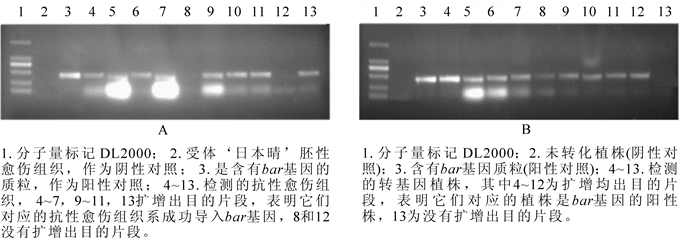
-
草丁膦(glufosinate,PPT)又叫草铵膦,是一种低毒且广谱性的有机磷类除草剂。它被植物吸收后,能有效抑制谷酰胺合成酶的活性,使铵在细胞里迅速积累,最后导致整个植物体死亡[1-2]。其抗性bar基因(bialaphos resistance gene)来自土壤吸水链霉菌Streptomyces hygroscopicus,它编码膦丝菌素乙酰转移酶(phosphinthricin acetyltransferase,PAT),PAT能使PPT的氨基发生乙酰化,进而解除PPT对植物的毒性[3]。因此,含有bar基因的植物对草丁膦除草剂具有一定的抗性[4]。将bar基因转入农作物,不仅有效去除杂草,而且对施用作物无毒害。转bar基因农作物的培育和应用受到育种者的广泛重视[5-7],转抗草丁膦的油菜Brassica campestris和玉米Zea mays已经商业化生产[8]。水稻Oryza sativa是世界性的粮食作物,中国是世界上最大的水稻生产和消费国。随着杂交育种和分子辅助选择育种技术的应用和发展,水稻产量不断提高,品质也得到了极大改良[9]。然而,目前仍有一些因素制约水稻生产。例如病虫害和田间杂草等生物逆境以及一些旱灾等非生物逆境都会造成水稻严重的减产。特别是杂草不仅与水稻争夺水分及养料,而且提高人工成本,已经成为水稻生产上的一大难题[10]。bar基因不仅可以作为选择标记基因,而且能使水稻具有抗除草剂的优良特征。转bar基因水稻能有效除去杂草,大大减少劳动力[11]。1996年美国对抗除草剂Liberty(草丁膦的商业名)转基因水稻进行抗性鉴定试验,喷施Liberty后,杂草被有效控制,而转基因水稻不受影响。与此同时,国内外开展了抗草丁膦基因转化水稻的相关研究。于志华等[12]采用基因枪法获得了抗bar基因旱稻植株,试图解决旱稻田间直播的杂草问题。CAO等[13]将bar基因作为筛选标记,利用微粒轰击法转化水稻的悬浮细胞,获得转化植株。PARK等[14]选用水稻茎尖分生组织作为转化受体,通过农杆菌介导法,获得几棵转基因植株,在后代中可以稳定遗传。黄大年等[15]用基因枪分别对水稻的幼胚进行转化,选取20.0~40.0 mg· L-1的草丁膦进行选择,获得6株转基因水稻,转化效率很低。简玉瑜等[16]通过基因枪将蚕抗菌肽基因转化发芽种胚、未成熟胚和愈伤组织3种组织,选用4.0 mg· L-1的Basta进行抗性筛选,只有发芽种胚获得5株抗性植株,分子检测3株为阳性株。许新萍等[17]用基因枪对水稻的愈伤组织进行转化,选取2.0~4.0 mg· L-1的Basta进行选择,并获得转基因植株,转化效率为89.6%。CHEN等[18]用农杆菌介导法对‘明恢63’的愈伤组织进行转化,先用25.0 mg· L-1的Basta筛选8~9周后,再用20.0 mg· L-1的Basta选1次,获得转基因植株的阳性率为70%。综上所述,所选的受体部位是悬浮细胞、茎尖分生组织、幼胚、成熟胚和愈伤组织等,草丁膦的筛选质量浓度为0.5~40.0 mg· L-1,没有一个确定的转化浓度。目前,以潮霉素作为选择压的水稻转基因体系已经非常成熟。但利用草丁膦作为筛选剂转化水稻胚性愈伤组织的报道较少。基于此,本研究选取具有成功再生体系的‘日本晴’‘秀水134’‘中花11’的胚性愈伤组织作为受体,筛选出最适的草丁膦转化质量浓度,再利用农杆菌介导法,将bar基因转化这些水稻的胚性愈伤组织,获得抗除草剂转基因水稻,建立以草丁膦为选择压的高效转化体系,为后续选育生产中农艺性状良好的抗性品种提供转基因材料。
HTML
-
粳稻‘日本晴’Oryza sativa‘Nipponbare’‘中花11’Oryza sativa‘Zhonghua 11’和‘秀水134’ Oryza sativa‘Xiushui 134’种子;具有草丁膦抗性的pKF111双元载体;纯度为99%草丁膦;EHA105农杆菌菌株。
-
‘日本晴’‘秀水134’和‘中花11’成熟种子用质量浓度为30%次氯酸钠表面消毒,无菌水洗涤2~3次,接种在胚性愈伤组织诱导的培养基R1[NB(N6培养基+B5维生素)+ 2.5 mg· L-1 2.4-D+0.5 g· L-1脯氨酸+0.3 g· L-1水解酪蛋白+30 g· L-1蔗糖+4.0 g· L-1非特胶(phytalgel)上,15 d后种子表面出现少量的愈伤组织,挑取出来继代在相同的培养基(2.4-D减为2 mg· L-1)上,胚性愈伤组织组织进一步增殖,待用做转化[19-20]。
-
将诱导获得的3种基因型胚性愈伤组织分别接种在含有0,5,10,15,20和50 mg· L-1草丁膦的胚性愈伤组织诱导培养基上,接种25块·培养皿-1大小均一的新鲜愈伤组织,3个重复,26 ℃培养25 d后,观察胚性愈伤组织的死亡数,并统计死亡率,确定合适的草丁膦筛选浓度。将含有bar基因载体的农杆菌菌液培养过夜,用菌液稀释培养基R2(NB+500 mg· L-1脯氨酸+300 mg· L-1水解酪蛋白+30 g· L-1蔗糖+20 mg· L-1乙酰丁香酮)稀释光密度值至0.6~0.8,侵染水稻的胚性愈伤组织15 min,期间不停晃动,之后用滤纸吸干多余的菌液,放在铺有滤纸的共培养培养基R3(NB+ 500 mg· L-1脯氨酸+300 mg· L-1水解酪蛋白+30 g· L-1蔗糖+4.0 g· L-1 phytalgel+20 mg· L-1乙酰丁香酮)上暗培养2 d,培养后的胚性愈伤组织用含有500 mg· L-1头孢霉素的无菌水洗2次,用滤纸吸干水分,转接抗性选择培养基R4(NB+2 mg· L-12.4-D + 500 mg· L-1脯氨酸+300 mg· L-1水解酪蛋白+500 mg· L-1谷氨酰胺+30 g· L-1蔗糖+4.0 g· L-1 phytalgel +500 mg· L-1头孢霉素+筛选出的合适草丁膦质量浓度)上筛选3次。每15 d继代1次。3轮选择后挑取抗性愈伤组织系,进行编号,并转接在分化培养基R5(NB + 0.5 mg· L-1NAA +3 mg· L-16-BA +500 mg· L-1脯氨酸+300 mg· L-1水解酪蛋白+500 mg· L-1谷氨酰胺+30 g· L-1蔗糖+4.0 g· L-1 phytalgel)上进行分化培养,同时提取抗性愈伤组织系的DNA,进行聚合酶链式反应(PCR)检测,统计转化率。胚性愈伤组织出现的绿点进一步分化出不定芽,待不定芽长到2~3 cm,转接在生根培养基R6(1/2 NB +20 g· L-1蔗糖+8.0 g· L-1琼脂)上,待苗长到10 cm,并有大量的根,移栽营养钵成活后,再移至大田成活,收种。
-
利用十六烷基三甲基溴化铵(CTAB)法提取抗性愈伤组织系和再生植株叶片的DNA,用bar基因的引物(F:ACCATCGTCAACCACTACATCG,R:GCTGCCAGAAACCCACGTCAT)进行PCR检测,扩增的目的片段是430 bp。
-
对照和转基因的植株的展开叶片用棉球蘸取质量浓度分别为50,100和300 mg· L-1的草丁膦,5 d后观察叶片的变化。
1.1. 材料
1.2. 方法
1.2.1. 胚性愈伤组织的诱导
1.2.2. 3种胚性愈伤组织的草丁膦敏感性试验和水稻遗传转化
1.2.3. 抗性愈伤组织系和叶片的DNA提取和PCR分子检测
1.2.4. T0代植株的田间草丁膦筛选
-
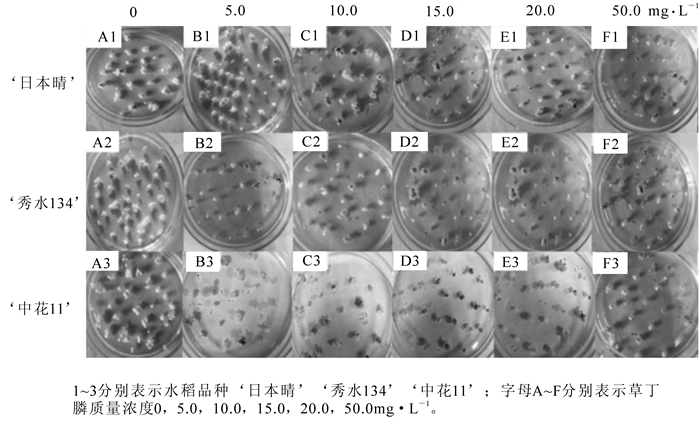
将‘日本晴’‘秀水134’和‘中花11’成熟胚诱导的米黄色且有光泽的胚性愈伤组织接种在分别含有6个质量浓度的草丁膦的选择培养基R4上,死亡率为50%~60%的愈伤组织对应的草丁膦质量浓度即可用作选择的质量浓度。结果如表 1和图 1所示:3个水稻品种的胚性愈伤组织在空白对照上全部存活,且大量增殖;在5.0 mg· L-1草丁膦的选择压下,死亡率为19%~23%,发生褐变的愈伤组织数量较少,且大部分愈伤组织有明显增殖,没有起到筛选作用;随着草丁膦质量浓度的增加,在10.0 mg· L-1时,‘秀水134’和‘中花11’2个品种死亡率为51%~56%,且与5.0 mg· L-1相比存在极显著性差异,达到选择效果;‘日本晴’在15.0 mg· L-1下,死亡率54.53%,且与10.0 mg· L-1相比存在极显著性差异,达到选择效果;但‘日本晴’在20 mg· L-1下,死亡率60%以上,选择效果偏大;‘秀水134’和‘中花11’在15.0 mg· L-1下,死亡率为55%~60%,选择效果偏大;而质量浓度50.0 mg· L-1时,3个水稻品种死亡率达到90%以上,表明该质量浓度已经超过临界质量浓度,选择压力太大。综上所述,‘日本晴’的草丁膦选择质量浓度应为15.0 mg· L-1,‘秀水134’和‘中花11’的草丁膦选择质量浓度都为10.0 mg· L-1。
水稻品种 不同质量浓度草丁膦下愈伤组织死亡率/% 0(ck) 5 10 15 20 50 mg.L-1 ‘日本晴’ 0.00±0.00 eD 19.29±0.71 dC 22.14±1.07 dC 55.79±1.11 cB 60.52±0.51 bB 90.67±1.33 aA ‘秀水134’ 0.00±0.00 fE 19.74±0.26 eD 55.79±1.90 dC 59.23±0.77 cC 81.00±0.24 bB 92.20±0.10 aA ‘中花11’ 0.00±0.00 fE 22.67±1.33 eD 51.28±1.28 dC 56.00±0.00 cC 81.33±1.33 bB 90.97±1.23 aA 说明:数值为均值±标准误差;小写字母不同表示0.05水平上的差异显著(P<0.05),大写字母不同表示0.01水平上的差异显著(P<0.01)。 Table 1. Mortality statistics of three kinds of rice EC (embryogenic callus) cultured on mediums containing 0, 5, 10, 15, 20, 50 mg·L-1 glufosinate after 25 d
-
用粳稻诱导出的米黄色且有光泽的胚性愈伤组织(图 2A)作为受体, 利用农杆菌介导法转化bar基因。将侵染后‘日本晴’‘秀水134’和‘中花11’的胚性愈伤组织分别在质量浓度为15,10和10 mg· L-1的草丁膦下进行3次选择培养。第1次选择后胚性愈伤组织大部分出现褐变(图 2B);第2次选择后,部分愈伤组织不抗继续褐化和变黑,部分周边长出新的抗性愈伤组织(图 2C);挑取新生的愈伤组织系进行第3轮选择,出现增殖(图 2D),抗性愈伤组织系分化出绿点(图 2E),进一步长出幼叶(图 2F),待叶片伸长到2~3 cm时,再生苗快速生根,获得完整的再生植株(图 2G),移栽成活(图 2H)。从胚性愈伤组织进行农杆菌介导法的转化到获得抗性胚性愈伤组织约2~3个月,抗性愈伤组织分化成苗约2个月。整个转化过程需要4~6个月。
-
抗性选择后,‘日本晴’‘秀水134’和‘中花11’分别获得86,40和90块抗性愈伤组织系,提取这些抗性愈伤组织系的DNA,进行PCR检测,分别有67,27和83块愈伤组织系均扩增出目的条带,转化率分别为77.9%,67.5%和92.2%(表 2)。图 3A是‘日本晴’随机选择的10块抗性愈伤组织的检测图,除了泳道8和13,均扩增出和阳性对照(泳道3)一样的条带,转化率均达到67%以上。说明筛选的草丁膦质量浓度是适合转化的质量浓度,同时淘汰非抗性愈伤组织系,减少后续的继代工作量。
品种 抗性愈伤组织系/块 阳性愈伤组织系/块 分化的愈伤组织系/块 分化绿点数/个 成苗数/株 阳性株数/株 转化率/% 分化率/% 成苗率/% 阳性率/% ‘日本暗’ 86 67 51 183 120 99 77.9 76.1 65.6 82.5 ‘秀水134’ 40 27 12 30 20 15 67.5 44.4 66.7 75.0 ‘中花11’ 90 83 72 195 60 52 92.2 86.7 30.8 86.7 说明:转化率=阳性愈伤组织系/抗性愈伤组织系;分化率=分化的愈伤组织系/阳性愈伤组织系;成苗率=成苗数/分化绿点个数;阳性率=阳性株数/成苗数。 Table 2. Transformation rate, differentiation rate and emergence rate in three rice genotypes
将这些阳性愈伤组织系进行分化培养发现:3个水稻品种的分化能力是不同的。‘秀水134’分化绿点最少,分化能力最弱(图 4A),共12块愈伤组织分化出绿点,分化率为44.4%;‘中花11’(图 4B)有72块愈伤组织系分化出绿点,分化率为86.7%;‘日本晴’分化绿点最多,分化能力最强(图 4C),51块愈伤组织系分化出绿点,分化率为76.1%(表 2)。
-
对获得的转基因植株叶片提DNA进行PCR扩增,以bar基因引物引物进行PCR扩增。‘日本晴’‘秀水134’和‘中花11’分别获得120株,20株和60株转基因小植株,其中bar基因阳性株有99株、15株和52株,阳性率为82.5%,75%和86.7%(表 2)。图 3B为部分转基因植株的检测结果。
-
对移栽成活的转基因植株进行草丁膦抗性检测,将蘸有不同质量浓度草丁膦的棉球粘附在转基因水稻的叶片上,5 d后,转基因和非转基因水稻出现了明显不同的反应。图 5是‘日本晴’叶片在低质量浓度为50.0 mg· L-1草丁膦处理时,未转化植株叶片上出现枯黄的斑点,在100.0 mg· L-1草丁膦处理后,出现较大面积枯黄的斑,在高质量浓度300.0 mg· L-1草丁膦处理后大面积变枯且严重灼伤。但转基因水稻叶片在50.0,100.0和300.0 mg· L-1草丁膦处理后仍无变化,表明转基因水稻抗性提高了约6倍。
‘日本晴’‘秀水134’和‘中花11’分别获得的99株、15株和52株PCR阳性株,其中分别有10株、5株和12株的叶片变黄枯,其余均表现绿色,草丁膦阳性率分别为89.9%,66.7%和76.9%(表 3),转基因水稻表现出草丁膦抗性。
水稻品种 PCR阳性植株/株 草丁膦抗性植株/株 草丁膦阳性率/% ‘日本晴’ 99 89 89.9 ‘秀水134’ 15 10 66.7 ‘中花11’ 52 40 76.9 Table 3. Glufosinate-resistant analysis of transgenic plants
2.1. 3个水稻品种的愈伤组织对草丁膦抗性筛选
2.2. 抗草丁膦bar基因转化3个水稻品种和植株再生
2.3. 转化率、分化率和成苗率的比较
2.4. 转基因植株分子检测结果
2.5. 转基因植株的草丁膦抗性鉴定结果
-
转基因水稻的获得依赖于筛选体系的选择效果。bar基因常作为选择标记基因,具有草丁膦抗性,除了选择效率高外,还可以带来抗除草剂的农艺性状。将bar基因或与其他目的基因串联在一起转化水稻,获得抗草丁膦水稻。确定一个合适的草丁膦筛选质量浓度,是提高转化效率和优化转化体系的关键步骤。本实验研究发现不同基因型水稻的胚性愈伤组织对草丁膦具有明显不同的抗性。为了确定以胚性愈伤组织为受体最佳的草丁膦选择质量浓度,本研究选取了5个5.0,10.0,15.0,20.0和50.0 mg· L-1草丁膦质量浓度梯度,对‘日本晴’‘秀水134’和‘中花11’的胚性愈伤组织进行草丁膦敏感试验,并确定有效的抗性筛选草丁膦质量浓度分别为15.0,10.0和10.0 mg· L-1(图 1),转化效率分别为77.9%,67.5%和92.2%,达到了一个很好的选择效果。本研究为以不同的水稻胚性愈伤组织为转化受体,提供可靠的草丁膦选择质量浓度。选取合适质量浓度的草丁膦进行选择,是成功转化的关键之一。
-
‘日本晴’‘秀水134’和‘中花11’的3个水稻品种均能获得成功的再生和转化体系,是目前水稻转基因常用的受体材料。本研究发现:当以草丁膦为选择标记时,它们的分化效率是不同的。其中‘中花11’的分化能力最强,分化率最高,高达86.7%;‘秀水134’的分化能力最差,分化率只有44.4%;‘日本晴’介于两者之间,分化率为76.1%。同时也对它们的再生效率进行了统计,研究发现,‘日本晴’有51个愈伤组织系分化,分化出183绿点,成苗数是120株,成苗率是65.6%;‘秀水134’有12个愈伤组织系分化,分化出30绿点,成苗数20株,成苗率是66.7%;‘中花11’有72个愈伤组织系分化,分化出195绿点,成苗数60株,成苗率是30.7%。‘中花11’分化率最高,成苗率最低;‘日本晴’的分化率居中,‘秀水134’的分化率最低;‘日本晴’和‘秀水134’的成苗率基本一样高。据此,当以草丁膦作为筛选标记时,首选‘日本晴’作为转化受体进行遗传转化。
-
水稻转基因除了常用的基因枪法[21-23],农杆菌介导法也是目前一种可行有效的方法。用农杆菌转化水稻的胚性愈伤组织,可以减少嵌合体,提高转化效率[24-30],但是仍然出现一些再生植株的变异问题。例如多分蘖,无花粉等[31]。采取以下措施:①缩短抗性选择时间,加强分化选择力度。经过1次抗性选择,进行分化培养,分化培养基上加入减半的草丁膦质量浓度,直到分化出绿点为止。②选用最新诱导出的、有光泽、淡黄色的胚性愈伤组织,刚从愈伤组织诱导出的胚性愈伤组织,及时挑出,增殖一定数量,用于转化[32];③减少继代次数。从转化到获得再生苗约4~5个月,期间继代5~6次;统计再生植株的变异率低于20%。本研究通过对水稻草丁膦敏感实验,成功建立了以草丁膦为筛选标记的农杆菌介导转化水稻胚性愈伤组织的体系。整个转化周期4~6个月。获得抗300 mg· L-1草丁膦的T0转基因植株。该体系为以除草剂作为选择压的水稻遗传转化提供了技术支持,获得的抗草丁膦水稻为水稻育种提供了材料。














 DownLoad:
DownLoad: