-
在遗传转化获得抗性植株时,转化体的抗性筛选是遗传转化能否取得成功的关键步骤。通常在选择培养基中加入合适种类和浓度的筛选剂,使其产生一定的筛选压起到抗性筛选的作用。转化体内选择标记基因的表达产物可对特定筛选剂产生抗性,使转化受体材料继续保持正常的生长发育[1]。目前的研究中,卡那霉素、潮霉素等抗生素被普遍作为筛选剂使用[2-3]。但是由于水稻Oryza sativa胚性愈伤组织对抗生素具有生理抗性,以抗生素为选择标记进行抗性筛选,不能起到很好的筛选效果,且经抗生素筛选后的转化体在分化和再生阶段易受抑制或产生白化苗[4-6]。以草甘膦作为筛选剂可以提高选择的灵敏度,消除转化体生理抗性对筛选结果的影响,克服了以往研究中抗生素筛选的局限性。可遗传的草甘膦抗性基因突变率低,并可在后代中稳定表达,因此进行抗草甘膦作物的培育是可行的[7]。籼稻Oryza sativa subsp. indica和粳稻Oryza sativa subsp. japonica是栽培稻的2个亚种,随着水稻遗传转化技术的发展,大部分粳稻品种已经建立了成熟的遗传转化体系,并成功引入抗虫、抗病、生长发育调控等诸多有利基因[8]。而大多数籼稻品种组培特性不佳,愈伤组织诱导率低,继代过程易褐化且分化再生频率低,导致籼稻的遗传转化效率低,有的品种甚至难以转化。尤其是对生产上广泛推广、农艺性状优良的重要品种而言,其改良与育种进程受到严重限制[9]。CHAN等[10]于1992年尝试利用农杆菌Agrobacterium tumefaciens介导法转化籼稻幼根愈伤组织,对转化体进行Southern印记杂交,结果表明:目的基因片段已成功转入转化体细胞中。后经酶活性检测,目的基因可在转化体中稳定表达。1994年,HIEI等[11]为建立高效稳定的农杆菌遗传转化体系,采用了“双超元”载体,并通过在菌液添加乙酰丁香酮(As)活化Vir基因提高转化效率等方法,推进了遗传转化技术在籼稻中的研究应用。目前,虽然已有转抗草甘膦基因的籼稻遗传转化体系的报道,但是转化效率低,还未建立一个高效的转化体系[12]。基于此,本研究选取具有成功再生体系的籼稻‘中恢161’ Oryza sativa subsp. indica ‘Zhonghui 161’为材料,利用农杆菌介导法,转入草甘膦抗性基因(CP4),探索适合的草甘膦质量浓度用于抗性筛选,并对农杆菌介导的转化过程进行了合理优化,建立‘中恢161’农杆菌介导的转化体系。
-
籼稻‘中恢161’成熟胚;农杆菌菌株EHA105;含CP4基因的表达载体p1300-HC。
-
将成熟种子去壳,进行消毒[13],接种于诱导培养基R1[NB(N6+B5)+3.0 mg·L−12.4-D+0.5 g·L−1脯氨酸+0.1 g·L−1肌醇+0.3 g·L−1水解酪蛋白+30.0 g·L−1蔗糖+4.0 g·L−1Gelrite]上,接种20 粒·皿−1。放入培养条件为28 ℃,光照16 h/黑暗8 h的组培室中诱导培养。5~7 d后,可观察到幼芽处有淡黄色愈伤组织,统计每皿的出愈数和出愈率。15 d后,剥下色泽鲜黄、结构紧密、生理状态良好的胚性愈伤组织,分散平铺于新鲜配制的胚性愈伤组织增殖培养基R1上进行增殖培养。继代2~4次后,增殖并产生大量的胚性愈伤组织,可用做后期转化的受体材料。
-
设置5组草甘膦质量浓度(100、200、300、400和500 mg·L−1),重复3次,设空白对照,接种20块·皿−1。15 d后,观察胚性愈伤组织的色泽、是否增殖等外观形态,统计胚性愈伤组织褐化率,选出合适的草甘膦质量浓度范围作为筛选压。
-
利用悬浮培养基R2(NB+0.5 g·L−1脯氨酸+0.1 g·L−1肌醇+0.3 g·L−1水解酪蛋白+30 g·L−1蔗糖+100 μmol·L−1乙酰丁香酮)将培养好的含CP4基因表达载体的农杆菌菌株EHA105稀释至D(600)为0.5~0.8,用其侵染胚性愈伤组织[14]。将转化好的胚性愈伤组织用无菌滤纸吸干多余的菌液,适当干燥后,用灭菌镊子夹取愈伤组织分散地平铺在铺有1层无菌滤纸的共培养培养基R3(NB+0.5 g·L−1脯氨酸+0.1 g·L−1肌醇+0.3 g·L−1水解酪蛋白+30.0 g·L−1蔗糖+100 μmol·L−1乙酰丁香酮+4.0 g·L−1Gelrite)上,20 块·皿−1。于25 ℃培养室中暗培养2~3 d。取出共培养后的愈伤组织,用含100 mg·L−1羧苄青霉素的无菌蒸馏水清洗3~4次,直至清洗液澄清透明。适度干燥后,用镊子夹取愈伤组织整齐均匀地平铺在筛选培养基R4(NB+3 mg·L−12.4-D+0.5 g·L−1脯氨酸+0.1 g·L−1肌醇+0.3 g·L−1水解酪蛋白+0.5 g·L−1谷氨酰胺+30.0 g·L−1蔗糖+4.0 g·L−1Gelrite+0.5 g·L−1头孢霉素+不同质量浓度草甘膦)上。抗性筛选培养基中草甘膦质量浓度分别为300、350和400 mg·L−1。
-
将抗性愈伤组织系移至分化培养基R5(NB+0.5 mg·L−1 NAA+3.0 mg·L−16-BA+0.5 g·L−1脯氨酸+0.1 g·L−1肌醇+0.3 g·L−1水解酪蛋白+0.5 g·L−1谷氨酰胺+30.0 g·L−1蔗糖+4.0 g·L−1Gelrite)上进行分化培养。约15~25 d,部分抗性愈伤组织长出绿点。待绿点进一步分化形成小苗,并长至2 cm左右时将其转移至生根培养基R6(1/2NB+20.0 g·L−1蔗糖+0.1 g·L−1肌醇+8.0 g·L−1琼脂)上生根培养。待幼苗生长出大量的茁壮根系,可将其从生根培养基中取出,小心洗净其根系附着的培养基,置于培养箱中炼苗,增强幼苗对环境的适应性,1周后将健壮的幼苗移至大棚成活。
-
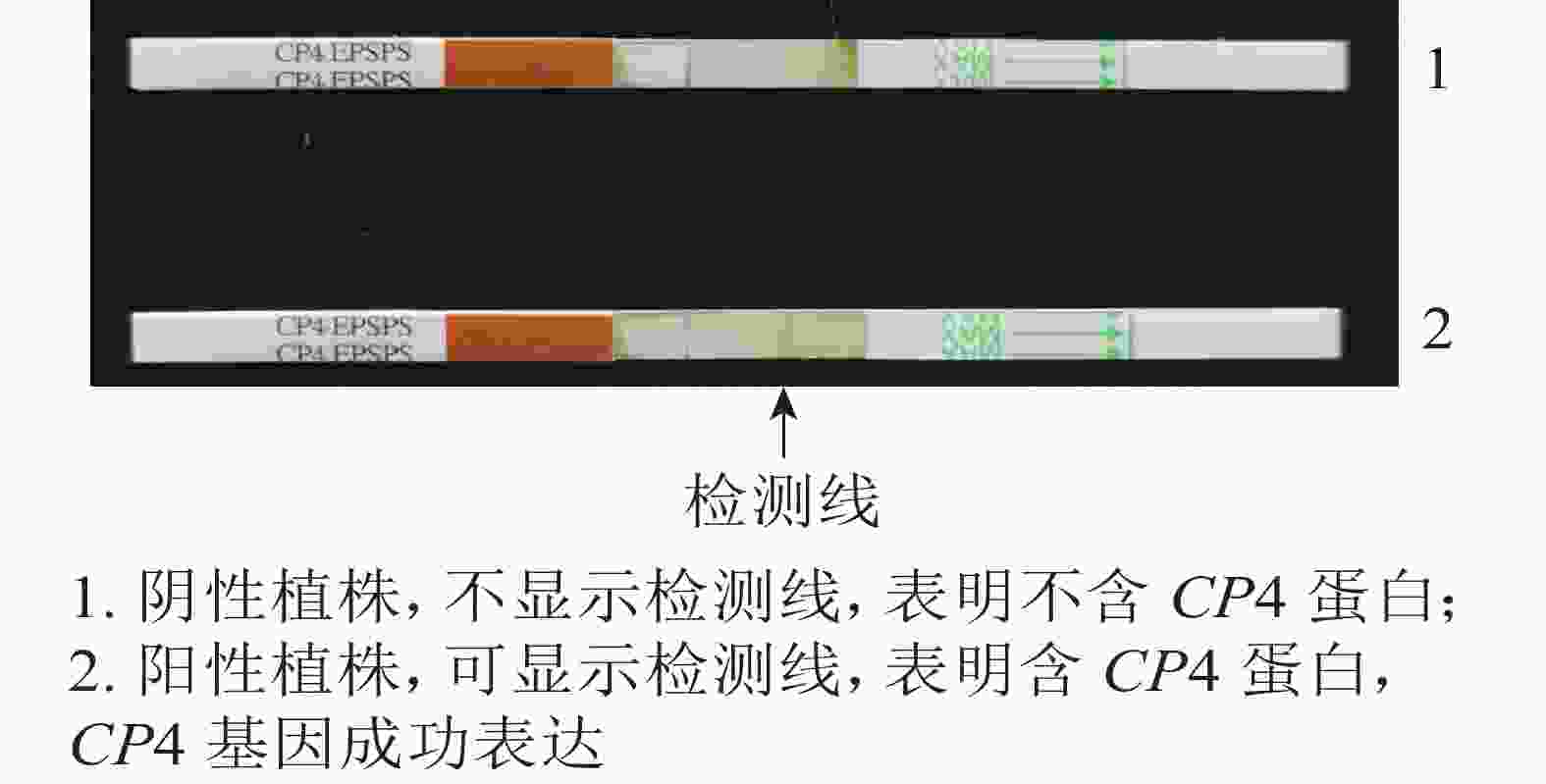
利用TPS法提取转基因植株叶片DNA。利用CP4基因引物(CP4-F: TTCCTTTAGGATTTCAGCATCAGTG, CP4-R: TCCTTCATGTTCGGCGGTCTC)进行CP4基因的PCR扩增,目的片段大小为400 bp。扩增后的产物经质量分数为1%琼脂糖凝胶电泳鉴定,统计阳性率。取阳性植株叶片,利用CP4基因表达检测试纸条进行再生植株抗性检测。
-
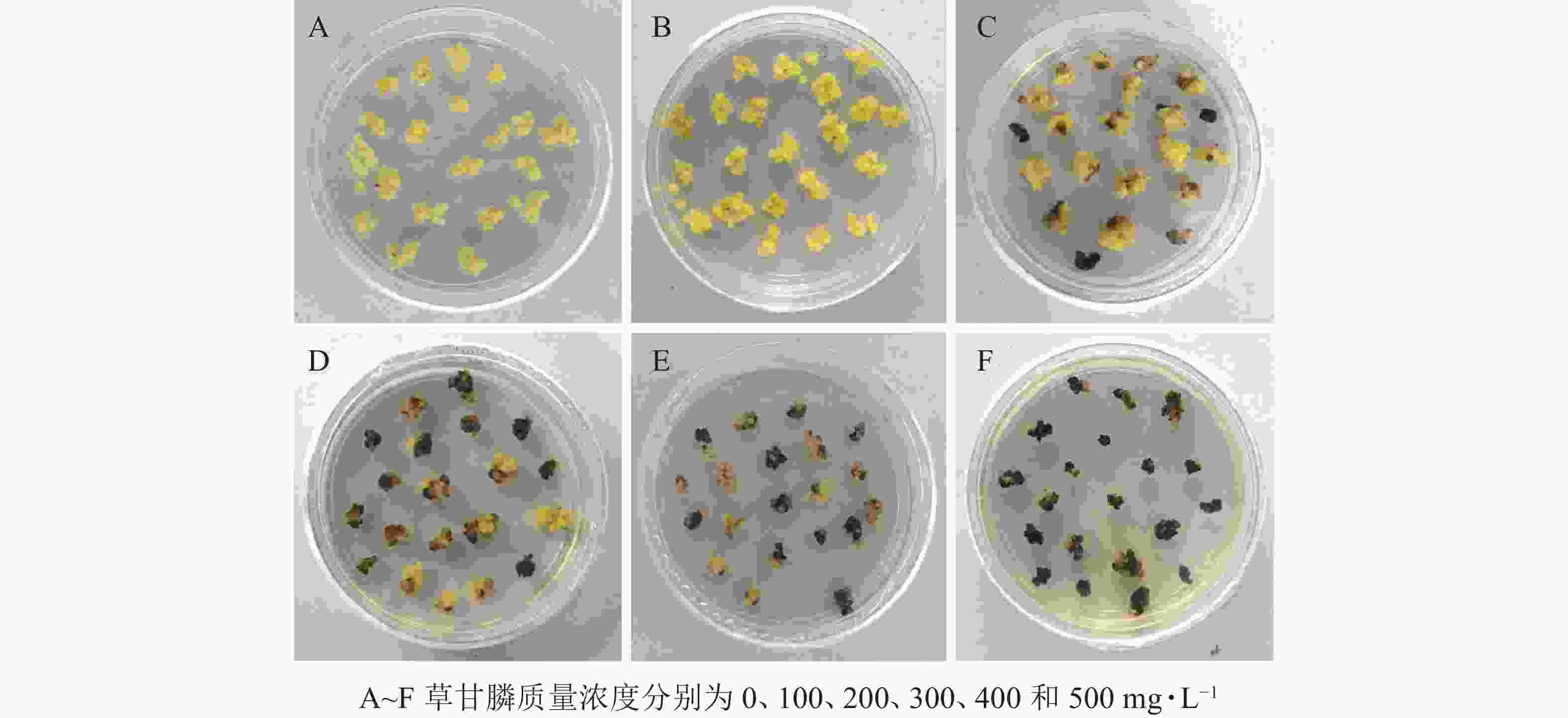
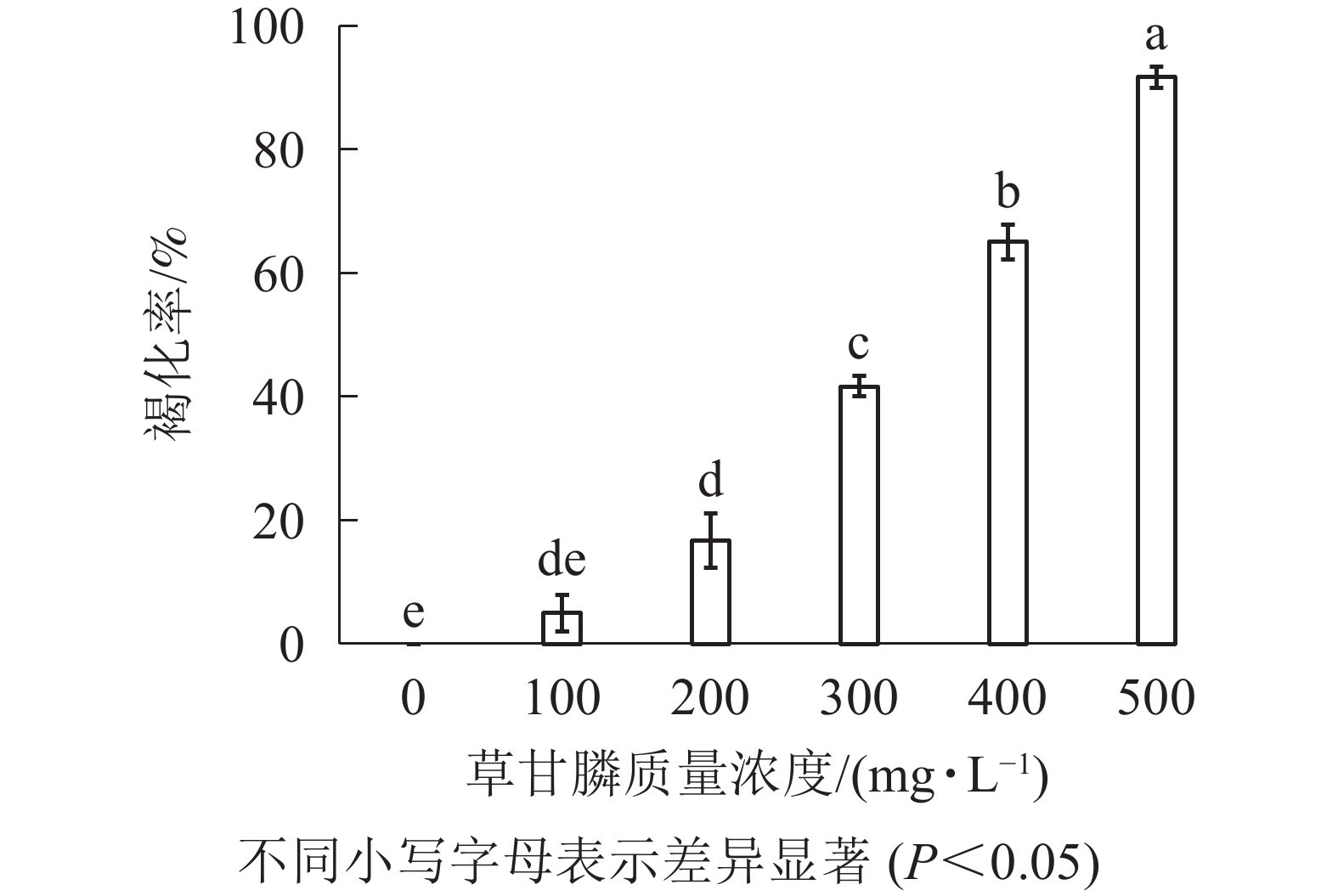
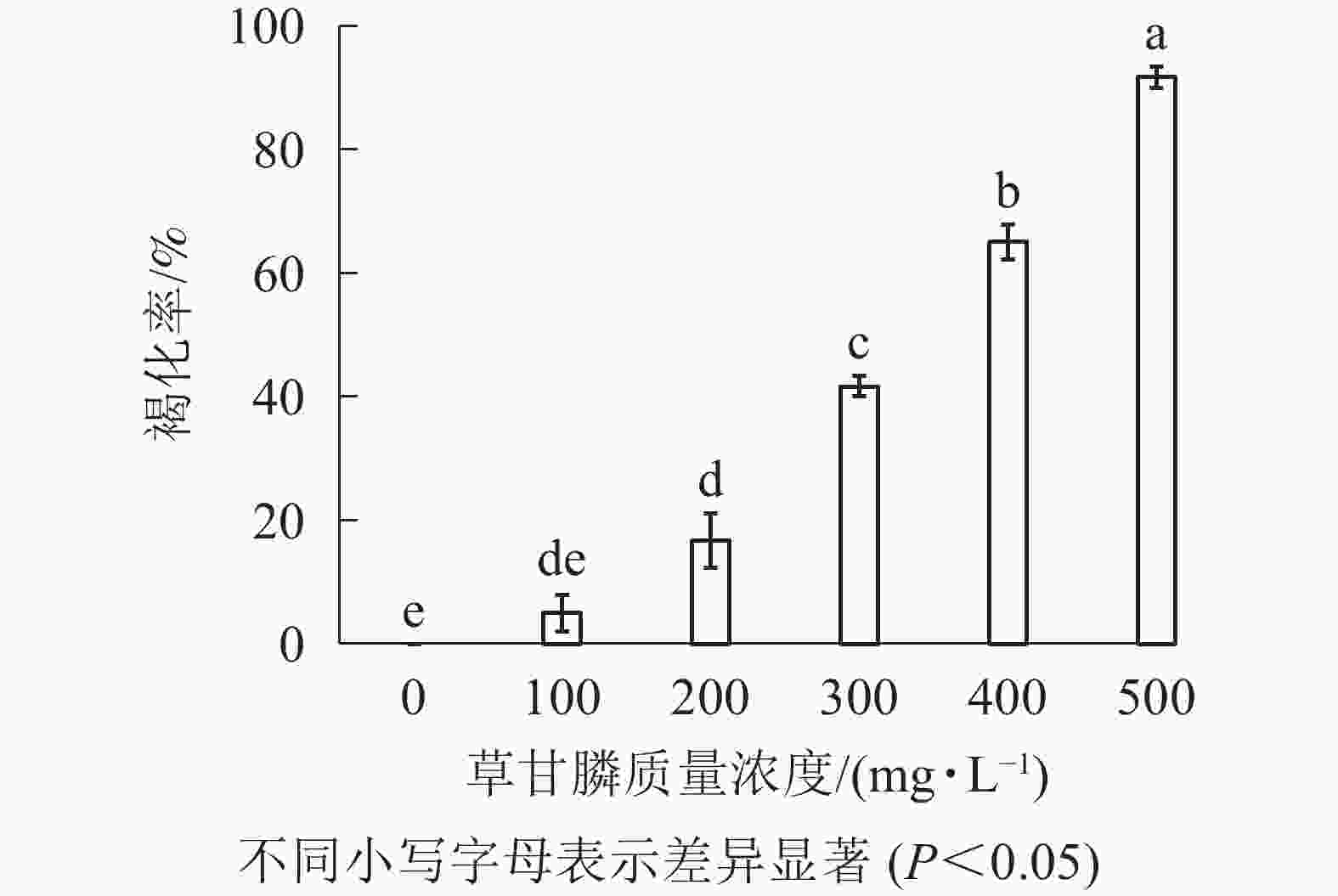
图1和图2所示:‘中恢161’的胚性愈伤组织在不含草甘膦的培养基中可正常生长且大量增殖,未发生褐化现象;在含100 mg·L−1草甘膦的培养基中,绝大多数胚性愈伤组织可正常生长增殖,褐化率低,仅为5.00%,未起到选择作用;在含200 mg·L−1草甘膦的培养基中褐化率为16.67%,选择效果不明显;在含300 mg·L−1草甘膦的培养基中,褐化率为41.67%,且与200 mg·L−1相比差异显著(P<0.05),选择效果好,适合作为筛选压;在含400 mg·L−1草甘膦的培养基中,大部分胚性愈伤组织发生褐化,少部分正常生长,褐化率为65.00%,选择效果明显;在含500 mg·L−1草甘膦的培养基中,胚性愈伤组织基本发生褐化,褐化率为91.67%,显著高于其他质量浓度下的褐化率(P<0.05),说明选择压过大。结果表明:草甘膦质量浓度为300~400 mg·L−1时,胚性愈伤组织褐化率约50%,具有很好的筛选效果。
-
转化后的胚性愈伤组织在含有300、350和400 mg·L−1草甘膦的选择培养基上进行抗性筛选,分别获得200、113、和84块抗性愈伤组织,提取抗性愈伤组织DNA进行CP4基因的PCR检测,阳性愈伤组织的PCR扩增产物经电泳可获得长度为400 bp的条带,与预期相符,表明CP4基因已成功整合到转化体内。进行3个草甘膦质量浓度抗性筛选后愈伤组织阳性率分别为40.16%、61.72%和84.04%。共获得67株再生植株,提取再生植株叶片DNA进行CP4基因的PCR检测。其中阳性植株43株,再生植株阳性率为64.18%(图3)。
-
抗性检测结果(图4)表明:检测的43株PCR阳性植株中,有25株表现为CP4基因表达,表达率为58.13%。
-
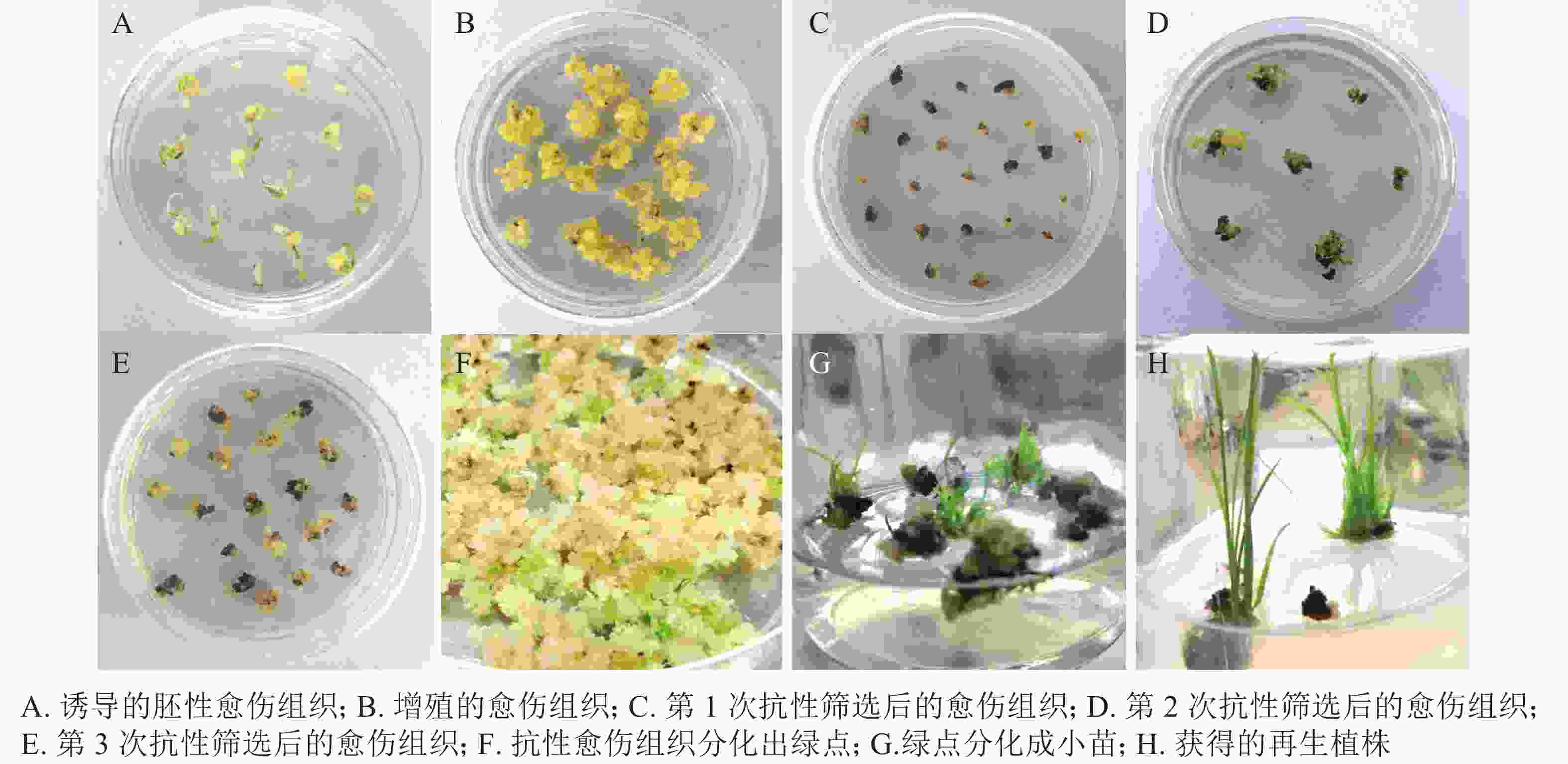
遗传转化再生过程如图5所示:对籼稻‘中恢161’成熟胚进行胚性愈伤组织诱导,约7 d可诱导出胚性愈伤组织(图5A)。胚性愈伤组织进行2~4次继代增殖(图5B),约40 d后进行遗传转化。转化后的胚性愈伤组织在选择培养基上进行抗性筛选(图5C~D),一段时间后,抗性愈伤组织系会出现增殖(图5E)。约50 d后,抗性愈伤组织于R5培养基上进行分化培养,约15~25 d,长出绿点(图5F)。1个月左右,长出小苗(图5G)。小心取出转移至R6培养基进行生根培养(图5H)。约15 d,幼苗长出大量的茁壮根系,将其从培养基中移出,小心洗净根部培养基。置于培养箱中炼苗,炼苗1周后可移至大棚成活。对再生植株进行CP4基因的PCR检测,保留阳性植株。从诱导胚性愈伤组织至获得抗草甘膦再生植株的整个过程需要4~6个月。
-
本研究建立了以草甘膦抗性基因CP4为选择标记的‘中恢161’遗传转化体系。非转化体的EPSPS酶活性较低,草甘膦可与S3P形成EPSPS-S3P-草甘膦复合体而竞争性抑制EPSPS酶活性,植物体内蛋白质合成受阻,生长受到抑制,不能正常生长分化[15]。而转化体抗草甘膦基因CP4的表达产物EPSPS酶具有高催化活性和低草甘膦亲和力不易与草甘膦结合,故能够进行正常的生长分化。因此,通过草甘膦筛选可获得转抗草甘膦基因CP4的再生植株。相较于以抗生素抗性基因为选择标记,草甘膦抗性基因不仅能作为筛选标记也能作为目的基因,使受体植物获得除草剂抗性,而且草甘膦比潮霉素等抗生素便宜[7]。不同植物细胞对草甘膦的抗性存在差异,选择合适的草甘膦质量浓度作为抗性筛选的筛选压是影响转化效率的关键因素。本研究将转化后的胚性愈伤组织分别在含有300、350和400 mg·L−1草甘膦的选择培养基上进行抗性筛选,进一步分化、成苗,共获得67株再生植株,进行CP4基因的PCR检测,其中阳性植株43株,再生植株阳性率为64.18%,达到很好的选择效果。
-
能否成功进行遗传转化的重要前提是选择适合的植物材料作为转化受体。水稻幼胚分裂能力强,易形成大量优质胚性愈伤组织,但受季节的影响,水稻幼胚利用不便,且在组织培养过程中易受微生物污染,转化效率不高,因此作为转化体存在一定的困难[16]。成熟胚方便储存与利用,不受季节限制和胚性愈伤组织诱导率较高,通常被作为遗传转化和再生的良好的外源体材料。苏军[17]比较了不同代龄的胚性愈伤组织,发现第4、5代的胚性愈伤组织转化效率较高,并且分化能力也较强。早代愈伤组织不易接受外源遗传物质。但晚代愈伤组织容易出现质地软、水渍化等现象,影响遗传转化成功率。本研究选择胚性愈伤组织代龄为3~4代,可有效减少愈伤组织老化、色泽暗黄、结构松散和褐化率高等问题,有效提高了遗传转化效率。
-
为提高遗传转化效率,本研究采取一系列措施对转化过程进行合理优化。①转化阶段选用色泽鲜黄、外观形态良好、结构紧致的愈伤组织与农杆菌共培养,淘汰外观发白发软发褐的愈伤组织。②在共培养基R2和悬浮培养基R3中加入100 μmol·L−1乙酰丁香酮,可诱导农杆菌Vir基因的活化,从而促进外源基因的整合,极大提高转化效率[18]。③农杆菌菌液经悬浮培养液R3稀释后,D(600)为0.5~0.8。此时为最适菌液浓度,既不会使农杆菌在转化体表面过多繁殖影响其正常生长,又具一定的侵染能力,提高了转化效率。④黑暗条件下共培养2~3 d为适合的共培养时长。共培养时间过短,目的基因不能成功整合至转化体细胞内[19-20]。
Agrobacterium-mediated transformation of CP4 gene into indica rice
-
摘要:
目的 建立籼稻‘中恢161’Oryza sativa subsp. indica ‘Zhonghui 161’农杆菌Agrobacterium tumefaciens介导的转化体系。 方法 以籼稻‘中恢161’的成熟胚为材料,设置了5个草甘膦质量浓度(100、200、300、400和500 mg·L−1)进行胚性愈伤组织的草甘膦敏感性试验。利用农杆菌介导法,将草甘膦抗性基因CP4-EPSPS导入‘中恢161’的胚性愈伤组织中,转化后的胚性愈伤组织分别在含有300、350和400 mg·L−1草甘膦的选择培养基上进行抗性筛选。抗性愈伤组织进一步分化、成苗。 结果 草甘膦质量浓度为300~400 mg·L−1时,愈伤组织褐化率约50%,具有很好的选择效果。经统计,300、350和400 mg·L−1草甘膦抗性筛选后,愈伤组织阳性率分别为40.16%、61.72%和84.04%,抗性愈伤组织的分化率为46.43%,成苗率为32.84%。共获得67株再生小苗,经PCR检测,43株成功转入CP4基因,再生植株阳性率为64.18%。 结论 建立了‘中恢161’农杆菌介导的转化体系。图5参20 Abstract:Objective The objective was to establish the Agrobacterium-mediated transformation system of Oryza sativa subsp. indica ‘Zhonghui 161’. Method 5 groups of glyphosate concentrations (100, 200, 300, 400 and 500 mg·L−1) were used to test the sensitivity of embryogenic callus to glyphosate. The glyphosate-resistant gene (CP4-EPSPS) was introduced into the embryogenic callus of ‘Zhonghui 161’ by Agrobacterium-mediated method. The transformed embryogenic callus was screened for glyphosate resistance on the selective medium containing 300, 350 and 400 mg·L−1 glyphosate. The resistant callus was further differentiated and seeded. Result When the concentration of glyphosate was 300−400 mg·L−1, the browning rate of callus was about 50%, showing a good selection effect. The positive rates of callus on 300, 350 and 400 mg·L−1 glyphosate were 40.16%, 61.72% and 84.04%, respectively. The further differentiation rate was 46.43%, and the seedling rate was 32.84%. A total of 67 regenerated plantlets were obtained, and 43 of them were successfully transformed into CP4 gene by PCR detection. The positive rate of regenerated plantlets was 64.18%. Conclusion Agrobacterium-mediated transformation system of ‘Zhonghui 161’ was established. [Ch, 5 fig. 20 ref.] -
-
[1] 李俊, 刘翠琼, 尹伟伦, 等. 转基因植物中标记基因研究概况[J]. 植物学报, 2009, 44(4): 497 − 505. LI Jun, LIU Cuiqiong, YIN Weilun, et al. A survey of marker genes in transgenic plants [J]. J Bot, 2009, 44(4): 497 − 505. [2] 王关林, 方宏筠. 植物基因工程原理与技术[M]. 北京: 科学出版社, 1998: 214 − 218. [3] 贾士荣. 转基因植物食品中标记基因的安全性评价[J]. 中国农业科学, 1997, 30(2): 1 − 15. JIA Shirong. Safety evaluation of marker genes in transgenic plant food [J]. Agric Sci China, 1997, 30(2): 1 − 15. [4] MULLINS M G, TANG F C A, FACCIOTTI D. Agrobacterium-mediated genetic transformation of grapevines: transgenic plants of Vitisrupestris Scheele and buds of Vitis vinifera L. [J]. Biotechnology, 1990, 8: 1041 − 1045. [5] NEHRA N S, CHIBBAR R N, LEUNG N, et al. Self-fertile transgenic wheat plants regenerated from isolated scutellar tissues following microprojectile bombardment with two distinct gene constructs [J]. Plant J, 1994, 5(2): 285 − 297. [6] ZHOU H, ARROWSMITH J W, FROMM M E, et al. Glyphosate-tolerant CP4 and GOX genes as a selectable maker in wheat transformation [J]. Plant Cell Rep, 1995, 15: 159 − 163. [7] 王和勇. 植物转化体草甘膦筛选技术平台的建立[D]. 广州: 中山大学, 2004. WANG Heyong. Establishment of Screening Technology Platform for Glyphosate in Plant Transformation[D]. Guangzhou: SunYat-sen University, 2004. [8] 苏军, 胡昌泉, 翟红利, 等. 农杆菌介导籼稻明恢86高效稳定转化体系的建立[J]. 福建农业学报, 2003, 18(4): 209 − 213. SU Jun, HU Changquan, ZHAI Hongli, et al. Establishment of a highly efficient and stable transforming system mediated by Agrobacterium tumefacien in indica rice [J]. J Fujian Agric, 2003, 18(4): 209 − 213. [9] 童普国, 欧阳解秀, 阎新, 等. 1个组培特性优良的籼稻品种的发现及其农杆菌转化体系的建立[J]. 湖南农业大学学报(自然科学版), 2016, 42(3): 225 − 230. TONG Puguo, OUYANG Jiexiu, YAN Xin, et al. Discovery of an indica rice variety with excellent tissue culture characteristics and establishment of its Agrobacterium transformation system [J]. J Hunan Agric Univ Nat Sci Ed, 2016, 42(3): 225 − 230. [10] CHAN Mingtsair, LEE Tsemin, CHANG Hsinhsiung. Transformation of indica rice(Oryza sativa L.)mediated by Agrobacterium tumefaciens [J]. Plant Cell Physiol, 1992, 33(5): 577 − 583. [11] HIEI Y, OHTA S, KOMARI T, et al. Efficient transformation of rice(Oryza sativa L.)mediated by Agrobacterium and sequence analysis of the boundaries of the T-DNA [J]. Plant J, 1994, 6(2): 271 − 282. [12] 温莉娴, 周菲, 邹玉兰. 抗除草剂转基因水稻的研究进展[J]. 植物保护学报, 2018, 45(5): 954 − 960. WEN Lixian, ZHOU Fei, ZOU Yulan. Research progress of herbicide resistant transgenic rice [J]. J Plant Prot, 2018, 45(5): 954 − 960. [13] 张秀香. 水稻成熟种子组织培养体系的优化及建立[J]. 安徽农学通报, 2012, 18(12): 39 − 40, 42. ZHANG Xiuxiang. Optimization and establishment of tissue culture system for mature rice seeds [J]. Bull Anhui Agric, 2012, 18(12): 39 − 40, 42. [14] 马永光, 于翠梅, 杨巍, 等. 水稻农杆菌转化体系中共培养方法的优化[J]. 园艺与种苗, 2011(4): 26 − 28. MA Yongguang, YU Cuimei, YANG Wei, et al. Optimization of co-cultivation methods in the transformation system of Agrobacterium tumefaciens [J]. Hortic Seedlings, 2011(4): 26 − 28. [15] 王慧, 闫晓红, 徐杰, 等. 我国抗草甘膦基因的发掘现状[J]. 农业生物技术学报, 2014, 22(1): 109 − 118. WANG Hui, YAN Xiaohong, XU Jie, et al. Current status of glyphosate resistant genes in China [J]. Acta Agric Biotech, 2014, 22(1): 109 − 118. [16] 邱萍. 籼稻浙恢7954遗传转化体系的优化及其应用[D]. 金华: 浙江师范大学, 2012. QIU Ping. Optimization and Application of Genetic Transformation System of Indica Rice Zhehui 7954[D]. Jinhua: Zhejiang Normal University, 2012 [17] 苏军. 淀粉合成相关基因转化籼型杂交稻亲本及育种利用研究[D]. 福州: 福建农林大学, 2005. SU Jun. Studies on the Transformation of Starch Synthesis Related Genes into Indica Hybrid Rice Parents and Their Breeding Utilization[D]. Fuzhou: Fujian Agricultural and Forestry University, 2005. [18] 董喜才, 杜建中, 王安乐, 等. 乙酰丁香酮在植物转基因研究中的作用[J]. 中国农学通报, 2011, 27(5): 292 − 299. DONG Xicai, DU Jianzhong, WANG Anle, et al. The role of acetosyringone in plant transgenic research [J]. China Agron Bull, 2011, 27(5): 292 − 299. [19] SAHOO K K, TRIPATHI A K, PAREK A, et al. An improved protocol for efficient transformation and regeneration of diverse indica rice cultivars [J]. Plant Methods, 2011, 7(1): 49. [20] 刘元风, 刘彦卓, 王金花, 等. 根癌农杆菌介导籼稻遗传转化影响因素研究[J]. 分子植物育种, 2005, 3(5): 737 − 743, 748. LIU Yuanfeng, LIU Yanzhuo, WANG Jinhua, et al. Study on the influencing factors of Agrobacterium tumefaciens mediated genetic transformation of indica rice [J]. Mol Plant Breed, 2005, 3(5): 737 − 743, 748. -

-
链接本文:
https://zlxb.zafu.edu.cn/article/doi/10.11833/j.issn.2095-0756.20200436






 下载:
下载: