-
香榧Torreya grandis ‘Merrillii’是红豆杉科Taxaceae榧树属Torreya的优质品种,其种子可食用,是著名的特色干果[1];种仁含油率较高[2],具有很高的经济价值[3]。随着各类栽培技术的开发,中国的香榧种植产业规模也在不断扩大,对于香榧植株及干果[4]的有关研究已经比较成熟,但对于香榧愈伤组织培养的研究还处在相对落后的阶段。
植物愈伤细胞的悬浮培养是一种通过调控细胞生长,快速获得大量细胞以及目的产物的技术,不受各类局限进行周年生产。建立好的培养体系以及优化培养条件,可以超过原有的生产方式[5]。王沐兰等[6]通过建立红豆杉Taxus chinensis高产悬浮细胞系,提高了紫杉醇的产量;赵文佳[7]诱导青钱柳Cyclocarya paliurus红色愈伤系,并利用细胞悬浮培养技术建立了花青素的生产体系。此外,国槐Sophora japonica[8]、南方红豆杉Taxus wallichiana[9]和落叶松Larix gmelinii[10]等植物的悬浮培养体系也已经建立。然而在香榧悬浮培养体系中,细胞生长动力学规律及最佳培养条件尚不清晰。
悬浮细胞的培养过程中需添加各类激素以促生长。赤霉素(GAs)是植物体内的一种重要激素,已被发现较多的种类,并按顺序命名为赤霉素A1 (GA1)、赤霉素A3 (GA3)等,在植物生长的各个环节都不可缺少[11−12]。在非生物胁迫或生物刺激下,外施赤霉素可以增加防御酶的数量或活性,进而影响植物抗性[13]。赤霉素还可对植物愈伤组织增殖过程中的褐化情况进行抑制,促进愈伤组织增殖[14]。
鉴于此,本研究通过优化香榧胚性愈伤组织悬浮培养基,探究其动力学特性,筛选最适合的培养条件,同时探究GA3处理下香榧胚性愈伤组织生长及差异基因表达的规律,为香榧愈伤组织的生长和遗传转化体系的构建提供科学依据。
-
以浙江农林大学省部共建亚热带森林培育国家重点实验室培育的香榧胚性愈伤组织为试验材料。使用SH液体培养基,其中添加脱落酸(ABA) 1.0 mg·L−1、水解酪蛋白(CH) 500.0 mg·L−1、蔗糖30.0 g·L−1、活性炭2.0 g·L−1和GA3 0.5 mg·L−1,设置pH 5.7,摇床转速为110 r·min−1,25 ℃暗培养。
-
在100 mL的锥形瓶中添置30 mL的SH液体培养基(配方同1.1),香榧胚性愈伤组织接种量为30 g·L−1,设置pH 5.7,转速为110 r·min−1,每3 d取样测定细胞鲜质量及细胞活力(氯化三苯四氮还原法,TTC),即波长为485 nm处的吸光度。
-
在100 mL锥形瓶中添置30 mL的SH液体培养基(配方及培养条件同1.1),设置转接愈伤组织接种量为10、20、30、40、50 g·L−1;pH为5.5、5.6、5.7、5.8、5.9;摇床转速为70、90、110、130、150 r·min−1。25 ℃摇床中振荡暗培养。每个处理设置3个重复,21 d时统计鲜质量,计算细胞增长率,细胞增长率=(收获量−接种量)/接种量×100%。
-
超氧化物歧化酶(SOD)活性采用氮蓝四唑光化还原法测定;过氧化物酶(POD)活性采用愈创木酚法测定;过氧化氢酶(CAT)活性采用紫外吸收法测定[15]。
-
将香榧胚性愈伤组织接种于SH液体培养基中(配方及培养条件同1.1),设置对照组(ck)和实验组(GA3+),对照组培养基中不添加GA3,实验组培养基中施加GA3。18 d后收集材料,使用多糖多酚植物总RNA提取试剂盒(北京天根生化科技有限公司),按说明书提取RNA,分别设置3个生物学重复,使用分光光度计检测浓度与纯度。
-
测序工作由北京诺禾致源科技有限公司完成,采用Illumina HiSeqTM 2000测序平台进行测序。由于香榧没有参考基因组,将聚类分析结果中最长的转录本作为参考序列单基因簇进行后续分析。
-
采用RSEM软件将每个样品过滤后的数据(clean reads)在参考序列上做比对。采用DESeq分析差异表达基因(DEG),以|log2Fc|>1 (Fc为表达量的差异倍数)且P<0.005为筛选条件[16]。将差异表达基因注释到基因本体论数据库(GO)和京都基因与基因组百科全书(KEGG),并进行GO和KEGG富集分析。
-
使用Primer 3.0 input在线软件设计引物(表1),以TgActin为内参基因[16]。用Takara生物科技有限公司的反转录试剂将提取的RNA反转录成cDNA进行RT-qPCR验证,每个样品3个生物学重复。反应体系为10.0 μL,包括5.0 μL的TB GREEN,0.2 μL的上下游引物,0.4 μL的模板cDNA,4.2 μL的ddH2O。PCR反应程序为:95 ℃ 10 min,95 ℃ 10 s,60 ℃ 1 min,95 ℃ 30 s,60 ℃ 15 s共39个循环。
表 1 差异表达基因RT-qPCR的特异性引物信息
Table 1. Gene-specific primer information for RT-qPCR
引物名称 正向引物(5′→3′) 反向引物(5′→3′) XM_024662224.1 TCCAAGGGAAGGGGAACATC TCCCCGGATTGCAGAAGATT XM_027242615.1 TGTACCCTCCACCCTTTTCC TCTATCAGGGAGGGAGCAGA XM_020665027.1 TACGACCAGTCAGAGGCTTG AAACACCCACCGTCTTTGAC XM_002991298.2 CACGCCCAATTTTCACGAGA CGAAAACTAGGCAGGGCATC XM_022920293.1 TCGACCTTGAGACCTGGAAC TGATGGTGCAGCAAATCAGG TgActin TGGCATCTCTCAGCACATTCCA TGCCAACATCTCAAGCAAGCAC -
使用Excel处理数据,采用SPSS 26进行单因素方差分析(one-way ANOVA)和多重比较(邓肯法),显著性水平为0.05,使用GraphPad Prism 9软件作图。
-
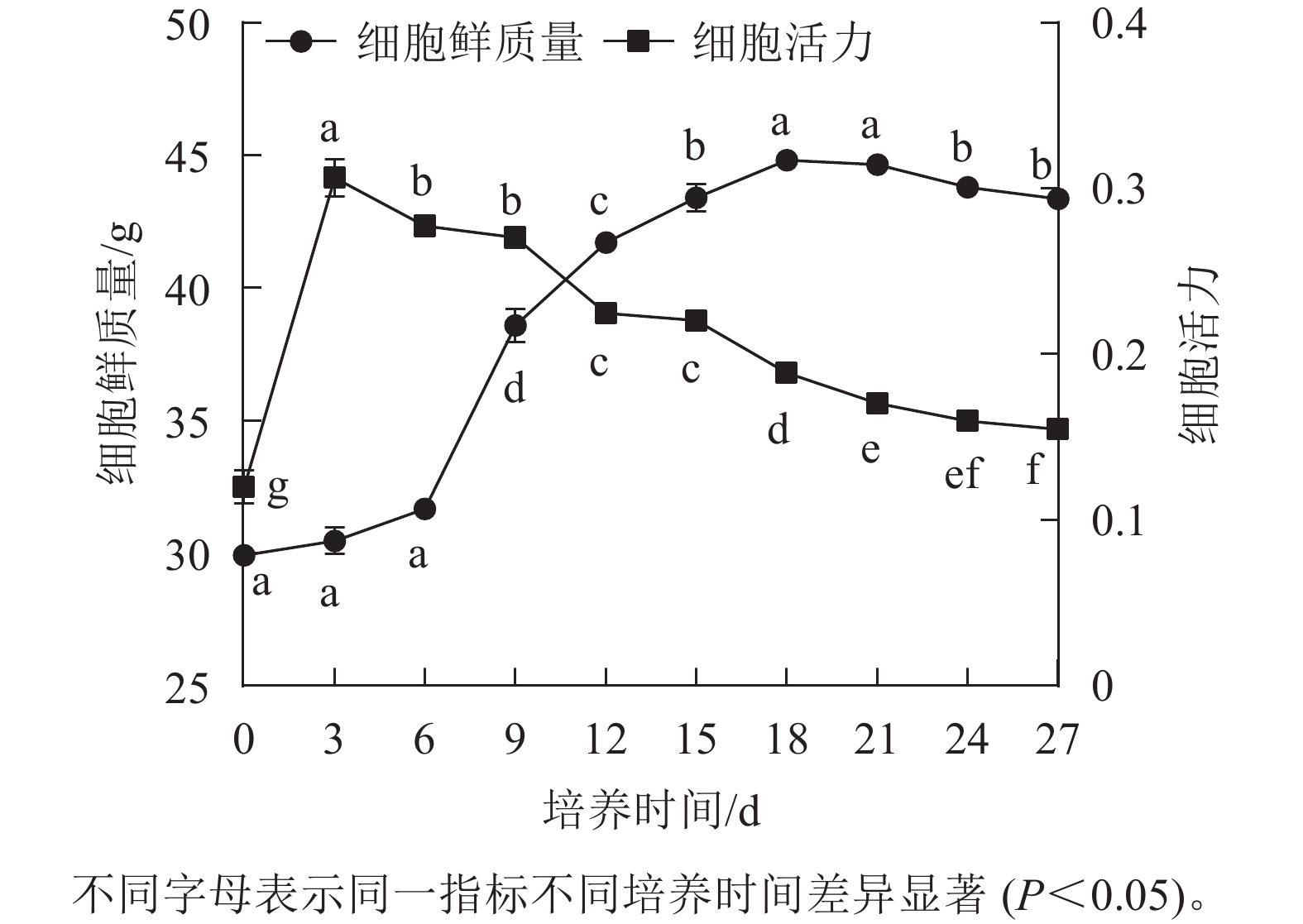
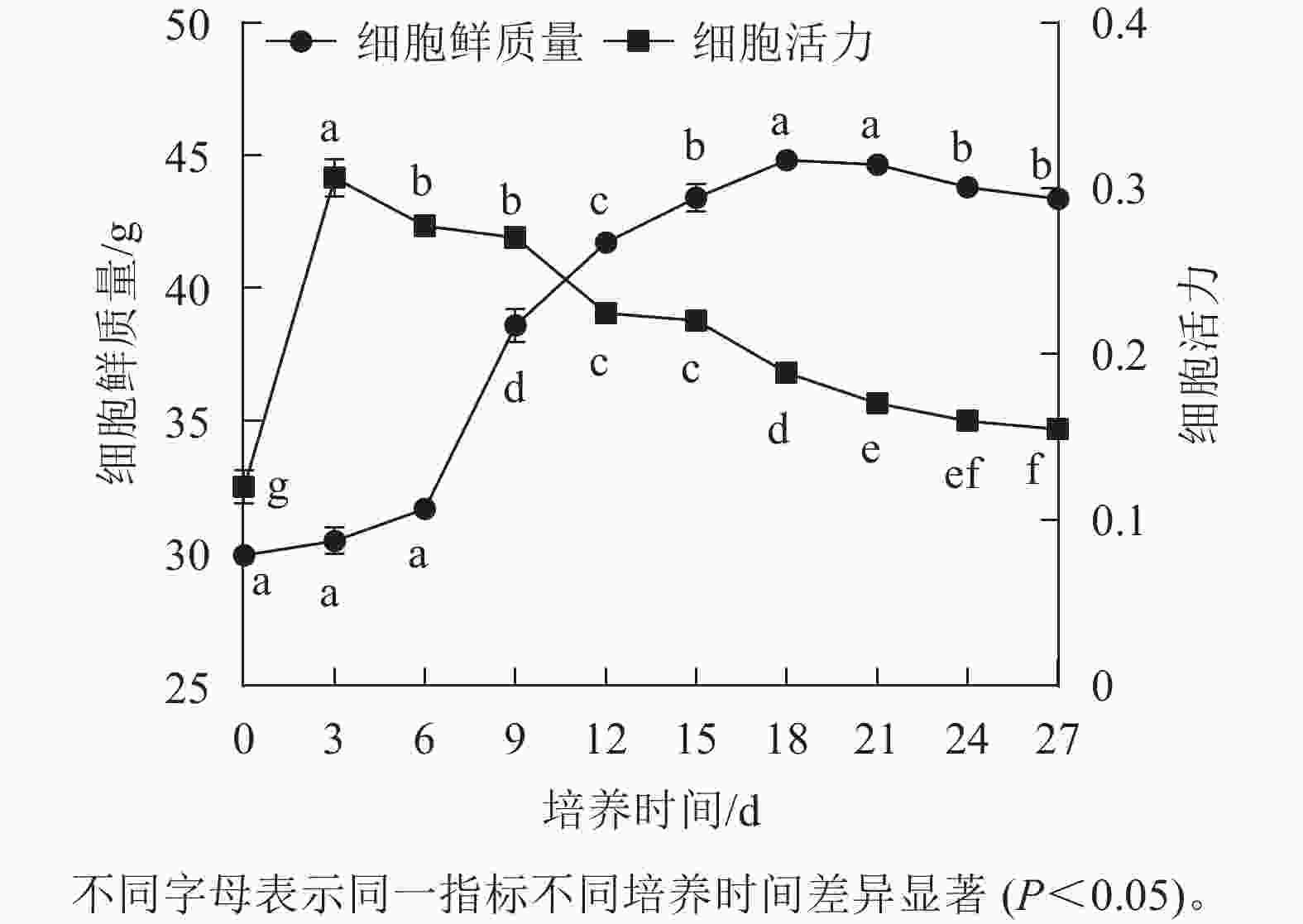
香榧胚性愈伤组织悬浮培养时,细胞鲜质量变化呈“S”型曲线。在0~6 d鲜质量增加缓慢;7~18 d鲜质量快速增加;18 d时达到最高值,为44.81 g;18~21 d进入稳定期,鲜质量基本不再增加;21 d后细胞进入衰退期,鲜质量下降。同时,在培养周期内香榧胚性细胞活力在0~3 d时快速增长,并在3 d时达到最高值(0.32),之后持续下降,并且随着培养时间的延长,变化趋势趋向平缓(图1),表明9~12 d为香榧胚性愈伤组织悬浮培养的最佳继代时期。
-
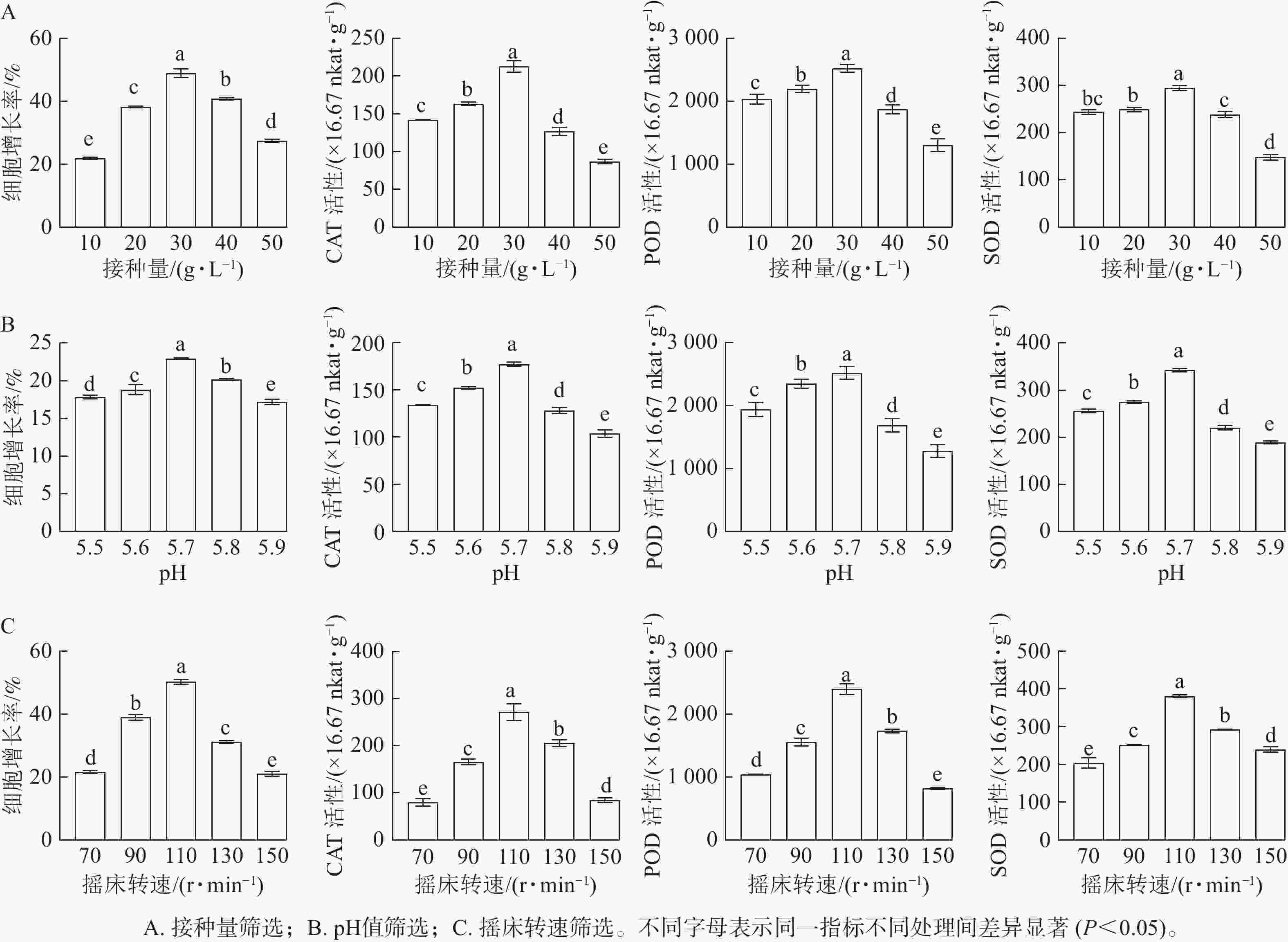
随着接种量的增加,胚性愈伤组织增长率呈先上升后下降的趋势。当愈伤组织接种量为10 g·L−1时,细胞增长率最低(21.78%);当接种量为30 g·L−1时,细胞增长率显著升高(P<0.05),达到最高值,为49.94%。SOD、POD和CAT活性均随接种量的增加而呈先升高后降低的趋势。当接种量为30 g·L−1时,SOD、POD和CAT活性均最高,显著高于其他接种量时的酶活性(P<0.05)(图2A)。
-
随着pH的增加,香榧悬浮细胞增长率呈先上升后下降的趋势。当培养基pH为5.7时,细胞鲜质量增殖效果最佳,细胞增长率最大,为22.95%,显著高于其他处理(P<0.05)。SOD、POD和CAT活性均随接种量的增加而呈先升高后降低的趋势。在pH为5.7时,悬浮细胞SOD、POD和CAT活性均最大,显著高于其他处理(P<0.05)(图2B)。
-
随着转速的增加,香榧悬浮细胞增长率呈逐渐上升的趋势,当转速为70 r·min−1时,细胞增长率较低,为20.33%;当转速达110 r·min−1时,细胞增长率最大,为50.84%,显著高于其他处理(P<0.05)。SOD、POD和CAT活性均随摇床转速的增加而呈先升高后降低的趋势。当转速为110 r·min−1时,SOD、POD和CAT活性最大,显著高于其他处理(P<0.05)(图2C)。
-
所有检测样品纯净碱基数均达6.66 Gb以上,质量为Q20 (每项碱基质量大于20的碱基数占总碱基数的比例)的碱基比例均大于97.47%,达Q30 (每项碱基质量大于30的碱基数占总碱基数的比例)以上的碱基比例大于92.70%,每个样本碱基GC含量为43.53%~44.45%,较为一致,且测序碱基平均错误率均在0.1%以下,表明转录组数据较为准确。
-
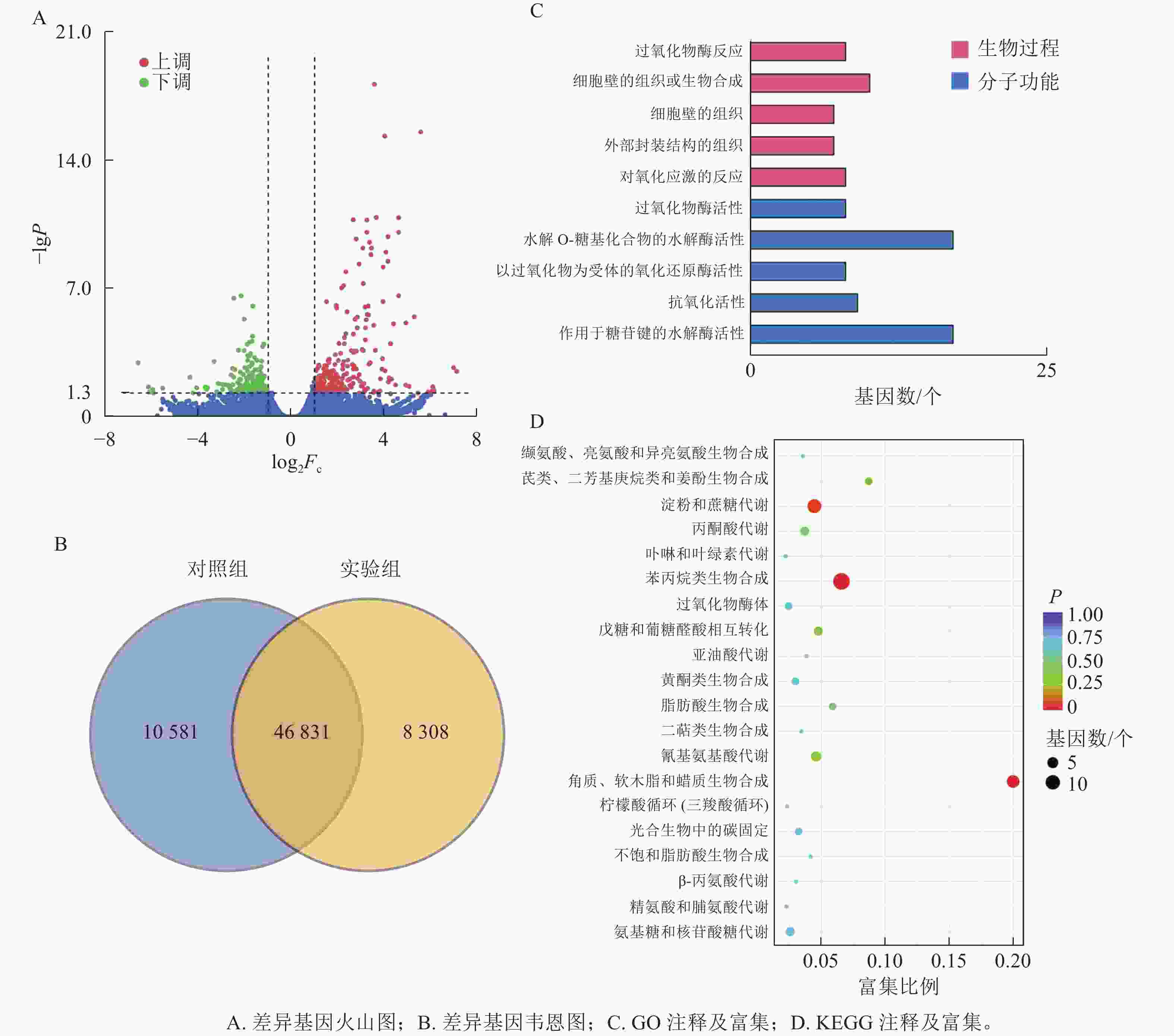
本研究共筛选到428个差异表达基因,其中236个基因上调表达,192个基因下调表达(图3A)。对照组与实验组共有表达的基因数为46 831个,对照组特有表达的基因数为10 580个,实验组特有表达的基因数为8 308个(图3B)。
-
GO功能注释与分类统计发现:差异表达基因被归类到2个功能分类组中,其中:分子功能中有17个基因涉及水解邻糖基化合物的水解酶活性;生物过程中细胞壁组织或生物合成过程注释到10个差异基因(图3C)。
-
将差异表达基因进行KEGG通路富集分析,对富集较多的前20个通路进行散点图绘制。差异基因富集最多的通路为苯丙烷生物合成;其次为淀粉和蔗糖代谢;再次为角质、木栓质和蜡的生物合成。而氰基氨基酸代谢、戊糖和葡萄糖醛酸相互转化、丙酮酸代谢、氨基糖和核苷酸糖代谢等通路富集较少(图3D)。
本研究有8个与苯丙烷生物合成相关的差异基因被富集到且呈上调表达,4个与过氧化物酶(POX)相关;2个与莽草酸-O-羟基肉桂酰转移酶(HCT)相关;2个与咖啡酸-O甲基转移酶(COMT)相关(图4)。本研究表明:GA3处理会使得香榧胚性愈伤组织细胞增加对苯丙氨酸的合成与利用,调控HCT、COMT、POX活性的基因上调表达,促进了对香豆酰辅酶A参与合成咖啡酰莽草酸、愈创木酰木质素等产物的过程,同时生成酚类物质参与其他途径物质合成。该过程产生的酚类物质还可能参与清除部分愈伤组织和培养基产生的有害活性氧,提高香榧胚性愈伤组织的抗性[17−19]。
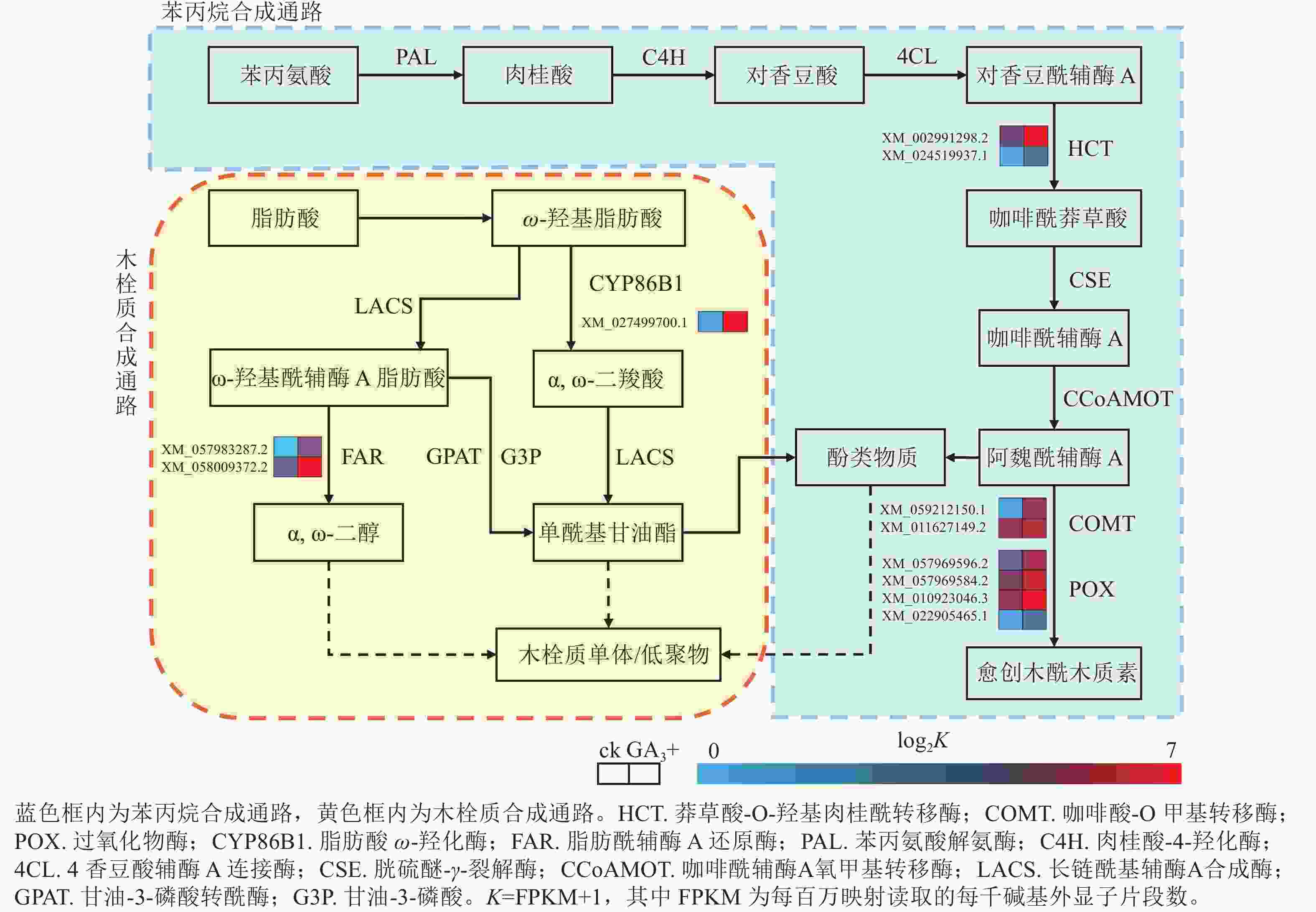
图 4 苯丙烷生物合成与木栓质合成相关通路及差异表达基因
Figure 4. Pathways and differentially expressed genes related to phenylpropanoid biosynthesis and suberin synthesis
本研究有3个与木栓质生物合成相关的差异基因被富集到且呈上调表达,其中1个与脂肪酸ω-羟化酶(CYPB6B1)相关,2个与脂肪酰辅酶A还原酶(FAR)相关(图4)。与对照组相比,GA3处理会使FAR与CYPB6B1上调表达,参与ω-羟基脂肪酸下游α,ω-二羧酸和α,ω-二醇合成途径,促进下游单酰基甘油酯的合成,共同参与形成木栓质单体或低聚物,同时产生酚类物质[20]。该过程可结合苯丙烷合成途径产生的酚类物质,促进木栓质单体或低聚物的合成,应对环境变化并进行调整[21−22]。
-
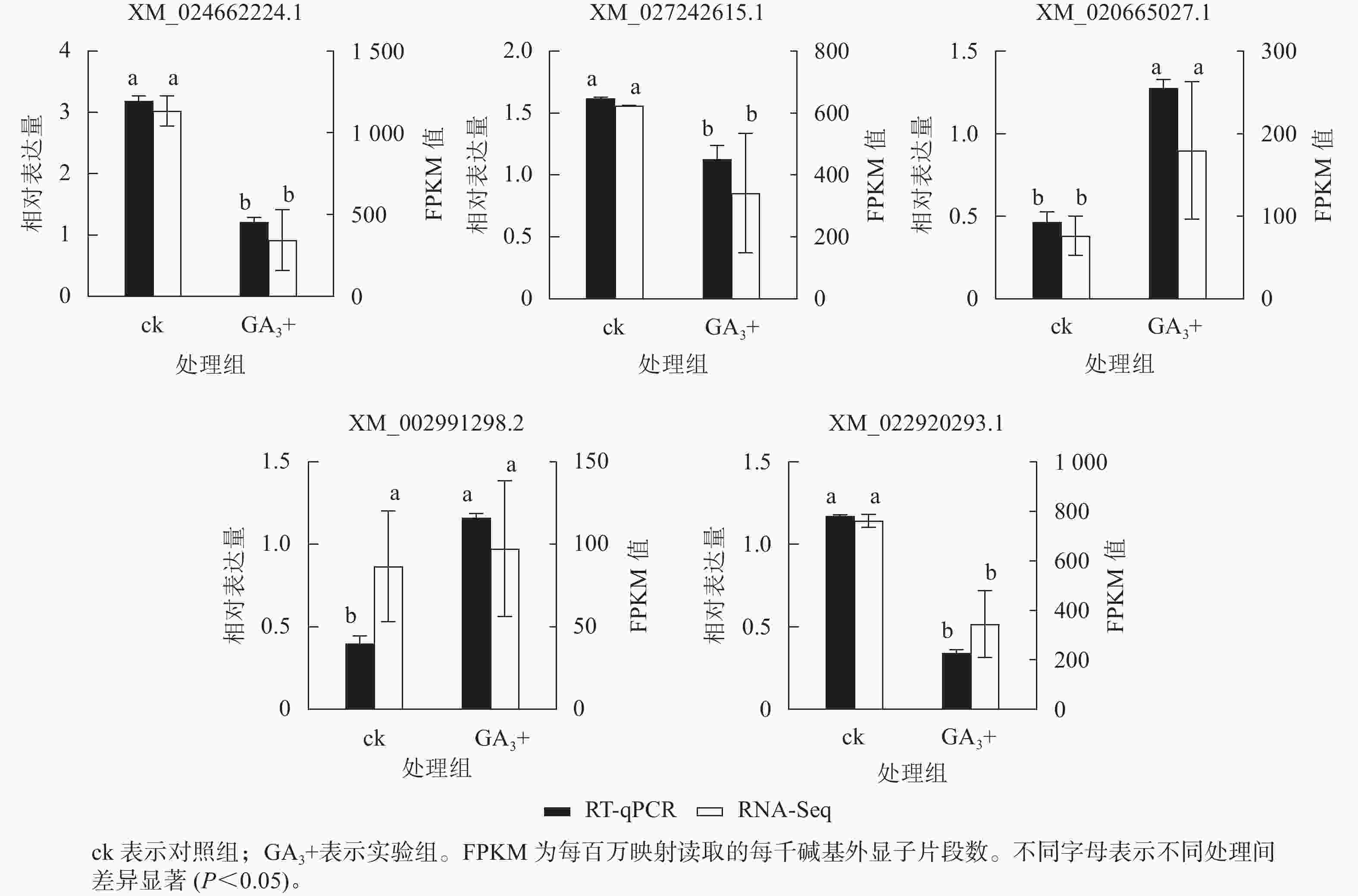
RT-qPCR验证结果(图5)表明:5个基因与转录组测序结果呈现相同的表达趋势,2个基因上调表达,3个基因下调表达,说明转录组测序结果可靠。
-
植物细胞悬浮培养是一个持续变化的过程。在悬浮细胞培养过程中,接种量直接影响细胞的生长。细胞需要足够的空间和营养物质支持其增殖,选择适当的接种量对于细胞的生长至关重要。已有研究表明:落叶松胚性愈伤组织的最佳接种量为4 g·L−1[6]。油茶Camellia oleifera愈伤细胞悬浮培养的最佳初始接种量为33 g· L−1[23]。本研究中,胚性愈伤组织接种量为30 g·L−1,有利于香榧悬浮细胞的生长,此时细胞增长率最大。在细胞生长过程中,细胞内部很多起关键调控作用的酶对pH有一定的要求。陈继光等[24]研究表明:青钱柳在pH为5时细胞活力较强,细胞质量增量达最大,当pH达6时,细胞活力和质量增量都开始下降。本研究中,pH为5.7时,香榧胚性愈伤组织悬浮细胞抗逆性最强,细胞增长率最大。悬浮培养中摇床转速对细胞生长也有较大的影响。较低的转速会使愈伤组织堆积导致褐化[4];转速较高时会使细胞间产生碰撞导致破裂死亡,只有合适的转速才能使得细胞快速增殖生长[25]。本研究表明:转速为110 r·min−1时,香榧悬浮细胞生长最快。悬浮培养过程中对多种条件进行优化,能显著提升细胞的生长能力及抗性。
植物细胞在离体悬浮培养过程中,还须通过外施激素来促进细胞的生长。赤霉素在植物体的生命过程中起着重要的作用。当植物体处于胁迫条件时,外源赤霉素可以有效缓解胁迫的危害[26]。有研究表明:加入外源GA3可以有效缓解牡丹Paeonia × suffruticosa愈伤组织的褐化,并且提高愈伤组织诱导率[14]。本研究表明:在差异基因的GO富集分析中,GA3处理后上调表达基因主要参与催化活性、氧化还原过程、氧化还原酶活性等过程,说明活性氧的清除是香榧胚性愈伤组织增殖发育时的一个重要过程。在代谢通路中,差异表达基因主要富集在苯丙烷生物合成、淀粉和蔗糖代谢、木栓质合成等途径中。KEGG通路富集结果表明:苯丙烷生物合成途径中注释的差异表达基因最多,说明GA3处理后,香榧胚性愈伤组织的次级代谢途径产生了一定的变化。
苯丙烷代谢途径是植物体内多种防御性次生代谢产物(如黄酮类、木质素及水杨酸等)的主要来源[27]。有关木栓质相关合成途径的研究集中于植株根系以及部分受创伤部位[28],对于其在植物愈伤组织内的合成与作用研究较少。本研究中,木栓质合成途径也有7个差异基因被显著富集,且呈上调表达。已有研究表明:细胞色素P450单加氧酶(CYP)家族与FAR主要参与木栓质的合成[29]。木栓质大分子的酚类组分已证明主要有阿魏酸、香豆酸等物质,通过苯丙烷生物合成途径产生,可继续生成木栓质单体中相应的底物物质,在一定程度上促进了木栓质单体的合成,影响细胞生长,以应对环境的胁迫[30]。这与本研究结果相似,推测外源GA3处理下香榧愈伤组织苯丙烷生物合成途径所产生的酚类物质不仅参与了活性氧的清除,还参与了木栓质单体的合成,对愈伤组织的生长及胁迫适应性产生一定的影响[31]。
-
香榧胚性愈伤组织悬浮细胞生长曲线呈“S”型,接种后9~12 d为最佳继代时期,设置接种量为30 g·L−1,pH为 5.7,摇床转速为110 r·min−1,可获得生长良好的香榧胚性愈伤组织细胞,同时也可以提高悬浮细胞的抗氧化能力。通过转录组分析发现:外源GA3处理下香榧胚性愈伤组织细胞苯丙烷生物合成、木栓质生物合成等相关代谢通路为差异基因的主要参与部分,且其中部分调空愈伤组织形成、生长和发育的关键基因表达量产生变化,可能在香榧愈伤组织的生长发育、适应环境变化以及应对胁迫相关过程中发挥重要作用。
Suspension culture dynamics of embryogenic callus from Torreya grandis ‘Merrillii’ and its response to gibberellin
-
摘要:
目的 以香榧Torreya grandis ‘Merrillii’胚性愈伤组织为试验材料,对其悬浮细胞的生长以及悬浮培养动力学进行研究,探究香榧胚性愈伤组织对赤霉素的响应。 方法 以实验室培育的香榧胚性愈伤组织为试验材料,通过测定细胞活力、细胞鲜质量进行悬浮培养动力学的研究,优化培养条件,并对施加外源赤霉素A3 (GA3)的愈伤组织进行转录组分析。 结果 ①在培养周期内,悬浮细胞的鲜质量增长呈“S”型曲线,细胞活力在3 d时达最高值后平稳下降。②香榧悬浮细胞培养最佳继代周期为9~12 d,最佳接种量为30 g·L−1,培养基最佳初始pH为5.7,最佳摇床转速为110 r·min−1。③对外源GA3处理后的香榧胚性愈伤组织进行转录组分析,共获得差异表达基因428个,其中上调基因236个,下调基因192个。在差异基因的GO富集分析中,GA3处理后上调表达基因主要参与催化活性、氧化还原过程、氧化还原酶活性等,在KEGG代谢通路中,差异表达基因主要富集在苯丙烷类生物合成、木栓质生物合成等途径中。 结论 香榧胚性愈伤组织培养动力学分析能获得最佳接种时期,优化培养条件能提高其细胞增长率及抗逆性,外源GA3处理下的香榧胚性愈伤组织细胞中,苯丙烷生物合成、木栓质生物合成等代谢通路为差异基因主要参与部分,且部分关键基因表达量变化可能在香榧愈伤组织生长发育、适应环境及应对胁迫中发挥重要作用。图5表1参31 Abstract:Objective This study, with embryogenic callus from Torreya grandis ‘Merrillii’ employed as the experimental material, is aimed to investigate the growth and suspension culture dynamics of its suspension cells, explore the response of T. grandis ‘Merrillii’ embryogenic callus to gibberellin treatment. Method Using the embryogenic callus of T. grandis ‘Merrillii’ cultivated in laboratory as the experimental material, the suspension culture dynamics were studied by measuring cell viability and fresh cell weight, and the culture conditions were optimized before a transcriptome analysis was performed on the callus treated with exogenous GA3. Result (1) Within the culture cycle, the fresh weight growth curve of the suspension cells exhibited an “S” shaped pattern, with cell viability reaching its peak at 3 days and then gradually decreasing steadily. (2) The optimal subculture period for T. grandis ‘Merrillii’ suspension cells was determined to be 9 to 12 days, with an optimal inoculation rate of 30 g·L−1, an initial pH of 5.7 for the culture medium, and an optimal shaking speed of 110 r·min−1. (3) transcriptome analysis of T. grandis ‘Merrillii’ embryogenic callus treated with exogenous GA3 revealed 428 differentially expressed genes, including 236 upregulated and 192 downregulated genes whereas GO enrichment analysis of these differentially expressed genes showed that the upregulated genes after GA3 treatment were mainly involved in catalytic activity, oxidation-reduction processes, and oxidoreductase activity, and in the KEGG metabolic pathways, the differentially expressed genes were primarily enriched in phenylpropanoid biosynthesis and suberin biosynthesis. Conclusion The kinetic analysis of T. grandis ‘Merrillii’ embryogenic callus culture can identify the optimal inoculation period and optimizing the culture conditions can enhance cell growth rate and stress resistance. In embryogenic callus of T. grandis ‘Merrillii’ under exogenous GA3 treatment, metabolic pathways such as phenylpropanoid biosynthesis and suberin biosynthesis are the main participating parts of differentially expressed genes. Moreover, changes in the expression levels of some key genes may play an important role in the growth and development, environmental adaptation, and stress response of T. grandis ‘Merrillii’ callus. [Ch, 5 fig. 1 tab. 31 ref.] -
Key words:
- Torreya grandis ‘Merrillii’ /
- embryogenic callus /
- suspension culture /
- gibberellin
-
表 1 差异表达基因RT-qPCR的特异性引物信息
Table 1. Gene-specific primer information for RT-qPCR
引物名称 正向引物(5′→3′) 反向引物(5′→3′) XM_024662224.1 TCCAAGGGAAGGGGAACATC TCCCCGGATTGCAGAAGATT XM_027242615.1 TGTACCCTCCACCCTTTTCC TCTATCAGGGAGGGAGCAGA XM_020665027.1 TACGACCAGTCAGAGGCTTG AAACACCCACCGTCTTTGAC XM_002991298.2 CACGCCCAATTTTCACGAGA CGAAAACTAGGCAGGGCATC XM_022920293.1 TCGACCTTGAGACCTGGAAC TGATGGTGCAGCAAATCAGG TgActin TGGCATCTCTCAGCACATTCCA TGCCAACATCTCAAGCAAGCAC -
[1] 黎章矩, 骆成方, 程晓建, 等. 香榧种子成分分析及营养评价[J]. 浙江林学院学报, 2005, 22(5): 540−544. LI Zhangju, LUO Chengfang, CHENG Xiaojian, et al. Component analysis and nutrition evaluation of seeds of Torreya grandis ‘Merrillii’ [J]. Journal of Zhejiang Forestry College, 2005, 22(5): 540−544. [2] 何慈颖, 娄和强, 吴家胜. 香榧油脂及其合成调控机制研究进展[J]. 浙江农林大学学报, 2023, 40(4): 714−722. HE Ciying, LOU Heqiang, WU Jiasheng. Research progress on synthesis and regulation mechanism of Torreya grandis ‘Merrillii’ kernel oil [J]. Journal of Zhejiang A&F University, 2023, 40(4): 714−722. [3] 余盛武, 吕赟薇, 蒋敏, 等. 香榧的营养和功能成分探析[J]. 南方农业, 2017, 11 (5): 75, 77. YU Shengwu, LÜ Yunwei, JIANG Min, et al. Analysis of nutritional and functional components of Torreya grandis ‘Merrillii’ [J]. South China Agriculture, 2017, 11 (5): 75, 77. [4] 胡渊渊, 鲍俊俊, 蔡晓郡, 等. 纳米复合膜对香榧坚果储藏过程中油脂品质的影响[J]. 浙江农林大学学报, 2024, 41(3): 447−456. HU Yuanyuan, BAO Junjun, CAI Xiaojun, et al. Effect of nanocomposite film on oil quality of Torreya grandis ‘Merrillii’ nuts during storage [J]. Journal of Zhejiang A&F University, 2024, 41(3): 447−456. [5] 陈林, 李永成. 海南粗榧细胞悬浮培养体系的建立[J]. 广东农业科学, 2014, 41(24): 54−57, 62. CHEN Lin, LI Yongcheng. Establishment of cell suspension culture system for Cephalotaxus mannii [J]. Guangdong Agricultural Sciences, 2014, 41(24): 54−57, 62. [6] 王沐兰, 杨生超, 郁步竹, 等. 红豆杉高产悬浮细胞系建立及其紫杉醇诱导的研究进展[J]. 广西植物, 2016, 36(9): 1137−1146. WANG Mulan, YANG Shengchao, YU Bubu, et al. Research progress in high yielding suspension cell lines and the induction of taxol in Taxus [J]. Guihaia, 2016, 36(9): 1137−1146. [7] 赵文佳. 青钱柳红色愈伤组织的诱导和筛选及细胞悬浮培养生产花青素[D]. 南昌: 江西农业大学, 2020. ZHAO Wenjia. Inducement and Screening of Cyclocarya Paliurus Red Callus and Cell Suspension Culture to Produce Anthocyanin [D]. Nanchang: Jiangxi Agricultural University, 2020. [8] 陈红贤, 于笑笑, 王晨阳, 等. 国槐槐角种胚细胞悬浮培养的动力学研究[J]. 浙江农林大学学报, 2016, 33(2): 272−279. CHEN Hongxian, YU Xiaoxiao, WANG Chenyang, et al. Kinetics of Sophora japonica embryo cells in a suspension culture system [J]. Journal of Zhejiang A&F University, 2016, 33(2): 272−279. [9] 徐志荣. 南方红豆杉细胞悬浮培养体系建立及培养条件优化[D]. 南昌: 江西农业大学, 2018. XU Zhirong. The Establishment of Cell Suspension Culture System and Optimization Culture Conditions of Taxus chinensis var. mairer [D]. Nanchang: Jiangxi Agricultural University, 2018. [10] 宋跃, 李淑娟, 张含国, 等. 落叶松胚性愈伤组织悬浮培养体系的优化[J]. 林业科学, 2018, 54(7): 146−154. SONG Yue, LI Shujuan, ZHANG Hanguo, et al. Establishment and optimization of embryogenic callus suspension culture system of Larix [J]. Scientia Silvae Sinicae, 2018, 54(7): 146−154. [11] KHAIPER M, DHANDA S K, AHLAWAT K S, et al. The effect of pre-sowing treatments with Glomus mosseae and GA3 on the leaves physiology of Melia azedarach seedling [J]. International Journal of Environment and Climate Change, 2023, 13(9): 2926−2937. [12] 马焕普, 刘志民. 赤霉素与果树的生长发育[J]. 植物学通报, 1998, 15(1): 27−36. MA Huanpu, LIU Zhimin. Gibberellins and fruit tree development [J]. Chinese Bulletin of Botany, 1998, 15(1): 27−36. [13] MOOSAVI M R. The effect of gibberellin and abscisic acid on plant defense responses and on disease severity caused by Meloidogyne javanica on tomato plants [J]. Journal of General Plant Pathology, 2017, 83(3): 173−184. [14] 程雨飞, 朱向涛, 季雯, 等. 牡丹愈伤组织增殖及褐化研究[J]. 广东农业科学, 2019, 46(6): 30−36. CHENG Yufei, ZHU Xiangtao, JI Wen, et al. Study on proliferation and browning of Peony callus [J]. Guangdong Agricultural Sciences, 2019, 46(6): 30−36. [15] 李合生. 植物生理生化实验原理和技术[M]. 北京: 高等教育出版社, 2000: 164−168. LI Hesheng. Principles and Techniques of Plant Physiology and Biochemistry Experiments [M]. Beijing: Higher Education Press, 2000: 164−168. [16] GRABHERR M G, HAAS B J, YASSOUR M, et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome [J]. Nature Biotechnology, 2011, 29(7): 644−652. [17] WANG Y, WEATHERS P J. Sugars proportionately affect artemisinin production [J]. Plant Cell Reports, 2007, 29(7): 1073−1081. [18] 钟巍然, 柴友荣, 张凯, 等. 苯丙烷代谢途径中细胞色素P450的研究[J]. 安徽农业科学, 2008, 36(13): 5285−5289. ZHONG Weiran, CHAI Yourong, ZHANG Kai, et al. Study on the cytochrome P450s in phenylpropanoid metabolic pathway [J]. Journal of Anhui Agricultural Sciences, 2008, 36(13): 5285−5289. [19] QIN Yuzhi, TAI G, XIE Kaiyun, et al. Ambient light alters gene expression pattern of enzymes and transcription factors involved in phenylpropanoid metabolic pathway in potato under chilling stress [J]. Agricultural Science & Technology, 2014, 15(11): 1899−1904. [20] FANG Lin, XU Xin, LI Ji, et al. Transcriptome analysis provides insights into the non-methylated lignin synthesis in Paphiopedilum armeniacum seed [J/OL]. BMC Genomics, 2019[2024-04-01]. DOI: 10.21203/rs.2.13152/v2. [21] COMPAGNON V, DIEHL P, BENVENISTE I, et al. CYP86B1 is required for very long chain ω-hydroxyacid and α, ω-dicarboxylic acid synthesis in root and seed suberin polyester [J]. Plant Physiology, 2009, 150(4): 1831−1843. [22] KOSMA D K, BOURDENX B, BERNARD A, et al. The impact of water deficiency on leaf cuticle lipids of Arabidopsis [J]. Plant Physiology, 2009, 151(4): 1918−1929. [23] LI Sufang, YE Tianwen, XU Xin, et al. Callus induction, suspension culture and protoplast isolation in Camellia oleifera [J/OL]. Scientia Horticulturae, 2021, 286 (1): 110193[2024-04-01]. DOI: 10.1016/j.scienta.2021.110193. [24] 陈继光, 上官新晨, 尹忠平, 等. 青钱柳悬浮细胞的培养及其基质消耗的规律[J]. 现代食品科技, 2014, 30(1): 44−49. CHEN Jiguang, SHANGGUAN Xinchen, YIN Zhongping, et al. Establishment of the cell suspension culture system of Cyclocarya paliurus and matrix consumption laws [J]. Modern Food Science and Technology, 2014, 30(1): 44−49. [25] PENG Chunxue, GAO Fang, WANG Hao, et al. Suspension culture and somatic embryogenesis of Korean pine [J]. International Journal of Experimental Botany, 2022, 91(1): 223−238. [26] GAO Jie, XUE Jingqi, XUE Yuqian, et al. Transcriptome sequencing and identification of key callus browning-related genes from petiole callus of tree peony (Paeonia suffruticosa cv. Kao) cultured on media with three browning inhibitors [J]. Plant Physiology and Biochemistry, 2020, 149: 36−49. [27] XUE Zhenglian, WANG Bingsheng, QU Changyu, et al. Response of salt stress resistance in highland barley (Hordeum vulgare L. var. nudum) through phenylpropane metabolic pathway [J/OL]. PLoS One, 2023, 18 (10): e0286957[2024-04-01]. DOI: 10.1371/journal.pone.0286957. [28] XIE Pengdong, YANG Yangyang, OYOM W, et al. Chitooligosaccharide accelerated wound healing in potato tubers by promoting the deposition of suberin polyphenols and lignin at wounds[J/OL]. Plant Physiology and Biochemistry, 2023, 199 : 107714[2024-04-01]. DOI: 10.1016/j.plaphy.2023.107714. [29] HU Bo, ZHANG Guifang, LIU Wu, et al. Divergent regeneration-competent cells adopt a common mechanism for callus initiation in angiosperms [J]. Regeneration, 2017, 4(3): 132−139. [30] POLLARD M, BEISSON F, LI Yonghua, et al. Building lipid barriers: biosynthesis of cutin and suberin [J]. Trends in Plant Science, 2008, 13(5): 236−246. [31] 魏晓博. 脱落酸与茉莉酸互作调控猕猴桃果实愈伤木栓质多聚酚类物质合成的作用机制[D]. 杭州: 浙江大学, 2022. WEI Xiaobo. The Mechanism of Abscisic Acid-jasmonic Acid Interaction in Regulating the Synthesis of Suberin Polyphenolics in Wounded Kiwifruit [D]. Hangzhou: Zhejiang University, 2022. -

-
链接本文:
https://zlxb.zafu.edu.cn/article/doi/10.11833/j.issn.2095-0756.20240291






 下载:
下载: