-
杜鹃花Rhododendron是举世公认的名贵观赏花卉,被誉为“世界之花”“花木之王”,也是国内的“十大名花”之一[1]。杜鹃花喜阴凉、潮湿环境,高温是限制杜鹃花正常生长和广泛利用的关键性因子,因此开展杜鹃花耐热等抗逆研究显得尤为重要[2-5]。丛枝菌根真菌(arbuscular mycorrhizal fungi,AMF)是陆地生态系统的重要成员[6],是一种能与90%以上的植物形成共生关系的微生物[7]。有研究表明:接种AMF可以显著促进植物的生长[8-9],提高植物抗连作障碍的能力[10],并能在一定程度上降低逆境胁迫,增强植物的抗逆性[11-14]。但接种AMF对杜鹃花抗热性影响的研究还未见报道。本研究对杜鹃花苗接种AMF,测定杜鹃花叶片的各项生理指标参数和解剖结构指标,以探讨在高温胁迫下,AMF对杜鹃花苗耐热性的影响,为杜鹃花在华北地区园林绿化中的应用提供科学依据。
-
选取生长健壮、长势一致的杜鹃花品种‘笔止’Rhododendron ‘Bi Zhi’扦插苗12盆,1株·盆-1。均栽培于陕西省杨凌新天地示范园。
-
3种供试丛枝菌根真菌:根内球囊霉Glimus intraradices(Gi),摩西球囊霉Glomus mosseae(Gm)和幼套球囊霉Glomus etunicatum(Ge)均由甘肃渤丰农林牧科技有限公司提供。将根内球囊霉、摩西球囊霉和幼套球囊霉的孢子经体积分数为30%过氧化氢消毒10 min,用无菌水漂洗数次,接种于三叶草Trifolium repens和苜蓿Medicago sativa,富集培养2个月后,去除地上部分,将孢子、菌根和根外菌丝制成AMF接种剂,孢子密度为70个·g-1。
-
试验土壤pH值6.0,主要原料为优质草炭、进口基质原料、珍珠岩、蛭石等。购于杨凌鑫方实验材料供应站。于烘箱中160 ℃高温灭菌2 h,自然冷却后继续160 ℃高温灭菌2 h后晾干备用。
-
试验于2017年1月至2018年5月在西北农林科技大学风景园林艺术学院实验室内进行。接种前,用质量浓度为5%的高锰酸钾溶液对所用花盆进行消毒。试验设4个处理:接种根内球囊霉(Gi),接种摩西球囊霉(Gm),接种幼套球囊霉(Ge)和不接种AMF的对照组(ck)。接种处理采用层施法,施接种菌剂80 g·盆-1。每处理重复3次。
接种后将杜鹃花苗放回杨凌新天地日光温室,温度为(25±1)℃,期间所有处理正常供水。接种50 d后将杜鹃花苗搬入(BIC-400)人工气候箱中进行预处理(25 ℃),光照时间为14 h/10 h(光照/黑暗),光照度12 000 lx,相对湿度80%,处理3 d。之后,将人工气候箱温度调为35 ℃/25 ℃(白天/晚上),光强和空气湿度不变,浇水1次·d-1,保持水分充足,高温胁迫处理10 d。
-
采集处理的杜鹃花幼苗的须根系,洗净,切成1~2 cm小段,浸入V(甲醛):V(冰醋酸):V(体积分数为50%乙醇)=13:5:200的固定液中,浸泡4 h,再浸入质量分数为8%氢氧化钾溶液,90 ℃水浴30 min,取出根段后洗净,于体积分数为5%乳酸溶液中浸泡5 min。用酸性品红溶液染色,最后在显微镜下观察杜鹃花根部是否被侵染,并计算AMF侵染率=(侵染根段长/观察根段长)×100%。
-
在高温处理0,5,10 d时,取各植株相同生长部位成熟的叶片采样,每次0.1 g,各重复3次。可溶性糖采用蒽酮法测定,游离脯氨酸、丙二醛、细胞膜透性、叶绿素等指标的测定均采用路文静等[15]描述的方法。利用总蛋白质测定试剂盒(南京建成生物工程研究所,A045)考马斯亮蓝G-250染色法测定可溶性蛋白质质量浓度。
-
生理指标测定结束后,将试验植株常温处理10 d。之后将不同处理植株分为常温对照组(25 ℃/18 ℃)和高温试验组(35 ℃/25 ℃),放入人工气候培养箱内培养5 d。处理结束后于早上10: 00采集健康植株叶片,迅速切成3 mm×3 mm,投入体积分数为4%戊二醛溶液中常温固定6 h,磷酸缓冲液(0.1 mol·L-1,pH 6.8)漂洗4次。用体积分数为30%,50%,70%,80%,90%乙醇依次脱水,100%乙醇脱水3次,乙酸异戊酯置换后,用二氧化碳临界点干燥仪(K-850),离子溅射仪(E-1045)喷金,再用钨灯丝扫描电镜(JSM-6360LV)对样本进行观察。随机选取10个视野对叶肉横切片观察、拍照。最后用Nano Measure 1.2测量软件测量叶片厚度、栅栏组织及海绵组织厚度,并计算栅栏海绵组织比、组织结构疏松度和组织结构紧密度。栅栏海绵组织比=(栅栏组织厚度/海绵组织厚度)×100%;组织结构紧密度=(栅栏组织厚度/叶片厚度)×100%;组织结构疏松度=(海绵组织厚度/叶片厚度)×100%。
-
耐热性综合评价采用隶属函数值对各项指标进行评价,与耐热性正相关采用公式B(xi)=(xi-xmin)/(xmax-xmin);负相关采用公式B(xi)=1-(xi-xmin)/(xmax-xmin)。其中:B(xi)为耐热隶属函数值;xi为指标测定值;xmin为测定指标最小值,xmax为测定指标最大值。根据上述公式,计算出杜鹃花品种‘笔止’各生理指标平均隶属函数值,然后进行排序,隶属函数值越大,耐热性越强[16]。
-
利用Excel 2010软件对数据进行处理分析,采用SPSS 22.0软件进行单因子方差分析,采用Duncan氏法进行差异显著性检验。图表中数据为平均值±标准误。
-
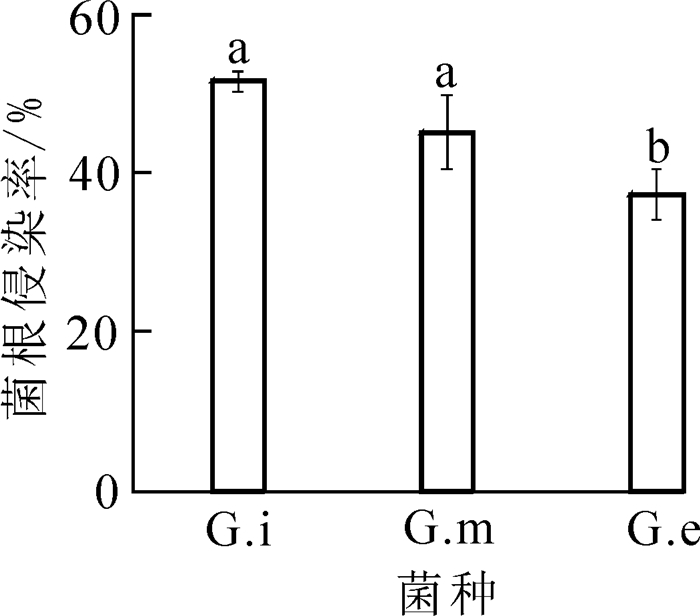
由图 1可知:常温条件下3种AMF对杜鹃花根系的根侵染率不同。其中Gi侵染率最高,为49.6%,而Ge(37.4%)侵染率较低。
-
由表 1可见,常温下接种AMF对杜鹃花叶片的可溶性糖的影响不显著,随着温度的增加,试验组和对照组的可溶性糖质量分数均有所增加。胁迫5 d,接种Gi和Gm的可溶性糖质量分数显著高于对照组(P<0.05),胁迫10 d,接种Gi的可溶性糖质量分数升高最为显著。
表 1 不同AMF对杜鹃花叶片生理参数的影响
Table 1. Effects of different arbuscular mycorrhizal fungi on physiological parameters of azaleas leaves
胁迫时间/d 处理 可溶性糖/(μg·g-1) 可溶性蛋白质/(g·L-1) 细胞膜透性/% 丙二醛/(μmol·g-1) 脯氨酸/(μg·g-1) 0 Gi 22.950 ± 1.345 a 3.307 ± 0.237 a 20.820 ± 1.049 c 3.473 ± 0.183 a 19.392 ± 2.455 b Gm 15.084 ± 1.154 b 3.601 ± 0.344 a 21.085 ± 0.996 c 4.025 ± 0.740 a 30.642 ± 1.982 a Ge 20.130 ± 2.602 ab 3.473 ± 0.075 a 27.715 ± 0.849 b 3.975 ± 0.344 a 28.726 ± 4.992 ab ck 14.716 ± 1.602 b 3.040 ± 0.256 b 33.557 ± 0.579 a 4.607 ± 0.144 a 19.231 ± 1.476 b 5 Gi 24.061 ± 0.814 a 3.977 ± 0.530 a 36.619 ± 0.842 b 3.980 ± 0.645 b 33.965 ± 5.216 b Gm 24.355 ± 0.972 ab 6.945 ± 0.483 b 36.838 ± 3.856 a 4.150 ± 0.838 b 41.802 ± 2.896 ab Ge 18.141 ± 1.460 b 4.856 ± 0.632 a 33.201 ± 1.905 c 5.592 ± 0.132 ab 46.653 ± 1.784 a ck 19.537 ± 2.286 b 4.174 ± 0.074 b 37.478 ± 0.932 a 5.768 ± 0.172 a 31.615 ± 1.247 b 10 Gi 31.410 ± 2.253 a 2.007 ± 0.129 a 37.922 ± 5.388 b 4.168 ± 0.166 d 46.833 ± 4.306 a Gm 25.178 ± 0.761 b 2.184 ± 0.208 a 38.416 ± 0.792 b 5.149 ± 0.141 c 54.546 ± 3.557 a Ge 23.615 ± 2.954 b 2.205 ± 0.320 a 37.049 ± 0.724 c 5.717 ± 0.235 b 47.249 ± 3.049 a ck 23.494 ± 1.444 b 1.528 ± 0.152 a 40.125 ± 1.896 a 6.508 ± 0.008 a 45.626 ± 1.953 a 说明:同列中不同小写字母表示差异显著(P<0.05) -
由表 1可见:常温下接种AMF对可溶性蛋白质质量浓度的影响不大,胁迫5 d,接种Gi,Gm,Ge和ck分别比常温下增加了20.3%,92.9%,39.8%,37.3%,其中接种Gm最为显著。随着胁迫天数增加,可溶性蛋白质质量浓度呈递减趋势,造成这一情况的原因可能是持续高温导致可溶性蛋白质合成所需的酶丧失活性,进而阻碍了可溶性蛋白质的合成。高温胁迫10 d,接种Ge的效果最好,比ck增加了40.3%。在高温胁迫5 d和10 d,Gm和Ge分别表现出较强的优势。
-
由表 1可见:随着胁迫天数的增加,脯氨酸质量分数随之增加。高温胁迫5 d,接种Gi,Gm和Ge的叶片脯氨酸质量分数分别比ck增加了7.4%,32.2%,47.6%,高温胁迫10 d,接种Gm表现最优,比ck增加了19.5%。
-
由表 1可见:随着胁迫天数的增加,细胞膜结构受到破坏,细胞膜透性增加。高温下接种AMF有效降低了细胞膜透性。高温胁迫5 d,接种Ge叶片细胞膜透性比ck降低了12.9%,高温胁迫10 d,接种Gi,Gm,Ge的杜鹃花叶片比ck各降低了5.8%,4.5%,8.3%。丙二醛是膜脂过氧化产物,它可以与细胞膜上的蛋白质和酶等结合,导致细胞膜完整性遭到破坏,丧失选择透性,引起电导率的上升。高温胁迫下,杜鹃花叶片的丙二醛质量摩尔浓度显著增加,对植物造成一定的伤害,接种AMF有限缓解了丙二醛的积累。其中接种Gi,Gm最为明显,5 d时,比ck减少了44.9%,39.0%。10 d,比ck减少了56.1%,26.4%。
-
随着温度的升高和胁迫时间的增加,接种AMF和对照组杜鹃花的叶绿素和类胡萝卜素质量分数均有所降低(图 2)。高温胁迫10 d,叶绿素和类胡萝卜素质量分数逐渐降低,而接种AMF的杜鹃花叶片叶绿素和类胡萝卜素质量分数均高于ck。接种Gi的杜鹃花叶片的叶绿素a、叶绿素和类胡萝卜素质量分数最高,分别比ck增加了39.7%,33.0%,16.3%,接种Gm的杜鹃花叶片的叶绿素b效果最为显著,比ck增加了33.3%,Gi次之。
-
从图 3可以看出:叶片解剖结构由叶表皮、栅栏组织、海绵组织及叶脉组成。叶表皮包括上表皮和下表皮,上、下表皮均为单层细胞且呈不规则扁长方形,排列紧密,表皮无毛。栅栏组织排列较为紧密,呈长柱形,海绵组织位于栅栏组织和叶片下表皮之间,且排列较为疏松。常温条件下,试验组栅栏组织排列较整齐,且呈长柱,ck栅栏组织整齐度变差。在高温下,ck和试验组栅栏组织和海绵组织厚度呈减小趋势,接种AMF的杜鹃花叶片解剖结构的部分栅栏组织细胞变短变粗,ck栅栏组织大部分发生短缩,排列参差不齐且海绵组织细胞排列零散,细胞间隙大。
由表 2可知:接种不同AMF并不同温度处理的杜鹃花叶片的叶肉细胞解剖结构指标差异显著(P<0.05)。常温条件下叶片平均厚度为186.49~201.75 μm,高温条件下叶片平均厚度126.41~187.49 μm,其中高温下ck叶片最薄(126.41 μm),常温下接种Gi菌的杜鹃花叶片最厚,为201.75 μm。海绵组织排列较为疏松,厚度均大于栅栏组织厚度,因此栅栏海绵组织比(简称栅栏比)均小于1。在高温条件下,接种AMF的杜鹃花叶片的栅栏比显著高于对照组(P<0.05),而细胞组织结构疏松度小于对照组。常温条件下,对照组和实验组表现不明显。
表 2 不同AMF对杜鹃花叶片叶肉组织结构的影响
Table 2. Effect of different arbuscular mycorrhizal fungi on mesophyll structure of Rhododendron
温度 处理 叶片厚度/μm 栅栏组织/μm 海绵组织/μm 栅栏组织/海绵组织 组织结构紧密度 组织结构疏松度 高温 Gi 187.49 ± 8.86 a 67.37 ± 3.56 a 97.32 ± 0.97 a 0.69 ± 0.06 a 0.35 ± 0.02 a 0.52 ± 0.03 ab Gm 180.81 ± 6.62 a 56.19 ± 3.19 a 81.78 ± 5.45 a 0.69 ± 0.15 a 0.31 ± 0.05 a 0.45 ± 0.04 c Ge 160.49 ± 7.17 a 67.31 ± 4.45 a 102.90 ± 4.92 a 0.66 ± 0.11 a 0.30 ± 0.03 ab 0.47 ± 0.03 b ck 126.41 ± 4.66 b 30.12 ± 2.80 b 60.44 ± 5.68 b 0.49 ± 0.06 b 0.26 ± 0.04 b 0.53 ± 0.04 a 常温 Gi 201.75 ± 6.15 a 59.14 ± 2.46 c 97.25 ± 6.59 a 0.62 ± 0.08 c 0.29 ± 0.02 b 0.48 ± 0.03 a Gm 191.68 ± 3.04 ab 58.50 ± 1.66 c 95.33 ± 3.02 a 0.61 ± 0.04 c 0.30 ± 0.02 b 0.50 ± 0.08 a Ge 186.49 ± 1.48 b 64.51 ± 1.03 a 86.61 ± 1.45 a 0.74 ± 0.02 a 0.35 ± 0.01 a 0.47 ± 0.01 a ck 186.52 ± 4.99 b 64.30 ± 6.08 b 107.11 ± 3.07 a 0.57 ± 0.06 b 0.32 ± 0.02 b 0.57 ± 0.02 a -
对以上生理指标(可溶性糖、可溶性蛋白质、脯氨酸、丙二醛、细胞膜透性、叶绿素)进行隶属函数分析(表 3)。根据平均隶属度排序,最终表明高温下,接种Gi效果最明显,其次是Gm,接种Ge的效果最差。
表 3 不同AMF耐热性综合评价
Table 3. Comprehensive evaluation on heat resistance of different arbuscular mycorrhizal fungi
处理 隶属度 平均隶属度 耐热性排序 可溶性糖 可溶性蛋 脯氨酸 丙二醛 细胞膜透性 叶绿素 高温Gi 1.000 0.447 0.135 1.000 0.290 1.000 0.645 1 高温Gm 0.213 0.611 1.000 0.581 0.587 0.742 0.619 2 高温Ge 0.015 0.613 0.182 0.307 1.000 0.182 0.036 3 高温ck 0.000 0.000 0.000 0.000 0.000 0.000 0.000 4 -
丛枝菌根真菌(AMF)是土壤微生物群落中生物量最大、最重要的成员之一,广泛分布于各种土壤生境中,对保持生态系统稳定具有重要作用[17]。大量研究表明:AMF能与植物根系形成共生菌根,有提高植物抗高温能力[18]。本试验也证明了接种丛枝菌根真菌可提高了杜鹃花‘笔止’的耐热性,表现为杜鹃花叶片组织结构受损较轻,叶片厚度、栅栏比、细胞结构密度均有所提高;可溶性糖、可溶性蛋白质、脯氨酸质量分数以及叶绿素质量分数均增加;此外,接种AMF,也能降低细胞膜透性和丙二醛质量摩尔浓度,且Gi对提高杜鹃花耐热性的效果最为显著,这可能与不同AMF对杜鹃花品种‘笔止’根系的侵染率高低有关,值得进一步研究。
在干旱情况下,接种AMF能使连翘Forsythia suspensa叶片的细胞膜透性降低[19]。从本试验结果来看,接种Gi,Gm和Ge真菌明显降低了杜鹃花叶片的细胞膜透性,其中接种Gi效果最明显,而接种Ge效果最差。说明在高温条件下,接种不同的丛枝菌根真菌对寄主植株影响不同。丙二醛质量摩尔浓度的多少能够反映细胞膜脂过氧化作用强弱和质膜破坏程度。本研究表明:高温胁迫下,接种AMF在一定程度上降低植物体内的丙二醛质量摩尔浓度,表明接种AMF使杜鹃花叶片的细胞膜过氧化程度减缓,从而提高了杜鹃花的耐热性。这和赵匠等[20]对黄檗Phellodendron amurense耐盐能力和曹岩坡等[21]对盐胁迫下芦笋Asparagus的研究结果一致。脯氨酸是一种渗透保护剂,其积累量与植物的抗逆能力呈正相关。可溶性糖是植物体内渗透调节的小物质分子,逆境条件下通过调节植物体内的渗透压来减轻细胞的机械损伤。可溶性蛋白质具有较高的保水性,可提高植物的抗性。许平辉等[22]研究证明:在干旱胁迫下,接种AMF能够增加茶树Camellia sinensis体内的渗透调节物质的质量分数。本试验也证明:高温胁迫下接种AMF,3种渗透调节物质的含量均随着温度增加而增加,表明杜鹃花在受到高温胁迫时,这些物质的积累可以减小细胞的伤害程度,从而提高植物的抗性。本研究结果表明:高温胁迫下,随着胁迫时间的增加,杜鹃花‘笔止’叶片的叶绿素质量分数均有所降低,但接种AMF植株叶片的叶绿素a,叶绿素b,类胡萝卜素和总叶绿素质量分数均高于未接种组。表明接种AMF能有效增强植株的光合能力。该试验结果与韩冰等[23]对苗期黄瓜Cucumis sativus和闫妍等[24]对低温下番茄Lycopersicon esculentum幼苗的研究结果一致。高温胁迫下,接种AMF的杜鹃花叶片厚度、栅栏比、细胞组织结构紧密度均大于ck,其中接种Gi处理的杜鹃花叶片厚度、栅栏组织和细胞结构紧密度与ck差异最显著,而细胞结构疏松度则小于ck。常温条件下,接种AMF的杜鹃花叶片与ck差异不大,该试验结果与郭邵霞等[13]研究的高温下牡丹Paeonia suffruticosa叶片解剖结果一致。
综合分析表明:高温胁迫对杜鹃花的叶片解剖结构和生理指标参数等均产生了显著影响。接种AMF,一定程度上能缓解高温胁迫对杜鹃花叶片组织结构、细胞膜物质、叶绿素、渗透调节物质等所造成的伤害,提高杜鹃花的耐热性。
Heat resistance of Rhododendron with arbuscular mycorrhizal fungi
-
摘要: 为研究丛枝菌根真菌对杜鹃花耐热性的影响,以杜鹃花品种‘笔止’Rhododendron ‘Bi Zhi’为试验材料,利用人工气候法,研究根内球囊霉Glimus intraradices(Gi),摩西球囊霉Glomus mosseae(Gm)和幼套球囊霉Glomus etunicatum(Ge)等3种丛枝菌根真菌(arbuscular mycorrhizal fungi,AMF)在高温胁迫下对杜鹃花幼苗生理生化指标的影响,比较高温胁迫下接种前后杜鹃花叶片解剖结构中栅栏组织厚度、海绵组织厚度、栅栏海绵组织比的差异,筛选出最佳AMF。结果表明:高温胁迫下,接种AMF不仅延缓了叶片的可溶性糖、可溶性蛋白质、游离脯氨酸和叶绿素质量分数的下降,并且使细胞膜透性和丙二醛摩尔质量分数保持相对较低的水平。此外,接种AMF后杜鹃花叶片解剖结构受损较轻,栅栏海绵组织比明显提高。根据平均隶属函数度对AMF提高杜鹃花的耐热性进行评价,其从强到弱排序为Gi,Gm和Ge,其中接种Gi的效果最为显著。因此,AMF能在一定程度上提高杜鹃花品种‘笔止’的耐热性。Abstract: To determine the effects of arbuscular mycorrhizal fungi (AMF) on heat resistance of Rhododendron and to choose the best AMF, Rhododendron 'Bi Zhi' was selected as the test material. Three AMF, Glomus intraradices (Gi), Glomus mosseae (Gm), and Glomus etunicatum (Ge) were used to study the effects on physiological and biochemical indexes of Rhododendron under high temperature stress. Analysis included the average subordinate function method to comprehensively evaluate the heat resistance with the three kinds of AMF and azalea. A comparison of the leaf anatomic structural characteristics before and after inoculation with the three fungi was conducted for heat stress by observing the performance. Results showed that under high temperature stress, the three AMF could colonize azalea roots. The colonization percentage of Gi was the highest (49.6%), while Ge the lest (37.4%). The contents of soluble sugar, soluble protein, chlorophyll, and proline increased compared to the control. At the same time the contents of malondialdehyde (MDA) and membrane permeability in azalea inoculated Gi stayed at a relatively low level. Furthermore, the anatomic structure of leaves inoculated with AMF was less damaged, and the ratio of palisade sponge tissue of the AMF inoculated leaf was much higher than that of the non-AMF control. According to the average subordinate function method, the heat resistance for azalea with AMF was in the order Gi > Gm > Ge. Thus, AMF could improve the heat resistance of the azalea cultivars to some extent.
-
表 1 不同AMF对杜鹃花叶片生理参数的影响
Table 1. Effects of different arbuscular mycorrhizal fungi on physiological parameters of azaleas leaves
胁迫时间/d 处理 可溶性糖/(μg·g-1) 可溶性蛋白质/(g·L-1) 细胞膜透性/% 丙二醛/(μmol·g-1) 脯氨酸/(μg·g-1) 0 Gi 22.950 ± 1.345 a 3.307 ± 0.237 a 20.820 ± 1.049 c 3.473 ± 0.183 a 19.392 ± 2.455 b Gm 15.084 ± 1.154 b 3.601 ± 0.344 a 21.085 ± 0.996 c 4.025 ± 0.740 a 30.642 ± 1.982 a Ge 20.130 ± 2.602 ab 3.473 ± 0.075 a 27.715 ± 0.849 b 3.975 ± 0.344 a 28.726 ± 4.992 ab ck 14.716 ± 1.602 b 3.040 ± 0.256 b 33.557 ± 0.579 a 4.607 ± 0.144 a 19.231 ± 1.476 b 5 Gi 24.061 ± 0.814 a 3.977 ± 0.530 a 36.619 ± 0.842 b 3.980 ± 0.645 b 33.965 ± 5.216 b Gm 24.355 ± 0.972 ab 6.945 ± 0.483 b 36.838 ± 3.856 a 4.150 ± 0.838 b 41.802 ± 2.896 ab Ge 18.141 ± 1.460 b 4.856 ± 0.632 a 33.201 ± 1.905 c 5.592 ± 0.132 ab 46.653 ± 1.784 a ck 19.537 ± 2.286 b 4.174 ± 0.074 b 37.478 ± 0.932 a 5.768 ± 0.172 a 31.615 ± 1.247 b 10 Gi 31.410 ± 2.253 a 2.007 ± 0.129 a 37.922 ± 5.388 b 4.168 ± 0.166 d 46.833 ± 4.306 a Gm 25.178 ± 0.761 b 2.184 ± 0.208 a 38.416 ± 0.792 b 5.149 ± 0.141 c 54.546 ± 3.557 a Ge 23.615 ± 2.954 b 2.205 ± 0.320 a 37.049 ± 0.724 c 5.717 ± 0.235 b 47.249 ± 3.049 a ck 23.494 ± 1.444 b 1.528 ± 0.152 a 40.125 ± 1.896 a 6.508 ± 0.008 a 45.626 ± 1.953 a 说明:同列中不同小写字母表示差异显著(P<0.05) 表 2 不同AMF对杜鹃花叶片叶肉组织结构的影响
Table 2. Effect of different arbuscular mycorrhizal fungi on mesophyll structure of Rhododendron
温度 处理 叶片厚度/μm 栅栏组织/μm 海绵组织/μm 栅栏组织/海绵组织 组织结构紧密度 组织结构疏松度 高温 Gi 187.49 ± 8.86 a 67.37 ± 3.56 a 97.32 ± 0.97 a 0.69 ± 0.06 a 0.35 ± 0.02 a 0.52 ± 0.03 ab Gm 180.81 ± 6.62 a 56.19 ± 3.19 a 81.78 ± 5.45 a 0.69 ± 0.15 a 0.31 ± 0.05 a 0.45 ± 0.04 c Ge 160.49 ± 7.17 a 67.31 ± 4.45 a 102.90 ± 4.92 a 0.66 ± 0.11 a 0.30 ± 0.03 ab 0.47 ± 0.03 b ck 126.41 ± 4.66 b 30.12 ± 2.80 b 60.44 ± 5.68 b 0.49 ± 0.06 b 0.26 ± 0.04 b 0.53 ± 0.04 a 常温 Gi 201.75 ± 6.15 a 59.14 ± 2.46 c 97.25 ± 6.59 a 0.62 ± 0.08 c 0.29 ± 0.02 b 0.48 ± 0.03 a Gm 191.68 ± 3.04 ab 58.50 ± 1.66 c 95.33 ± 3.02 a 0.61 ± 0.04 c 0.30 ± 0.02 b 0.50 ± 0.08 a Ge 186.49 ± 1.48 b 64.51 ± 1.03 a 86.61 ± 1.45 a 0.74 ± 0.02 a 0.35 ± 0.01 a 0.47 ± 0.01 a ck 186.52 ± 4.99 b 64.30 ± 6.08 b 107.11 ± 3.07 a 0.57 ± 0.06 b 0.32 ± 0.02 b 0.57 ± 0.02 a 表 3 不同AMF耐热性综合评价
Table 3. Comprehensive evaluation on heat resistance of different arbuscular mycorrhizal fungi
处理 隶属度 平均隶属度 耐热性排序 可溶性糖 可溶性蛋 脯氨酸 丙二醛 细胞膜透性 叶绿素 高温Gi 1.000 0.447 0.135 1.000 0.290 1.000 0.645 1 高温Gm 0.213 0.611 1.000 0.581 0.587 0.742 0.619 2 高温Ge 0.015 0.613 0.182 0.307 1.000 0.182 0.036 3 高温ck 0.000 0.000 0.000 0.000 0.000 0.000 0.000 4 -
[1] 林斌.中国杜鹃花(园艺品种及应用)[M].北京:中国林业出版社, 2008. [2] 申惠翡, 赵冰.杜鹃花品种耐热性评价及其生理机制研究[J].植物生理学报, 2018, 54(2):335-345. SHEN Huifei, ZHAO Bing. Study on evaluation of heat tolerance and its physiological mechanisms in Rhododendron cultivars[J]. Plant Physiol J, 2018, 54(2):335-345. [3] 梁雯, 赵冰, 黄文梅.热锻炼对杜鹃花耐热性的影响[J].浙江农林大学学报, 2018, 35(2):284-290. LIANG Wen, ZHAO Bing, HUANG Wenmei. Heat-resistance of Rhododendron with a heat acclimation pretreatment[J]. J Zhejiang A&F Univ, 2018, 35(2):284-290. [4] 高晓宁, 赵冰, 刘旭梅, 等. 4个杜鹃花品种对干旱胁迫的生理响应及抗旱性评价[J].浙江农林大学学报, 2017, 34(4):597-607. GAO Xiaoning, ZHAO Bing, LIU Xumei, et al. Physiological response to drought stress and drought resistance evaluation of four Rhododendron cultivars[J]. J Zhejiang A&F Univ, 2017, 34(4):597-607. [5] 高晓宁, 梁雯, 赵冰.外源水杨酸对2个杜鹃花品种抗旱性的影响[J].西北林学院学报, 2018, 33(3):131-136. GAO Xiaoning, LIANG Wen, ZHAO Bing. Effects of exogenous salicylic acid on heat-resistance of Rhododendron hybridum[J]. J Northwest For Univ, 2018, 33(3):131-136. [6] 刘润进, 焦惠, 李岩, 等.丛枝菌根真菌物种多样性研究进展[J].应用生态学报, 2009, 20(9):2301-2307. LIU Runjin, JIAO Hui, LI Yan, et al. Advances in the study of arbuscular mycorrhizal fungi onspecies diversity[J].China J Appl Ecol, 2009, 20(9):2301-2307. [7] 马通, 刘润进, 李敏.丛枝菌根真菌对生菜耐热性的效应[J].植物生理学报, 2015, 51(11):1919-1926. MA Tong, LIU Runjin, LI Min. Effects of arbuscular mycorrhizal fungi on heat-tolerance of Lactuca satica L.[J]. Plant Physiol J, 2015, 51(11):1919-1926. [8] 刘慧, 陈薇, 周勇, 等.内生真菌和丛枝菌根真菌对羊草生长的影响[J].植物生态学报, 2015, 39(5):477-485. LIU Hui, CHEN Wei, ZHOU Yong, et al. Effects of endophyte and arbuscular mycorrhizal fungi on growth of Leymus chinensis[J]. Chin J Plant Ecol, 2015, 39(5):477-485. [9] 马放, 苏蒙, 王立, 等.丛枝菌根真菌对小麦生长的影响[J].生态学报, 2014, 34(21):6107-6114. MA Fang, SU Meng, WANG Li, et al. Effects of arbuscular mycorrhizal fungi (AMF) on the growth of wheat[J]. Acta Ecol Sin, 2014, 34(21):6107-6114. [10] 陈可, 孙吉庆, 刘润进, 等.丛枝菌根真菌对西瓜嫁接苗生长和根系防御性酶活性的影响[J].应用生态学报, 2013, 24(1):135-141. CHEN Ke, SUN Jiqing, LIU Runjin, et al. Effects of arbuscular mycorrhizal fungus on the seedling growth of grafted watermelon and the defensive enzyme activities in the seedling root[J]. Chin J Appl Ecol, 2013, 24(1):135-141. [11] 刘爱荣, 陈双臣, 刘艳英, 等.丛枝菌根真菌对低温下黄瓜幼苗光合生理和抗氧化酶活性的影响[J].生态学报, 2011, 31(12):3497-3503. LIU Airong, CHEN Shuangchen, LIU Yanying, et al. Effects of AM fungi on leaf photosynthetic physiological parameters and antioxidant enzyme activities under low temperature[J]. Acta Ecol Sin, 2011, 31(12):3497-3503. [12] 张珊珊, 康洪梅, 杨文忠, 等.干旱胁迫下AMF对云南蓝果树幼苗生长和光合特征的影响[J].生态学报, 2016, 36(21):6850-6862. ZHANG Shanshan, KANG Hongmei, YANG Wenzhong, et al. Effects of arbuscular mycorrhizal fungi on growth and photosynthetic characteristics of Nyssa yunnanensis seedlings under drought stress[J]. Acta Ecol Sin, 2016, 36(21):6850-6862. [13] 郭绍霞, 王政军, 赵廷武.高温胁迫下AM真菌对牡丹叶片解剖结构和蒸腾特性的影响[J].青岛农业大学学报(自然科学版), 2011, 28(3):165-167. GUO Shaoxia, WANG Zhengjun, ZHAO Tingwu. Effects of Arbuscular mycorrhizal fungi on leaf anatomical structure and transpiration of Paeonia suffruticosa under high temperature stress[J]. J Qingdao Agric For Univ Nat Sci Ed, 2011, 28(3):165-167. [14] 孙吉庆, 刘润进, 李敏.丛枝菌根真菌提高植物抗逆性的效应及其机制研究进展[J].植物生理学报, 2012, 48(9):845-852. SUN Jiqing, LIU Runjin, LI Min. Advances in the study of increasing plant stress resistance and mechanisms by arbuscular mycorrhizal fungi[J]. Plant Physiol J, 2012, 48(9):845-852. [15] 路文静, 李奕松.植物生理学实验教程[M].北京:中国林业出版社, 2012. [16] 魏望, 施富超, 王东玮, 等.多倍体植物抗逆性研究进展[J].西北植物学报, 2016, 36(4):846-856. WEI Wang, SHI Fuchao, WANG Dongwei, et al. Advances in polyploid plant resistance[J]. Acta Bot Boreal-Occident Sin, 2016, 36(4):846-856. [17] 李素美, 王银桥, 刘润进.特殊生境中丛枝菌根真菌多样性[J].应用生态学报, 2013, 24(11):3325-3332. LI Sumei, WANG Yinqiao, LIU Runjin. Diversity of arbuscular mycorrhizal fungi in special habitats[J]. Chin J Appl Ecol, 2013, 24(11):3325-3332. [18] 田密, 陈应龙, 李敏, 等.丛枝菌根结构与功能研究进展[J].应用生态学报, 2013, 24(8):2369-2376. TIAN Mi, CHEN Yinglong, LI Min, et al. Structure and function of arbuscular mycorrhiza[J]. Chin J Appl Ecol, 2013, 24(8):2369-2376. [19] 赵平娟, 安锋, 唐明.丛枝菌根真菌对连翘幼苗抗旱性的影响[J].西北植物学报, 2007, 27(2):396-399. ZHAO Pingjuan, AN Feng, TANG Ming. Effect of arbuscular mycorrhizal fungi on drought resistance of Forsythia suspensa[J]. Acta Bot Boreal-Occident Sin, 2007, 27(2):396-399. [20] 赵匠, 徐佳晶, 李霞.丛枝菌根真菌对黄檗耐盐能力的影响[J].东北林业大学学报, 2016, 44(11):74-77. ZHAO Jiang, XU Jiajing, LI Xia. Effects of Arbuscular mycorrhizal fungi on salt tolerance ability of Phellodendron amurense[J]. J Northeast For Univ, 2016, 44(11):74-77. [21] 曹岩坡, 代鹏, 戴素英.丛枝菌根真菌(AMF)对盐胁迫下芦笋植株渗透调节物质及抗氧化酶活性的影响[J].西南大学学报(自然科学版), 2017, 39(5):43-48. CAO Yanpo, DAI Peng, DAI Suying. Effects of arbuscular mycorrhiza fungi (AMF) on osmoregulation substances and antioxidant enzyme activities of Asparagus plant under salt stress[J]. J Southwest Univ Nat Sci Ed, 2017, 39(5):43-48. [22] 许平辉, 王飞权, 齐玉岗, 等.丛枝菌根真菌对茶树抗旱性的影响[J].西北农业学报, 2017, 26(7):1033-1040. XU Pinghui, WANG Feiquan, QI Yugang, et al. Effect of arbuscular mycorrhiza fungi on drought resistance in tea plant[J]. J Northwest Agric, 2017, 26(7):1033-1040. [23] 韩冰, 徐刚, 郭世荣, 等.丛枝菌根真菌对苗期黄瓜生长及生理特性的影响[J].江苏农业学报, 2012, 28(6):1392-1397. HAN Bing, XU Gang, GUO Shirong, et al. Effects of arbuscular mycorrhiz fungi on growth and physiological characteristics of cucumber seedlings[J]. J Jiangsu Agric Sci, 2012, 28(6):1392-1397. [24] 闫妍, 孙超, 于贤昌, 等.低温胁迫对接种丛枝菌根真菌番茄幼苗生理特性的影响[J].中国农业大学学报, 2011, 16(6):64-69. YAN Yan, SUN Chao, YU Xianchang, et al. Effects of arbuscular mycorrhizal fungi on physiological properties of tomato seedlings under low temperature stress[J]. J China Agric Univ, 2011, 16(6):64-69. -

-
链接本文:
https://zlxb.zafu.edu.cn/article/doi/10.11833/j.issn.2095-0756.2019.04.013







 下载:
下载: