酰]-葡萄糖苷(Cy3GZpC),峰P13为矢车菊素-3-O-[6-O-(E)-p-香豆酰]-半乳糖苷(Cy3GaEpC),峰P16为矢车菊素-3-O-[6-O-(E)-p-香豆酰]-葡萄糖苷(Cy3GEpC)。
-
在杜鹃红山茶、越南抱茎茶及其18个杂交后代中共检测到16种花青苷(图2和表2),杜鹃红山茶中质量分数较高的花青苷有8种(表3),其他花青苷质量分数均低于1 μg·g−1。在越南抱茎茶中检测到8种含葡萄糖苷的花青苷,未检测到含半乳糖苷的花青苷(图2),其中Cy3GX、Cy3G、Cy3GECafX、Cy3GECaf、Cy3GEpCX和Cy3GEpC这6种花青苷与杜鹃红山茶中花青苷相同,Cy3GZpCX和Cy3GZpC仅在越南抱茎茶中发现;越南抱茎茶中Cy3G、Cy3GX、Cy3GEpC和Cy3GEpCX的质量分数较高,其余均较低。在杜鹃红山茶与越南抱茎茶18个杂交后代花瓣中检测到7~8种质量分数较高的花青苷,其余成分质量分数均较低或未被检测到。
样品 质量分数/(μg·g−1) Cy3GaX Cy3Ga Cy3GX Cy3G Cy3GaEpC Cy3GaEpCX Cy3GEpCX Cy3GEpC 总量 D 141.54±0.47 5.75±0.00 466.23±1.57 44.88±0.16 2.07±0.01 23.38±0.07 40.05±0.12 11.9±0.03 735.81±2.43 Y − − 1.50±0.01 8.26±0.33 − − 2.88±0.04 68.73±2.47 81.37±2.17 DY9 14.29±0.33 − 124.47±4.00 10.14±0.36 5.28±0.09 24.83±0.71 135.63±4.31 25.72±1.34 340.38±11.15 DY12 5.99±0.04 − 180.81±3.05 10.16±0.03 17.57±0.32 16.88±0.53 149.3±3.92 40.02±1.83 420.72±9.65 DY31 9.52±0.14 − 187.92±2.58 11.96±0.25 10.75±0.16 22.26±0.32 170.97±2.52 69.00±1.25 482.38±7.22 DY34 9.51±0.15 0.65±0.03 89.98±2.35 24.42±0.69 8.89±0.21 16.81±0.59 90.52±2.90 71.49±3.81 312.26±10.72 DY35 6.31±0.08 − 251.83±2.74 42.14±0.46 0.70±0.02 3.88±0.06 39.58±0.37 15.35±0.09 359.79±3.78 DY41 13.38±0.52 − 382.97±14.91 36.78±1.27 2.12±0.08 23.57±0.91 189.78±7.08 105.88±3.56 754.47±28.32 DY43 3.18±0.07 − 114.04±3.07 8.51±0.10 17.36±0.46 16.31±0.41 166.08±4.57 93.51±2.22 419.01±10.89 DY44 7.87±0.15 1.66±0.05 83.32±1.93 41.60±1.16 2.83±0.06 11.90±0.39 65.32±2.04 92.14±4.71 306.65±10.48 DY45 5.21±0.09 0.56±0.02 118.98±2.60 31.47±0.72 10.28±0.27 15.40±0.55 140.4±4.76 102.39±5.49 424.70±14.50 DY46 7.47±0.00 0.87±0.01 101.58±0.86 32.60±0.33 5.99±0.05 11.43±0.22 67.15±1.14 72.97±2.61 300.05±5.22 DY47 9.39±0.28 − 275.28±9.31 24.70±0.63 3.03±0.11 18.16±0.58 135.44±4.49 119.67±3.45 585.67±18.84 DY49 5.99±0.23 − 100.55±1.38 10.39±0.09 5.18±0.08 6.75±0.16 57.46±1.29 26.06±1.05 212.37±4.26 DY51 1.66±0.04 − 66.88±0.92 8.65±0.05 6.91±0.16 4.81±0.13 36.82±1.09 16.20±0.78 141.92±3.16 DY52 9.42±0.28 0.36±0.04 159.55±3.79 33.28±1.04 2.78±0.05 2.90±0.09 18.34±0.53 7.42±0.36 234.06±6.10 DY53 3.17±0.13 − 122.24±2.93 19.79±0.62 1.77±0.03 1.42±0.04 11.20±0.30 2.86±0.12 162.45±4.17 DY56 7.69±0.14 0.28±0.07 133.01±2.40 51.66±1.16 1.71±0.03 4.40±0.13 8.31±0.23 13.72±0.62 220.78±4.79 DY63 2.46±0.06 0.23±0.01 123.42±2.64 32.72±0.88 6.14±0.11 7.79±0.23 86.22±2.41 46.55±2.15 305.53±8.46 DY64 3.65±0.03 − 210.03±0.44 30.31±0.07 3.41±0.00 10.82±0.01 153.25±0.19 106.55±0.34 518.02±0.37 说明:−表示未鉴定出。数值为平均值±标准误。D. 杜鹃红山茶,Y. 越南抱茎茶,DY. 二者的杂交后代。 Table 3. Contents of main anthocyanins in hybrids between C. azalea and C. amplexicaulis
杜鹃红山茶主要花青苷总量为735.81 μg·g−1,越南抱茎茶为81.37 μg·g−1,前者为后者的9.04倍。18个杂交后代中,仅DY41的主要花青苷总量(754.47 μg·g−1)高于杜鹃红山茶;其余后代花青苷总量均介于双亲之间,其中DY51的花青苷总量最低,约为越南抱茎茶的1.74倍。杜鹃红山茶中质量分数较高的为含2-O-木糖基的花青苷Cy3GX和Cy3GaX,分别为466.23、141.54 μg·g−1,而越南抱茎茶中质量分数较高的为不含2-O-木糖基的花青苷Cy3GEpC、Cy3G,分别为68.73、8.26 μg·g−1。杂交后代中Cy3GaX等4种花青苷来源于杜鹃红山茶,其中Cy3GaX、Cy3Ga质量分数远低于杜鹃红山茶,Cy3GaEpC的质量分数高于杜鹃红山茶(除个别杂交后代外)。在杂交后代的主要花青苷中,除DY56的Cy3G质量分数高于杜鹃红山茶外,其余杂交后代的Cy3GX、Cy3G质量分数均介于双亲之间;13个杂交后代的Cy3GEpCX质量分数高于杜鹃红山茶,9个杂交后代Cy3GEpC质量分数高于越南抱茎茶。
根据花青苷的结构将8种主要花青苷分为含2-O-木糖基的花青苷和不含2-O-木糖基的花青苷,含2-O-木糖基的花青苷包括Cy3GaX、Cy3GX、Cy3GaEpCX和Cy3GEpCX,不含2-O-木糖基的花青苷为相应的Cy3Ga、Cy3G、Cy3GaEpC和Cy3GEpC。杜鹃红山茶花瓣中含2-O-木糖基的花青苷总量为671.21 μg·g−1,为不含2-O-木糖基花青苷的10.39倍,而越南抱茎茶中不含2-O-木糖基的花青苷总量是含2-O-木糖基花青苷的17.58倍,18个杂交后代中含2-O-木糖基的花青苷总量均高于不含2-O-木糖基的花青苷。含葡萄糖苷的花青苷有Cy3G、Cy3GX、Cy3GEpCX和Cy3GEpC,含半乳糖苷的花青苷为Cy3GaX、Cy3Ga、Cy3GaEpCX和Cy3GaEpC。杜鹃红山茶中含葡萄糖苷的花青苷总量为563.07 μg·g−1,为含半乳糖苷花青苷的3.26倍,越南抱茎茶中未检测到含半乳糖苷的花青苷;18个杂交后代含葡萄糖苷的花青苷总量均高于含半乳糖苷的花青苷。
-
以亲本及其18个杂交后代中的8种主要花青苷Cy3GaX、Cy3Ga、Cy3GX、Cy3G、Cy3GaEpC、Cy3GaEpCX、Cy3GEpC和Cy3GEpCX为自变量,分别对应x1、x2、x3、x4、x5、x6、x7、x8,以5个花色指标L*、a*、b*、C*和h为因变量,进行多元逐步回归分析,研究杜鹃红山茶、越南抱茎茶及其杂交后代花色与花青苷关系。回归方程式L*=−0.299x6+53.985,r=0.560,P=0.010;a*=0.210x6+49.841,r=0.565,P=0.009;b* = 2.051x2+9.812,r=0.566,P=0.009;C*=0.275x6+50.329,r=0.591,P=0.006。回归方程的相关系数r及显著性P表明:杜鹃红山茶、越南抱茎茶及其杂交后代L*与花青苷之间显著相关(P<0.05),其中a*与b*、C*与花青苷极显著相关(P<0.01)。
回归方程表明:Cy3GaEpCX与L*呈负相关,L*随着Cy3GaEpCX的质量分数增加而减小,表现为花色亮度降低。Cy3GaEpCX与a*呈正相关,a*随着Cy3GaEpCX的质量分数增加而增大,当a*较大时(a*>30),b*越大,花色越红[19];Cy3Ga与b*呈正相关,b*随Cy3Ga的质量分数增加而增大,b*越大花色越红。Cy3GaEpCX与C*呈正相关,随着Cy3GaEpCX质量分数的增加,花瓣由浅色变为深色。因此,Cy3GaEpCX和Cy3Ga是影响杜鹃红山茶、越南抱茎茶及其杂交后代花色的主要成分,这2种主要花青苷的积累使花色从浅粉渐变为深红。
-
植物花色色素主要包括叶绿素、类黄酮、类胡萝卜素和生物碱[28],山茶花色色素成分主要为类黄酮,其中黄色系山茶花主要为黄酮醇类[29],红色系山茶花为花青素类[30]。花青素作为天然的水溶性色素广泛存在于植物体内,主要分为6种,即矢车菊素、飞燕草素、天竺葵素、芍药素、矮牵牛素和锦葵色素[31]。花青素种类及含量是植物花朵呈现丰富花色的重要原因,如天竺葵素通常显砖红色,矢车菊素通常显红色,飞燕草素通常显蓝紫色[32]。植物花瓣中可能含一种或多种花青素,如换锦花Lycoris sprengeri花中含有矢车菊素、天竺葵素和飞燕草素[33],山茶花中含有矢车菊素和飞燕草素[4, 34]。
研究植物花色遗传规律有助于了解杂交后代的选育过程,对提高杂交后代品质以及培育不同色系的新品种有重要意义。刘玉琪等[35]发现夏蜡梅Sinicalycanthus chinensis、光叶红蜡梅Calycanthus floridus var. glaucus及其杂交后代花瓣花色表型与代谢物质存在明显差异,3种植物之间主要以类黄酮为主的差异代谢物。本研究从杜鹃红山茶、越南抱茎茶及其杂交后代中共检测到16种矢车菊素类花青苷。杂交后代中含半乳糖苷的花青苷Cy3GaX、Cy3Ga、Cy3GaEpC、Cy3GaEpCX未在越南抱茎茶中检测到,表明其全部来源于杜鹃红山茶,而含葡萄糖苷的花青苷则来源于双亲。杂交后代中含2-O-木糖基的花青苷总量高于不含2-O-木糖基的花青苷,这与杜鹃红山茶和山茶‘媚丽’杂交后代的研究结果一致[15]。越南抱茎茶中不含2-O-木糖基的花青苷总量是含2-O-木糖基花青苷的17.58倍,且含2-O-木糖基的花青苷中质量分数最高的Cy3GEpCX仅为2.88 μg·g−1,远低于杜鹃红山茶中的Cy3GEpCX(40.05 μg·g−1),表明杂交后代中含2-O-木糖基的花青苷主要来源于杜鹃红山茶,而不含2-O-木糖基的花青苷来源于双亲,具体情况有待进一步研究。
花青苷为山茶花色形成的物质基础,总花青苷和主要花青苷质量分数决定其花色,花色随花青苷质量分数的增加而加深[36−37]。本研究中杜鹃红山茶主要花青苷总量为越南抱茎茶的9.04倍,杂交后代花青苷总量总体上介于双亲之间;杜鹃红山茶花鲜红色,越南抱茎茶花紫红色,杂交后代花色深浅不同,从浅粉红色、红色至深红色,表明花色变化与花青苷质量分数相关。多元逐步回归结果表明:Cy3GaEpCX与L*显著负相关,与a*和C*极显著正相关,Cy3GaEpCX质量分数升高,花色趋向于更红更深;Cy3Ga与b*极显著正相关,Cy3Ga的积累也使花色趋向于红色。
-
本研究从杜鹃红山茶、越南抱茎茶及其杂交后代的花瓣中共鉴定出16种花青苷,在越南抱茎茶中首次检测到8种含葡萄糖苷的花青苷。杜鹃红山茶主要花青苷总量高于越南抱茎茶,杂交后代花青苷总量基本介于双亲之间,Cy3GaEpCX和Cy3Ga是决定杜鹃红山茶、越南抱茎茶及其杂交后代花色的主要花青苷,其质量分数的差异影响花瓣的红色程度。
Analysis of anthocyanin variability characteristics of Camellia hybrids
doi: 10.11833/j.issn.2095-0756.20250441
- Received Date: 2025-08-20
- Accepted Date: 2025-09-24
- Rev Recd Date: 2025-09-19
- Available Online: 2025-10-29
- Publish Date: 2025-10-20
-
Key words:
- Camellia azalea /
- C. amplexicaulis /
- hybrids /
- anthocyanins /
- variation
Abstract:
| Citation: | ZHANG Ying, YANG Meiying, LI Jianbin, et al. Analysis of anthocyanin variability characteristics of Camellia hybrids[J]. Journal of Zhejiang A&F University, 2025, 42(5): 994−1002 doi: 10.11833/j.issn.2095-0756.20250441 |











 DownLoad:
DownLoad: