-
谷胱甘肽过氧化物酶(glutathione peroxidase,GPXs)是一种重要的过氧化物分解酶,能清除活性氧,阻止脂膜氧化,起到保护细胞免受氧化胁迫的作用,具有重要的生理功能[1]。自CRIQUI等[2]从烟草Nicotiana tabacum中获得GPX基因后,先后从柑橘Citrus[3],番茄Lycopersicon esculentum[4],水稻Oryza sativa[5],香蕉Musa nana[6],荷花Nelumbo nucifera[7],枣Ziziphus jujube[8]等植物中获得GPX基因。近年来,对于植物GPXs的研究主要集中在生物胁迫和非生物胁迫(如高温、低温、干旱、重金属毒害、耐盐等)方面,尤其是GPXs在非生物胁迫中的功能作用,证实了GPX基因与植物抗逆性紧密相关。在非生物胁迫下,GPX基因在转录水平发生变化,参与相关氧化还原途径[9]。如苹果Malus MdGSTU1提高苹果的耐盐能力[10],过量表达NtGPX基因提高烟草的抗冻性[11],番茄LePHGPX基因的过量表达提高植株耐高温能力[12]。研究人员对拟南芥Arabidopsis thaliana GPX基因的功能研究取得较大进展,新的GPX被不断发掘(拟南芥目前已知8个),对其中7个GPX基因的研究证实,全部对非生物胁迫有应答反应,转基因可提高植物抵御胁迫的能力[13],如AtGPX3能显著增强植株的抗旱性[14],AtGPX1具有抗高温下的氧化胁迫能力[15]。目前虽有多种植物GPX基因被发现,并开展相关功能研究,但主要集中于单子叶和双子叶植物,对裸子植物的研究较少。马尾松Pinus massoniana属裸子植物松科Pinaceae松属Pinus植物,是中国南方重要的用材林树种[16],但在马尾松生长过程中常受到病虫害的危害,给林业经济带来严重损失,制约了马尾松人工林的健康发展。本研究在高通量测序分析基础上结合cDNA末端快速扩增(RACE)技术,获得谷胱甘肽过氧化物酶基因,对其进行生物学分析,利用实时定量聚合酶链式反应(qRT-PCR)技术研究其不同组织、不同抗性材料、不同时间表达模式,为探求马尾松虫害防御机制提供依据。
HTML
-
植物材料包括抗虫马尾松品种‘松韵’‘Songyun’和对照GC101无性系,材料选自广西马尾松桐棉种源,采自广西马尾松种质资源库。该库栽植于2007年,树龄8年生,栽植株行距为4 m × 4 m,根、茎、叶、花、果采自普通马尾松无性系,雌雄球花样品采自2015年2月,其他组织采自2015年4月。日变化叶和茎的材料采自2015年5月,采样时间分别在当日的7:00,10:00,13:00,16:00,19:00,所有样品采摘后放入液氮中保鲜,带回实验室存放于-80 ℃冰箱中备用。
-
实验所有样品的RNA采用多酚多糖植物RNA提取试剂盒(天根公司)进行提取,具体步骤按说明书进行。提取完成后进行凝胶电泳检查质量,参照M-MLV逆转录酶合成cDNA,进行凝胶检查和浓度测定(采用核酸定量仪)后备用。根据前期马尾松转录组测序结果中PmGPX的部分cDNA序列进行比对,根据获得的序列在2端设计特异引物,3′端引物(5′-ATGGCATCCTCTTTCGCAGCATCT-3′),5′端引物(5′-TGCAGCAAGCAGATTCTGTATATCC-3′)。以不同组织等量混合cDNA为模板进行扩增。设定退火温度为56.0 ℃,具体聚合酶链式反应(PCR)扩增体系和参数参照Taq DNA聚合酶说明书进行操作,扩增产物经凝胶电泳检查获得目的条带后,用胶回收试剂盒进行回收纯化,连接、转化后进行菌液PCR检查正确后测序。
-
采用在线SOSUI和Plant-mPLoc亚细胞定位,利用Proteomics Server,SignalP 4.1 Server,SOPMA,TargetP 1.1 Server,DAS软件分析基因等电点、信号肽、二级结构、亲水性和跨膜结构,利用Motif Scan和SMART分析基因的功能结构域。将PmGPX基因完整氨基酸序列在美国生物技术信息中心(NCBI)进行覆盖率和同源性比较,利用DNAMAN软件构建PmGPX与其他植物的进化树。
-
根据马尾松内参基因筛选结果[17],利用UBI4基因和CYP基因作为内参基因,内参引物分别为UBI4F: AGCTCCGACACCATTGATAA,UBI4R: CCAAAGTACGTCCAT CTTCCA,CYPR: CAAGGGTTCGTCGTTCCAC,CYPF: GGCAAACTTCTCGCCGTA,同时设计PmGPX基因引物,PmGPX1F: TCAAACTCCCCGTCTTATGG,PmGPX1R:GCTCCAACTGAATCCT TGC。参照SYBR Premix Ex Taq Ⅱ(Prefect real-time)试剂盒,构建qRT-PCR体系和扩增程序,其中退火为58 ℃,采用45个循环作熔解曲线。设生物学重复3个·样品-1,根据2-ΔΔCt法[18]计算相对表达量。
1.1. 实验材料
1.2. 实验方法
1.2.1. RNA提取及PmGPX克隆
1.2.2. 序列分析
1.2.3. 基因表达模式分析
-
对马尾松不同抗虫材料高通量测序比对发现,有个基因在抗虫材料中大量表达(log2.Fold_1.1491),该基因在KEGG代谢途径中只参与谷胱甘肽代谢(glutathione metabolism)和花生四烯酸代谢(arachidonic acid metabolism)途径。这2个途径中只有该基因上调表达,通过对序列在NCBI进一步比对,确定为谷胱甘肽过氧化物酶(glutathione peroxidase)基因。通过进行扩增、比对,获得了该基因完整开放阅读框(ORF)序列,命名为PmGPX。该基因完整开放阅读框(ORF)共包括741 bp,编码246个氨基酸(图 1)。
-
利用生物信息学软件对PmGPX编码的氨基酸序列进行分析,结果显示:该基因不含信号肽,有6个丝氨酸(Ser)和1个色氨酸(Try)磷酸化位点,2个糖基化位点;二级结构预测中:α-螺旋占30.49%,随机卷曲占38.62%,延伸链占20.33%,β-转角占10.57%;该基因等电点为8.29,蛋白分子量为27.08,在113~117个氨基酸处存在1个跨膜结构,亚细胞定位于叶绿体。
对PmGPX蛋白进行功能结构域显示,具有3个N-糖基化位点,4个N-酰基化位点,2个酪蛋白激酶Ⅱ磷酸化位点,4个蛋白激酶c磷酸化位点,二硫键氧化还原酶结构域、硫氧还蛋白结构域、固氮铁蛋白、抗氧化蛋白AhpC/TSA家族结构域、谷胱甘肽过氧化物酶结构域,以及谷胱甘肽氧化酵素活性部位和信号位点(图 1)。
-
PmGPX核酸序列进行比对发现,序列同源性在62%~100%,相似度在74%~94%,与白云杉Picea glauca和北美云杉Picea sitchensis相似度最近,与葡萄Vitis vinifera,烟草,龙眼Dimocarpus longan等植物相似度为74%~78%。氨基酸序列比对发现,序列同源性在64%~100%,除与北美云杉相似度在91%外,与其他相似度为58%~81%,大部分为64%~80%,与山嵛菜Eutrema yunnanense,亚麻芥Camelina sative,菜豆Phaseolus vulgaris的相似度低,总体上除与针叶类同源性较高外,其他都较低。但对谷胱甘肽过氧化物酶保守域同源性比对时发现,PmGPX氨基酸序列与其他植物具有很高的同源性,特别是在3个特征性结构域G1,G2,G3高度保守,PmGPX构成GPX催化三联体的3个氨基酸(Cys119,Gln150,Trp209),具有植物GPX蛋白活性中心的3个保守半胱氨酸(Cys)残基Cys119,Cys148,Cys167(图 2)。
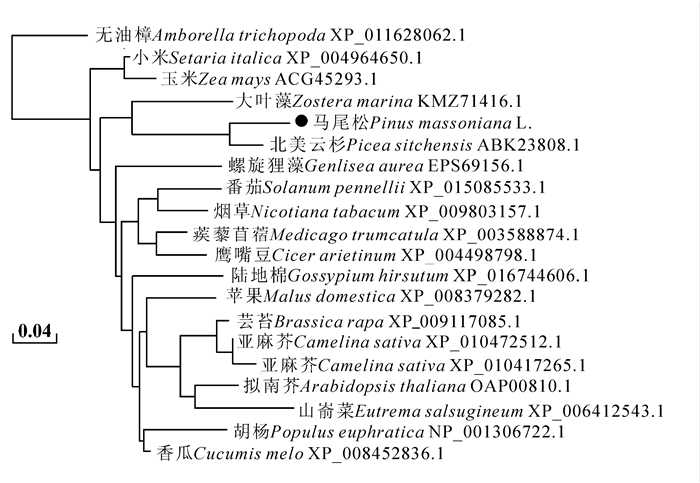
采用邻接法(neighbor-joining method)对20个植物的GPX基因构建进化关系树,马尾松与北美云杉聚为一类,并且与大叶藻的同源关系较近,与山嵛菜,亚麻芥,菜豆,无油樟Amborella trichopoda,小米Setaria italica和玉米Zea mays的同源关系较远。推测马尾松PmGPX基因与针叶植物,尤其是与云杉的该基因在进化过程是相似的,而与其他植物则存在较大差异,单子叶植物玉米、香瓜Cucumis melo同源关系较远,双子叶植物间相对的分化较小(图 3)。
-
实时荧光定量对根、茎、叶、花、果中PmGPX基因的表达特征进行分析表明,PmGPX基因在所有组织中都有表达,但表达量存在明显的差异。PmGPX基因主要表达于针叶和茎中,在针叶中的表达量要高于其他组织,在嫩叶中的表达量为根中表达量的33.45倍,针叶成熟度的不同表达量差异也明显,嫩叶中表达量是成熟叶的2.72倍。PmGPX基因在不同成熟茎中的表达量差异不明显,虽仅次于在叶中的表达量,但相差11.04倍,在根、雌雄花、果等4个组织中的表达量相差不大,表达量均较少(图 4)。
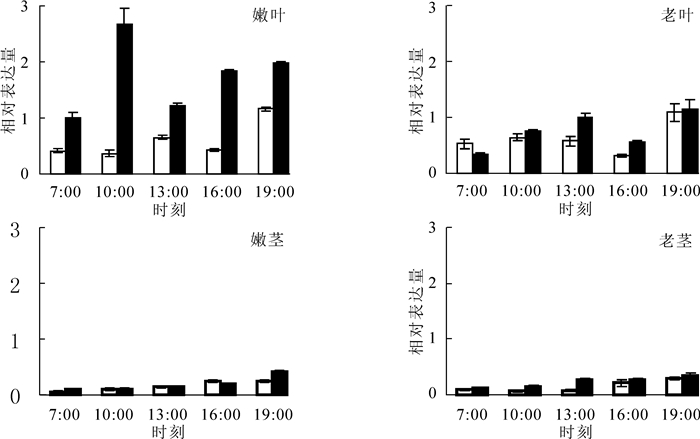
为进一步研究PmGPX基因在不同材料1 d内的变化模式,采用qRT-PCR进行分析, 结果如图 5所示。在嫩叶和老叶中,2个材料PmGPX基因表达变化趋势相似,都是“升—降—升”模式。在嫩叶中抗虫材料在10:00时表达量达到最大值,是7:00表达量的2.65倍,而对照中该基因从7:00-16:00的表达量变化不大,只有到19:00时才达到峰值。老叶中2种材料的表达规律一致,不同的是PmGPX在抗虫材料中的表达要高于对照,在13:00时表达量要高于对照。从茎中的表达模式分析,PmGPX在不同成熟茎和不同抗性材料中的变化趋势一致,随时间推移,表达量不断上升。不同的是在嫩茎中前4个时间点,2种材料的表达量基本相同,在19:00时抗虫材料的表达量才出现明显的上升。而在老茎抗虫材料中PmGPX的表达量始终高于对照,在13:00时两者的表达量相差3.48倍。
2.1. 基因全长获得
2.2. PmGPX基因序列分析
2.3. PmGPX蛋白同源性及进化
2.4. PmGPX基因表达分析
-
在马尾松抗虫材料转录组测序差异分析发现,在谷胱甘肽代谢存在唯一大量上调表达的基因,并且参与了花生四烯酸代谢途径,通过克隆比对确定为谷胱甘肽过氧化物酶基因,命名为PmGPX。该基因与针叶植物云杉同源性较高,在谷胱甘肽过氧化物酶保守域与其他植物同源性也较高,说明植物GPX基因在进化过程中虽然N端序列发现了改变,但在GPX基因功能结构域中的变化不大。另外,在PmGPX序列具有GPX中3个保守的特征性结构域,及GPX蛋白活性中心的3个保守半胱氨酸(Cys)残基。目前已知的植物GPX的活性位点由Cys作为催化残基来代替动物体内的硒代半胱氨酸[6, 19],马尾松PmGPX也在Cys119处替代了动物PHGPXs中的硒代半胱氨酸残基,进一步证明PmGPX是GPX的家族成员。
PmGPX存在二硫键氧化还原酶结构域、硫氧还蛋白结构域、谷胱甘肽过氧化物酶结构域等功能结构域,在植物体内GPXs是含有巯基的过氧化物酶类,通过催化GSH来减少过氧化氢或有毒化合物,起到解毒和保护细胞免受破坏[20-21]。
MILLA等[13]推测拟南芥中的GPX基因分布于3条染色体上,定位在叶绿体、线粒体、胞质等亚细胞器中,如AtGPX2定位于细胞质。本研究PmGPX预测定位于叶绿体中,推测AtGPX1与抵御光氧化胁迫有关。活性氧在植物细胞代谢中主要来源于叶绿体、线粒体,亚细胞定位与功能有直接关系,马尾松PmGPX发挥的作用可能与光合或产生活性氧有关。
本研究中马尾松PmGPX在所有组织中均有表达,与已有研究结果一致,说明PmGPX在马尾松生长过程中起到清除细胞内活性氧(ROS)的作用[13],但PmGPX基因在叶和茎中的表达最高,这与拟南芥[13]和油菜Brassica napus[22]的研究结果一致,而香蕉MaGPX在花和根中表达量较高[6],白菜Brassica pekinensis BrGPX6a在早期子叶胚中的表达量较高,在成熟胚中表达较低[23]。不同植物或同一植物GPX在不同组织中的表达均有所差别,可能与不同组织中活性氧的分布有关联[7, 13, 24]。由于PmGPX基因在叶中,尤其是嫩叶中的表达量最高,而在雌雄花和根中表达量极低,进一步验证了该基因亚细胞定位于叶绿体的可能。
PmGPX基因在不同抗性材料同一组织中的表达模式基本一致,主要区别在于抗性品种PmGPX基因表达量始终高于对照,并且随时间延长,表达量提升速度和达到峰值的时间均比对照要早。从选出的抗逆性材料来看,耐铝小麦Triticum sestivum和水稻品种,在受到铝胁迫后GPX活性明显提高,而对照则出现下降趋势[25-26],同样耐盐品种在盐胁迫下,本身含有或能够迅速提高植株的抗氧化能力,对照则较低[27]。从非生物胁迫下,香蕉MaGPX在盐、干旱等胁迫下表达量上升[7],拟南芥AtGPX1表达量的上升,会提高抵御光氧化胁迫[15]。
目前,有关GPX基因在植物抗虫功能的研究还较少,马尾松PmGPX基因在抗虫材料中的高表达,现有研究表明:植物中GSH合成和酶活性提高,能够增加植物对多种胁迫的抵抗能力,相反在环境胁迫下GPXs的mRNA水平也会提高,在谷胱甘肽代谢中GPX基因表达量提高,能够提高氧化谷胱甘肽量,从而转化成GSH,提高植物抵御环境胁迫的能力[28-29]。马尾松PmGPX在抗虫材料中高表达,提高了防御虫害的能力,另外,PmGPX基因在抗虫材料花生四烯酸代谢中高量表达,推测该基因可能提高该途径15-oxoETE和5-oxoETE代谢物含量,从而提高丙酮含量,提高了防御抗虫的能力,但通过何种信号传导途径参与来起作用[14],有利于抵御虫害次生代谢物质的产生,起到抗虫效果还有待进一步研究。















 DownLoad:
DownLoad: