-
抗生素因其广谱的抗菌特性和促进生长作用广泛应用于医疗卫生、畜牧及水产养殖等领域[1]。然而,进入机体的抗生素仅有小部分发挥作用,剩下约30%~90%会随着代谢排出体外[2-4],并且以原药的形式输入周边环境[5]。一些化学性质稳定的抗生素会在环境中长期残留,不仅对周边植物[6]、鱼类[7]造成不利影响,其产生的选择压力还能够诱导耐药菌群的出现和抗性基因的传播[8]。鉴于其巨大的风险和威胁,抗生素以及抗性基因已经被世界卫生组织列为21世纪最重要的污染源之一。残留抗生素的去除和抗生素污染环境的修复成为保障生态安全和生命健康的重要举措。残留抗生素的去除主要有理化处理和生物降解2种途径,其中生物降解因为价格低廉、操作简便且不会造成二次污染而备受青睐[9]。当前关于利用微生物进行抗生素生物降解的研究日益增多,并已积累不少优良的菌种资源[10-11]。但抗生素被降解并不能确保转化产物的安全性,一些原药被代谢后可能会产生毒性更高的中间产物[12]。因此对降解菌株代谢产物的生物毒性评估和生态安全性评价是菌株能够实际应用的必要保证。土霉素(oxytetracycline,OTC)是当今应用范围最广、用量最多的抗生素之一[13],由于性状稳定已在陆地、水体等多种环境中被高频次检出[14-15]。本研究前期分离获得1株OTC高效降解菌Arthrobacter nicotianae ZAF-05,该菌株环境适应性好,能在液体体系、粪肥和土壤中发挥积极的降解作用[16]。本研究以该菌株为实验对象,利用高效液相色谱-电喷雾质谱联用技术(HPLC-Q-TOF-MS)初步解析菌株作用下OTC的降解产物,并以对OTC敏感的大肠埃希菌Escherichia coli、枯草芽孢杆菌Bacillus subtilis和水生生物斜生栅藻Scenedesmus obliquus作为指示生物,评估OTC降解产物的生物毒性,以期为菌株的安全使用提供初步理论依据。
-
OTC高效降解菌A. nicotianae ZAF-05于2019年分离自制药厂活性污泥。OTC敏感菌革兰氏阳性菌枯草芽孢杆菌No 1.821、革兰氏阴性菌大肠埃希菌No 1.12882均来自中国普通微生物菌种保藏管理中心(CGMCC),OTC敏感斜生栅藻购自上海光宇生物技术有限公司。
-
OTC购于Solarbio公司,纯度>99%。LB培养基用于细菌的培养,主要配方为胰蛋白胨10 g·L−1;酵母提取物5 g·L−1;氯化钠5 g·L−1,pH 7.2。BG11合成培养基用于供试斜生栅藻的培养,购自上海光宇生物技术有限公司并按说明书指导方法使用。质谱所用甲醇和乙腈等相关试剂均购自Merck公司(Kenilworth, 美国)。
-
制备不同密度降解菌菌悬液,绘制细胞数量与悬液600 nm波长下吸光度[D(600)]的标准曲线,根据标准曲线得到合适密度的细菌悬液。在OTC终质量浓度为100 mg·L−1的LB培养基中接入终密度为1.5×105 CFU·mL−1的降解菌。培养2 d后,取样菌悬液经过滤除菌、过柱萃取后利用HPLC-Q-TOF-MS技术检测OTC降解产物。质谱数据由Agilent 6545 Q-TOF质谱仪获得,使用电喷雾电离源,以正离子和负离子模式工作。具体工作条件:离子化压力3 500 V,裂解电压75 V,碰撞能30 eV,质荷比50~1 200。筛选获得可能的转化产物以TPs表示。以添加OTC但不接种降解菌的处理为阳性对照,以不添加OTC但接种降解菌的处理为阴性对照,所有处理均设置3个平行。
-
降解产物毒性测定设置4个处理。对照A(ckA):添加300 mg·L−1 OTC的LB液体培养基。T1处理:添加300 mg·L−1 OTC的LB培养基,接种降解菌;培养4 d后20 000 r·min−1高速离心2 min,取过滤除菌后的上清备用。T2处理:添加300 mg·L−1OTC的LB液体培养基,自然降解4 d。对照B(ckB):LB液体培养基。①OTC降解产物对细菌的生物毒性。取2组各4支试管,按1∶1(v/v)加入LB培养液和上述4个处理样品,定容至体积5 mL。分别依次接入1.5×105 CFU·mL−1的枯草芽孢杆菌和大肠埃希菌新鲜菌悬液,菌液密度确定方法同1.3.1。37 ℃下震荡培养,每隔24 h取样测定菌液吸光度[D(600)],用以表征细菌数量。②OTC降解产物对供试藻的生物毒性。取1组4个三角瓶,依次加入16 mL BG11培养基和2 mL上述4个处理样品,混匀后接种终密度为1.2×103个·mL−1新鲜藻种。于25 ℃、12 h (光照) /12 h (黑暗)光周期条件下静置培养,每天定时摇晃4~5次,每隔24 h取样测定吸光度[D(680)]。培养4 d后取样藻液,参考武鹏鹏等[17]的方法测定藻液叶绿素质量浓度,参考邹宁等[18]的方法观察藻细胞结构。
-
各处理均3个平行,所得数据利用SPSS 16.0进行方差分析,在0.01水平下确认差异显著性。
-
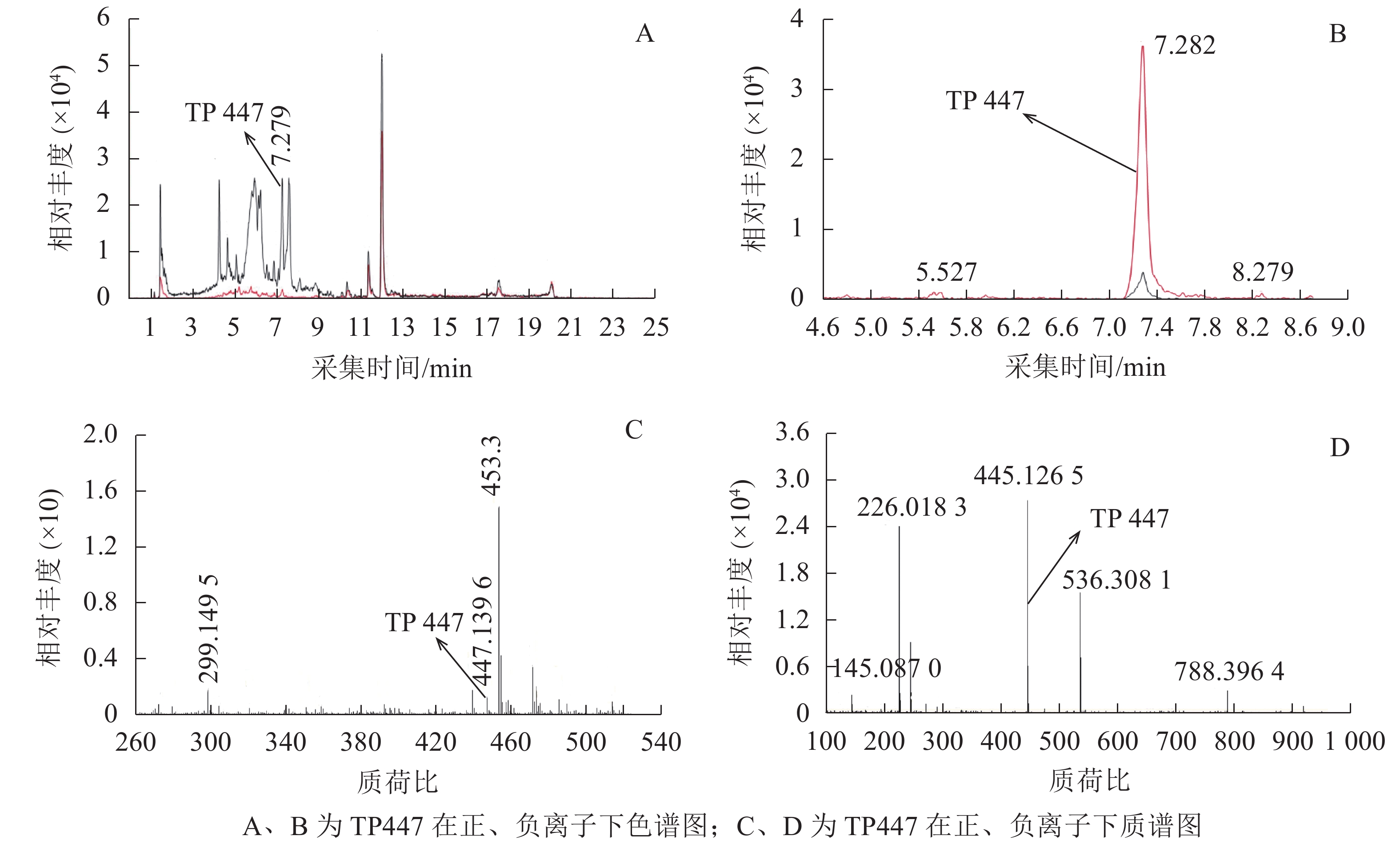
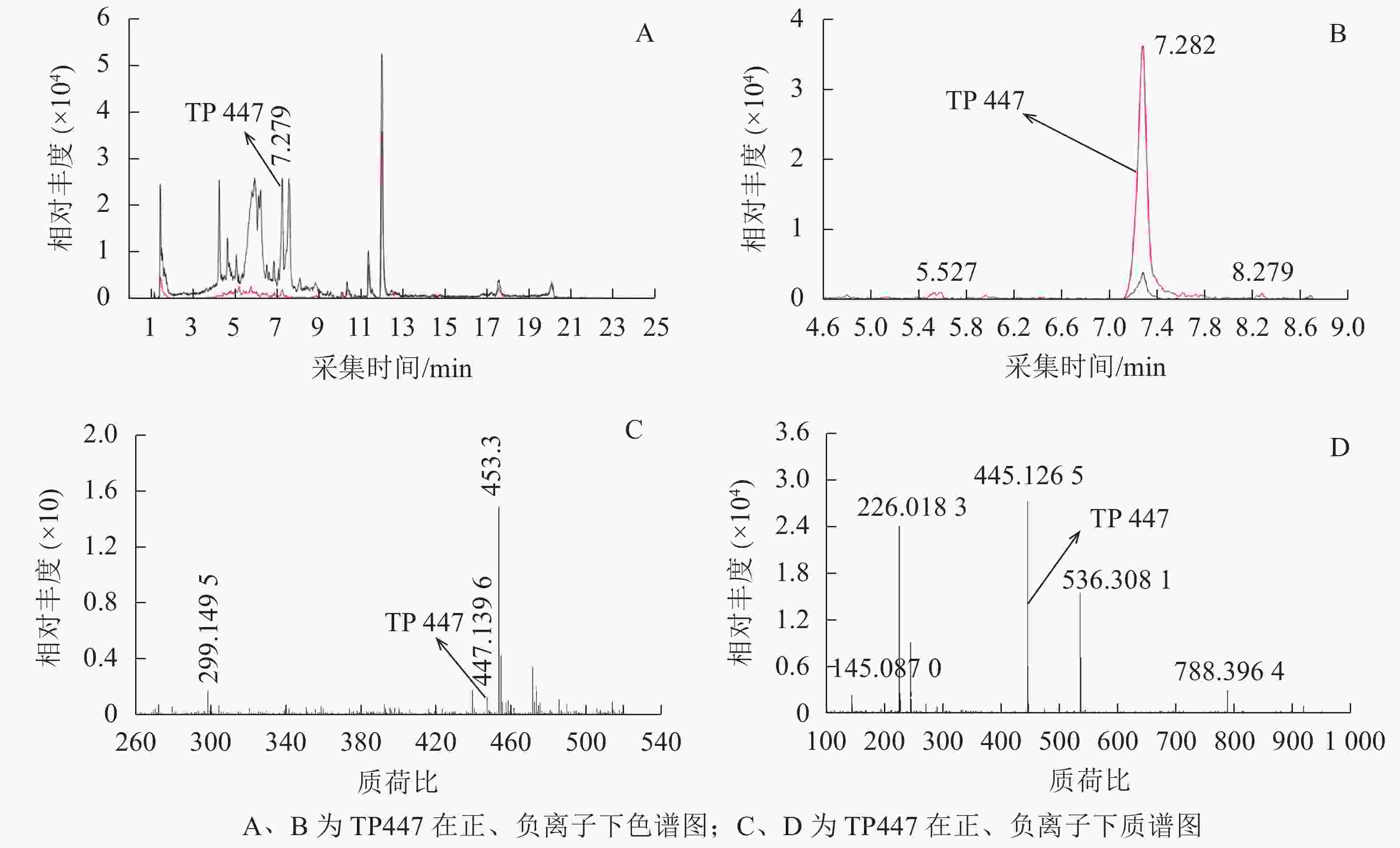
OTC降解产物的生物毒性既取决于残留的OTC,也取决于降解过程中产生的中间代谢产物。培养2 d后,对各处理悬液产物进行HPLC-Q-TOF-MS分析发现:降解菌组和阳性对照中均检出了TP447和TP461,阴性对照中则未检出;推测TP447和TP461可能为OTC降解相关中间产物。图1 A和图1 B可见:在7.279 min时TP447出现了明显的特征峰,其图谱特征以及出峰时间与QI等[19]发现的脱甲基OTC一致;由图1 C和图1 D可见:TP447在正、负离子作用下均出现了脱甲基OTC特有的离子碎片图谱,推测TP447可能是OTC水解产物——脱甲基OTC。TP461在4~7 min区段内出现了多个特征峰(图2 A),参考文献[20]可知:OTC出峰时间在5.434 min时,差向异构OTC(epioxytetracycline,EOTC)出峰时间在4.893 min时,同分异构OTC(isomeric oxytetracyline,ISO-OTC)出峰时间在6.550 min时;此3个化合物出峰时间、质荷比和分子离子峰图谱与TP461相同,推测TP461可能为OTC(图2 B)、EOTC (图2 C)和ISO-OTC (图2 D)的混合物。由表1可知:降解菌培养体系中OTC、EOTC和脱甲基OTC相对丰度极显著低于阳性对照(P<0.01),表明菌株ZAF-05在加速OTC分解的同时,还可能抑制了OTC向脱甲基OTC和EOTC的转化,使得体系中EOTC和脱甲基OTC丰度降低。
表 1 OTC及其推定代谢产物的相对丰度
Table 1. Relative abundance of OTC and the putative degradation products
可能的化合物 保留时间/min 分子式 相对丰度 阳性对照 降解菌处理 OTC 5.434 C22H24N2O9 13.872 7.254 EOTC 4.893 C22H24N2O9 20.931 5.231 ISO-OTC 6.550 C22H24N2O9 2.727 3.157 脱甲基OTC 7.279 C21H22N2O9 37.278 4.142 -
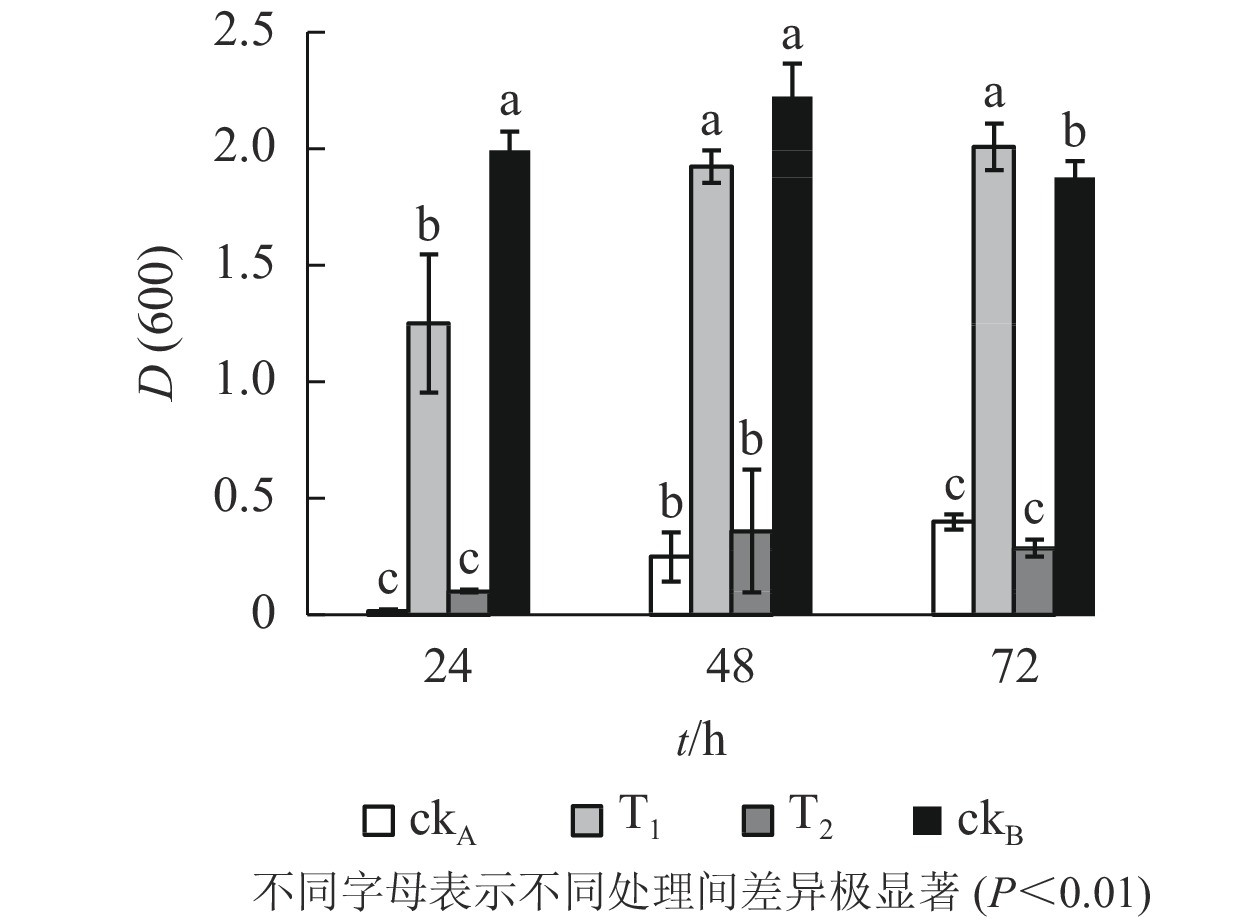
图3显示:72 h的培养期内,枯草芽孢杆菌在各处理中均有不同程度的生长,随时间推移细胞数量逐渐增加。具体来看,ckB处理下枯草芽孢杆菌生长良好,不同培养时间下细胞的吸光度[D(600)]始终最大,培养72 h时达2.563。相比之下,ckA处理72 h时D(600)仅0.334,表明受试细菌的生长受到明显抑制,极显著低于T1处理[D(600)=2.336]和ckB(P<0.01)。同时T2处理下吸光度一直处于较低水平[D(600)=0.424,72 h],与T1差异极显著(P<0.01)。提示OTC的自然水解过程并不能明显降低液体中OTC及其水解产物的毒性,降解菌ZAF-05能有效分解OTC,降低OTC及中间代谢产物的毒性;T1处理下细菌细胞数量与ckB最为接近,说明降解菌减轻了OTC及中间代谢产物对枯草芽孢杆菌生长的不利影响。
OTC的降解产物对大肠埃希菌生长的影响呈现出与枯草芽孢杆菌一致的态势(图4)。T2处理下,大肠埃希菌生长受阻,72 h培养期内,与ckA处理差异均不显著,说明OTC自然水解的解毒作用甚微。T1处理下,整个培养期内大肠埃希菌细胞数量始终处于较高水平,仅次于ckB,培养后期(48~72 h)甚至超过后者,推测可能是经ZAF-05降解后,OTC中间代谢产物为细菌生长提供了额外的碳源。
-
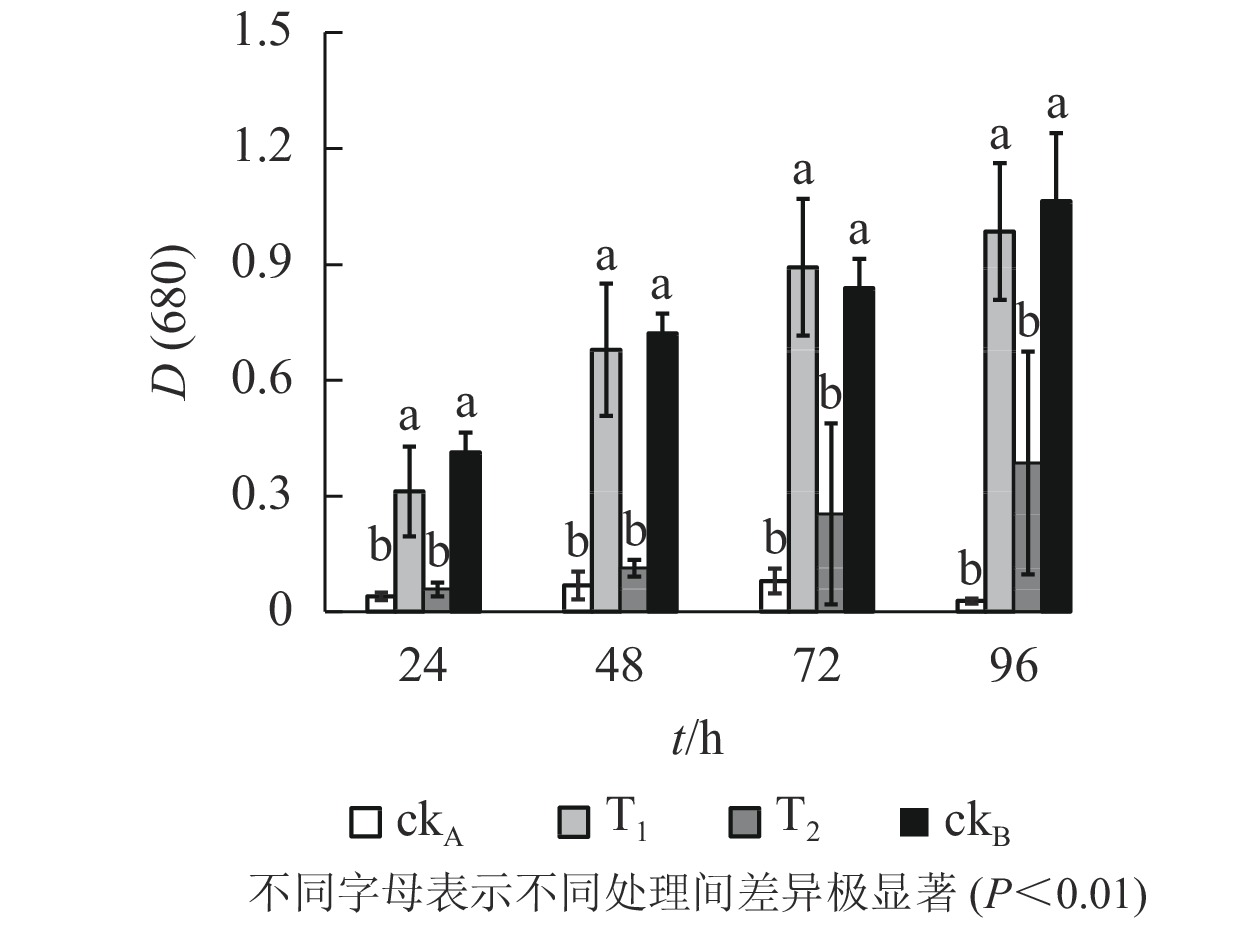
从图5可以看出:斜生栅藻在4个不同处理中的长势不同,ckB处理下供试藻生长良好[D(680)=1.064,96 h],而ckA中培养96 h后斜生栅藻吸光度[D(680)]仅0.028,表明OTC抑制斜生栅藻生长,生理毒性较强。T1处理下,斜生栅藻细胞数量与ckB无显著差异,远高于T2和ckA,说明降解菌株ZAF-05有效缓解了OTC对斜生栅藻的生物毒害。培养0~48 h,T2处理的吸光度与ckA比较差异不明显,尽管48 h后有所增加但远不及T1,说明OTC能发生自然水解,但解毒作用有限。
-
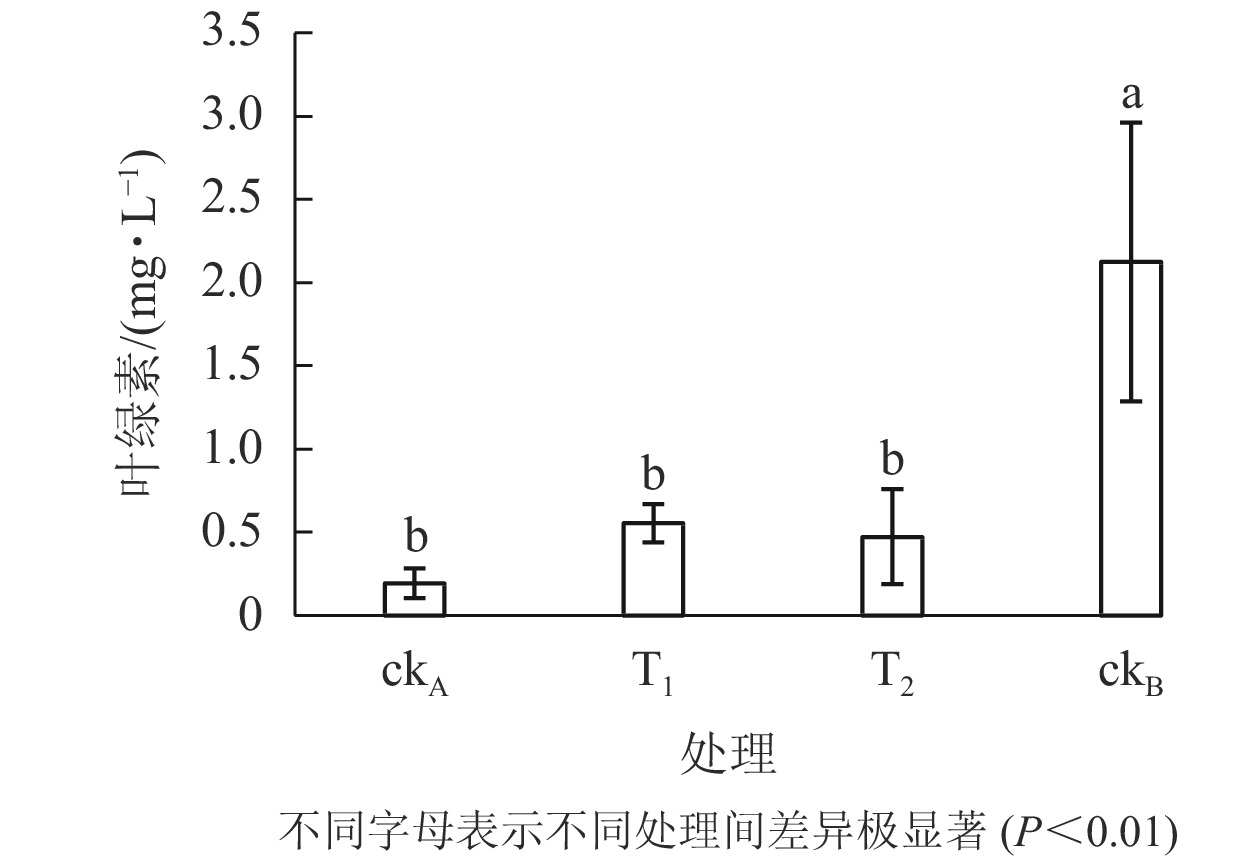
从图6可以看出:ckB处理下斜生栅藻叶绿素质量浓度为2.126 mg·L−1,ckA为0.193 mg·L−1。可见,OTC的存在大大降低了藻的叶绿素质量浓度。与ckA相比,T1处理一定程度上缓解了叶绿素的减少(0.555 mg·L−1),但仍极显著低于ckB (P<0.01)。结合图5认为:尽管T1与ckB的藻类细胞数量相差不大,但其叶绿素质量浓度却显著降低,说明尽管降解菌ZAF-05能高效降解OTC,但短期(96 h)内,仍不能完全转化毒性物质,培养体系中残留的毒性可能会影响斜生栅藻的叶绿素合成。
-
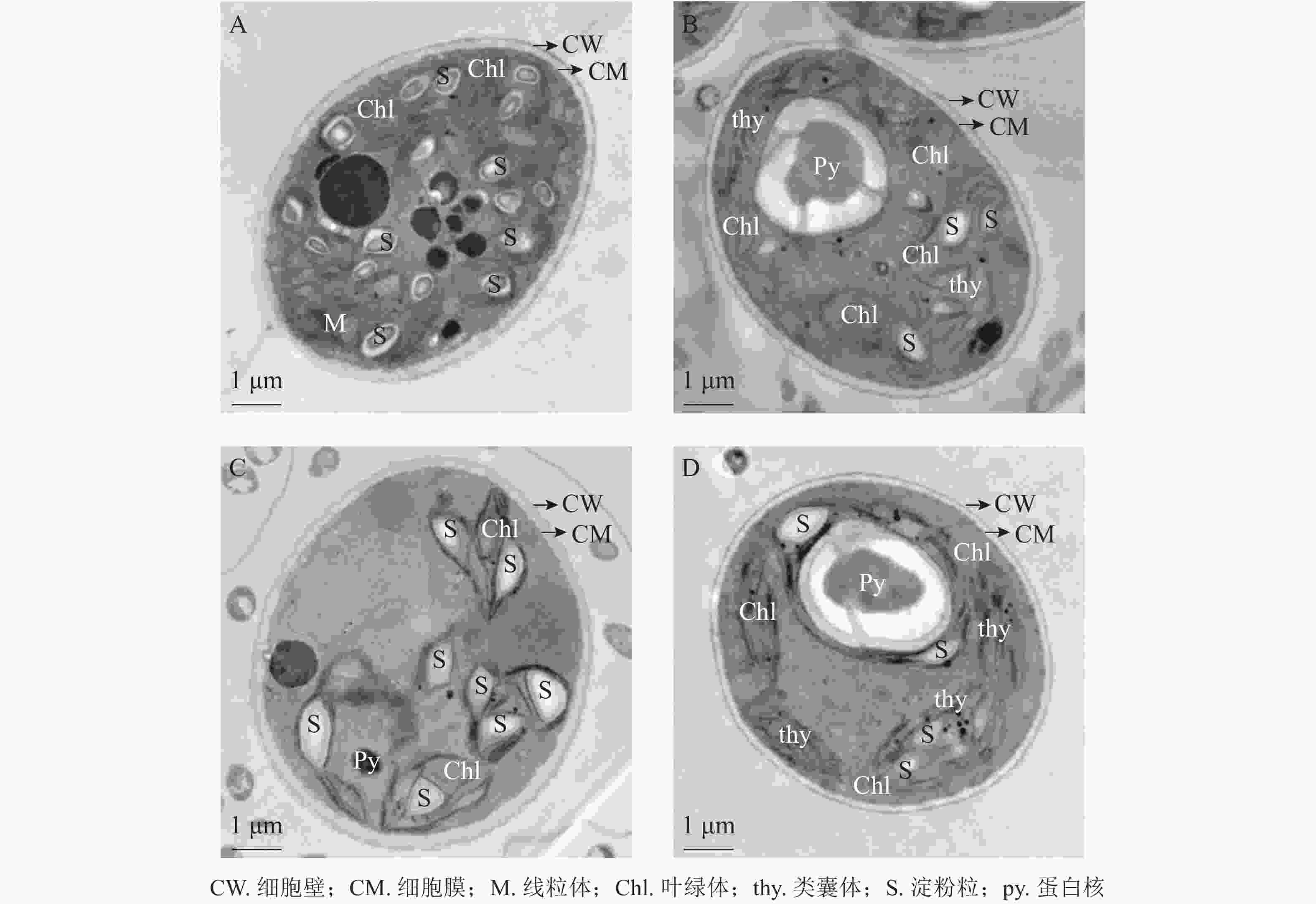
将斜生栅藻置于电镜下放大12 000倍观察细胞超微结构,由图7可见:ckB处理下斜生栅藻细胞截面为典型的椭圆形,细胞壁边缘整齐,细胞器界限清晰,叶绿体数量多,几乎布满整个细胞,平行排列的类囊体整齐地堆叠在叶绿体内。在叶绿体片层间的基质中可见电子密度较大的淀粉粒,淀粉粒数目较多(图7A)。相比之下ckA(图7B)和T2处理(图C)中的细胞壁边缘不整齐,略有缺刻,叶绿体数量少,且结构松散甚至破裂变形。叶绿体中基粒类囊体片层结构减少,形态模糊不清,且排列无序无方向。T1处理下(图7D),斜生栅藻细胞叶绿体少于ckB,但结构仍然完整,整齐地分布在细胞壁周围,类囊体的片层结构清晰可见且平行排列整齐,细胞结构完整无明显损伤。以上结果证明:与T2和ckA相比,T1处理对斜生栅藻的亚细胞结构影响更小,再次证明降解菌ZAF-05能够有效降解并降低OTC的生物毒性。
-
A. nicotianae ZAF-05是实验室前期分离获得的1株OTC高效降解菌,该菌株不仅能良好降解OTC,还对四环素和金霉素具有一定的去除能力,具有广泛的应用潜力。有些环境残留抗生素在消解时可能会被转化成毒性更高的中间代谢产物。如LI等[21]发现:经臭氧处理5~30 min后OTC大量分解,但产生的代谢产物比OTC的生物毒性更高,其生态危害也更大。因此明确降解菌株的代谢产物生物毒性是菌株能实际发挥作用的前提和保障。本研究采用HPLC-Q-TOF-MS技术解析了OTC的降解产物,最终获得脱甲基OTC、EOTC和ISO-OTC 3种代谢中间产物,其中脱甲基OTC和EOTC为自然水解产物,在降解菌体系和对照中均有出现,但对照中相对丰度远高于降解菌处理体系。研究[22-23]表明:四环素类抗生素的差向异构体,往往具有比母体化合物更高的毒性,而OTC水解形成的ISO-OTC毒性却较OTC及其衍生物(如EOTC)要小得多[23]。本研究中,对照中EOTC相对丰度为降解菌体系的4倍,而ISO-OTC在降解菌体系的相对丰度略高于对照,因此推测A. nicotianae ZAF-05促进了OTC及其高毒代谢产物EOTC向低毒物质(如ISO-OTC)的转化,降低了体系生物毒性。
本研究以大肠埃希菌、枯草芽孢杆菌和模式藻斜生栅藻为靶标生物,考察了OTC及其降解产物对供试细菌和藻类的影响。结果表明:OTC不仅抑制细菌和供试藻增殖,还导致藻细胞变形,引起叶绿体片层结构破裂,与同属四环素族的金霉素的细胞毒性表现一致[24]。OTC被降解后,细菌和藻细胞生长的抑制作用减轻,藻叶绿素合成和叶绿体超微结构的伤害作用缓解。说明降解菌ZAF-05促进体系中OTC及其高毒代谢产物EOTC向低毒物质转化,从而降低了生物毒性。
叶绿体是藻进行光合作用的重要细胞器,内部密布排列整齐的基粒内囊体,基粒内囊体既是叶绿素也是光合系统存在的场所,光合作用过程中的光反应在此进行[25-26],因此基粒类囊体结构的完整性和有序性是叶绿体进行正常、高效的光能转换的首要前提,对于保障光合作用的顺利进行具有重要意义。本研究中OTC的存在破坏了藻细胞叶绿体中基粒内囊体片层结构的完整,影响了光合作用。这可能是OTC导致藻细胞数量减少的一个重要原因。而添加菌株ZAF-05后,藻内叶绿体仍整齐地分布在细胞壁周围,类囊体片层结构清晰可见且平行排列整齐,细胞结构完整无明显损伤,再一次证实,ZAF-05的降解作用能降低OTC的生物毒性,提高其生态安全性。
Reduction of toxicity of oxytetracycline by highly efficient degrading bacterium Arthrobacter nicotianae ZAF-05
-
摘要:
目的 明确1株土霉素(oxytetracycline, OTC)高效降解菌降低OTC生物毒性的效应,为菌株的实际应用提供初步理论依据。 方法 以前期获得的OTC高效降解菌Arthrobacter nicotianae ZAF-05为对象,利用高效液相色谱-电喷雾质谱联用技术(HPLC-Q-TOF-MS)分析了OTC的降解产物。以对OTC敏感的大肠埃希菌Escherichia coli、枯草芽孢杆菌Bacillus subtilis和斜生栅藻Scenedesmus obliquus作为指示生物,评估OTC降解产物的生物毒性。 结果 HPLC-Q-TOF-MS分析发现:经菌株ZAF-05作用后,体系中的高毒性OTC以及其自然水解产物差向异构土霉素(EOTC)含量显著降低,而低毒性的同分异构土霉素(ISO-OTC)含量略有上升。供试细菌和藻类在添加ZAF-05菌株的OTC体系中的生长情况明显好于OTC自然水解的处理和未添加降解菌的阴性对照,且差异极显著(P<0.01)。与原药和自然水解物相比,经过ZAF-05菌株降解的OTC代谢产物生物毒性大大降低。菌株的降解作用缓解了OTC对斜生栅藻细胞超微结构的伤害,细胞叶绿体及细胞壁受损减轻。 结论 降解菌ZAF-05能降低OTC残留的环境生物毒性,而毒性的降低可能与体系中高毒性物质被转化成无毒或低毒的代谢产物有关。图7表1参26 Abstract:Objective The objective is to determine the effect of a degrading bacterium which can effectively reduce the bio-toxicity of oxytetracycline (OTC) metabolites, so as to provide a preliminary theoretical basis for practical application of the strain. Method Taking Arthrobacter nicotianae ZAF-05, an efficient OTC-degrading bacterium obtained in the previous studies, as the object, the degradation products of OTC were analyzed by high-performance liquid chromatography-quadrupole-time-of-flight mass spectrometry (HPLC-Q-TOF-MS). OTC sensitive Escherichia coli, Bacillus subtilis, and Scenedesmus obliquus were used as indicators to evaluate the bio-toxicity of degradation products. Result HPLC-Q-TOF-MS analysis showed that the content of highly toxic OTC and its natural hydrolysate epioxytetracycline(EOTC) decreased significantly after treatment with strain ZAF-05, while the content of low toxic isomeric oxytetracyline(ISO-OTC) increased slightly. The growth of tested bacteria and alga in OTC system treated by strain ZAF-05 was significantly better than that in OTC natural hydrolysis treatment and negative control without degrading bacteria, and the difference was significant (P<0.01). The results indicated that the bio-toxicity of OTC metabolites degraded by strain ZAF-05 was greatly reduced compared with the active drug and its natural hydrolysates. Besides, the degradation effect of the strain alleviated the damage of OTC to the ultrastructure of algal cells, as well as the damage to chloroplasts and cell walls. Conclusion Degrading bacterium ZAF-05 can reduce bio-toxicity of OTC residues, which may be related to the conversion of highly toxic substances into non-toxic or low-toxic metabolites in the system. [Ch, 7 fig. 1 tab. 26 ref.] -
Key words:
- oxytetracycline /
- degrading bacterium /
- degradation products /
- algae /
- bio-toxicity
-
表 1 OTC及其推定代谢产物的相对丰度
Table 1. Relative abundance of OTC and the putative degradation products
可能的化合物 保留时间/min 分子式 相对丰度 阳性对照 降解菌处理 OTC 5.434 C22H24N2O9 13.872 7.254 EOTC 4.893 C22H24N2O9 20.931 5.231 ISO-OTC 6.550 C22H24N2O9 2.727 3.157 脱甲基OTC 7.279 C21H22N2O9 37.278 4.142 -
[1] TURKER G, AKYOL Ç, INCE O, et al. Operating conditions influence microbial community structures, elimination of the antibiotic resistance genes and metabolites during anaerobic digestion of cow manure in the presence of oxytetracycline [J]. Ecotoxicology Environ Saf, 2018, 147: 349 − 356. [2] LI Xuewen, XIE Yunfeng, WANG Jinfeng, et al. Influence of planting patterns on fluoroquinolone residues in the soil of an intensive vegetable cultivation area in northern China [J]. Sci Total Environ, 2013, 458/460: 63 − 69. [3] HALLER M Y, MULLER S R, MCARDELL C S, et al. Quantification of veterinary antibiotics (sulfonamides and trimethoprim) in animal manure by liquid chromatography-mass spectrometry [J]. J Chromatogr A, 2002, 952(1): 111 − 120. [4] HALLING-SØRENSEN B. Inhibition of aerobic growth and nitrification of bacteria in sewage sludge by antibacterial agents [J]. Arch Environ Contam Toxicol, 2001, 40(4): 451 − 460. [5] XIE Yunfeng, LI Xuewen, WANG Jinfeng, et al. Spatial estimation of antibiotic residues in surface soils in a typical intensive vegetable cultivation area in China [J]. Sci Total Environ, 2012, 430: 126 − 131. [6] CHENG Dengmiao, LIU Xinhui, WANG Liang, et al. Seasonal variation and sediment-water exchange of antibiotics in a shallower large lake in North China [J]. Sci Total Environ, 2014, 476/477: 266 − 275. [7] BARROS-BECKER F, ROMERO J, PULGAR A, et al. Persistent oxytetracycline exposure induces an inflammatory process that improves regenerative capacity in zebrafish larvae[J]. PLoS One, 2012, 7(5): e36827. doi: 10.1371/journal.pone.0036827. [8] CZEKALSKI N, BERTHOLD T, CAUCCI S, et al. Increased levels of multiresistant bacteria and resistance genes after wastewater treatment and their dissemination into Lake Geneva, Switzerland[J]. Front Microbiol, 2012, 3. doi: 10.3389/fmicb.2012.00106. [9] GUO Xiaohong, XIE Chengyun, WANG Lijuan, et al. Biodegradation of persistent environmental pollutants by Arthrobacter sp. [J]. Environ Sci Pollut Res, 2019, 26(9): 8249 − 8443. [10] MAKI T, HASEGAWA H, KITAMI H, et al. Bacterial degradation of antibiotic residues in marine fish farm sediments of Uranouchi Bay and phylogenetic analysis of antibiotic-degrading bacteria using 16S rDNA sequences [J]. Fisheries Sci, 2006, 72(4): 811 − 820. [11] SHAO Sicheng, HU Yongyou, CHENG Ce, et al. Simultaneous degradation of tetracycline and denitrification by a novel bacterium, Klebsiella sp. SQY5 [J]. Chemosphere, 2018, 209: 35 − 43. [12] LI Kuixiao, YEDILER A, YANG Min, et al. Ozonation of oxytetracycline and toxicological assessment of its oxidation by-products [J]. Chemosphere, 2008, 72(3): 473 − 478. [13] GRANADOS-CHINCHILLA F, RODRIGUEZ C. Tetracyclines in food and feeding stuffs: from regulation to analytical methods, bacterial resistance, and environmental and health implications[J]. J Anal Methods Chem, 2017. doi: 10.1155/2017/1315497. [14] HU Xiangang, ZHOU Qixing, LUO Yi. Occurrence and source analysis of typical veterinary antibiotics in manure, soil, vegetables and groundwater from organic vegetable bases, northern China [J]. Environ Pollut, 2010, 158(9): 2992 − 2998. [15] HOU Jie, WANG Chong, MAO Daqing, et al. The occurrence and fate of tetracyclines in two pharmaceutical wastewater treatment plants of Northern China [J]. Environ Sci Pollut Res, 2016, 23: 1722 − 1731. [16] SHI Yanke, LIN Hui, MA Junwei, et al. Degradation of tetracycline antibiotics by Arthrobacter nicotianae OTC-16[J]. J Hazard Mater, 2021, 403: 123996. doi: 10.1016/j.jhazmat.2020.123996. [17] 武鹏鹏, 王雅学, 沈洪艳. 土霉素对斜生栅藻的毒性效应研究[J]. 生态毒理学报, 2020, 15(4): 215 − 223. WU Pengpeng, WANG Yaxue, SHEN Hongyan. Toxic effects of oxytetracycline on Scenedesmus obliquus [J]. Asian J Ecotoxicology, 2020, 15(4): 215 − 223. [18] 邹宁, 魏丕伟, 肖波, 等. 扁藻细胞电镜观察的制样技术[J]. 烟台教育学院学报, 2004, 10(3): 73 − 75, 97. ZOU Ning, WEI Piwei, XIAO Bo, et al. Sample preparation methods of Platymonas subcordiformis for the submicroscopic structure observation by transmission electric microscope (TEM) [J]. J Yantai Coll Educ, 2004, 10(3): 73 − 75, 97. [19] QI Weining, LONG Jian, FENG Changqing, et al. Fe3+ enhanced degradation of oxytetracycline in water by Pseudomonas [J]. Water Res, 2019, 160: 361 − 370. [20] HALLING-SØRENSEN B, LYKKEBERG A, INGERSLEV F, et al. Characterisation of the abiotic degradation pathways of oxytetracyclines in soil interstitial water using LC-MS-MS [J]. Chemosphere, 2003, 50(10): 1331 − 1342. [21] LI Zhaojun, QI Weining, FENG Yao, et al. Degradation mechanisms of oxytetracycline in the environment [J]. J Integr Agric, 2019, 18(9): 1953 − 1960. [22] HALLING-SØRENSEN B, SENGELØV G, TJRØNELUND J. Toxicity of tetracyclines and tetracycline degradation products to environmentally relevant bacteria, including selected tetracycline-resistant bacteria [J]. Arch Environ Contam Toxicol, 2002, 42(3): 263 − 271. [23] XU Dongmei, XIAO Yingping, PAN Hua, et al. Toxic effects of tetracycline and its degradation products on freshwater green algae [J]. Ecotoxicology Environogy Saf, 2019, 174: 43 − 47. [24] 张迪, 厉圆, 沈忱思, 等. 金霉素及其异构体降解产物对斜生栅藻的毒性效应研究[J]. 农业环境科学学报, 2019, 38(4): 756 − 764. ZHANG Di, LI Yuan, SHEN Chensi, et al. Understanding the toxic effects of chlortetracyclineandits and its isomer degradation products on Scenedesmus obliquus [J]. J Agro-Environ Sci, 2019, 38(4): 756 − 764. [25] STAEHELIN L A. Chloroplast structure: from chlorophyll granules to supra-molecular architecture of thylakoid membranes [J]. Photosynth Res, 2003, 76(1/3): 185 − 196. [26] ALBERTSSON P. A quantitative model of the domain structure of the photosynthetic membrane [J]. Trends Plant Sci, 2001, 6(8): 349 − 354. -

-
链接本文:
https://zlxb.zafu.edu.cn/article/doi/10.11833/j.issn.2095-0756.20200819






 下载:
下载: