-
淹水胁迫对植物生长的抑制作用除了低氧环境引起的根系活力下降、呼吸抑制以及矿质元素吸收受阻外,长时间淹水胁迫引起的叶绿素合成受阻与降解加速导致了叶光合色素含量下降、光能利用与转化活性改变,进而引起光合能力的大幅度下降[1]。光合作用是植物生存和繁衍的物质基础,在这个复杂的生理生化过程中,受到伤害的最原初部位是与光系统Ⅱ(PSⅡ)紧密联系的[2-5]。植物淹水后会导致PSⅡ光化学活性和电子传递速率降低[6],PSⅡ捕光色素蛋白复合物(LHCⅡa,LHCⅡb,LHCⅡc)各组分的变化,从而引起光合二氧化碳同化效率的降低[7]。另一方面,植物也可以以热的形式耗散过剩光能[8],PSⅡ反应中心的失活和周转[9]及Mehler反应[10]等减轻光抑制过程,从而保护光合机构免受破坏。叶绿素荧光参数最大光化学效率(Fv/Fm),PSⅡ实际光化学效率(Fv′/Fm′),光化学荧光猝灭系数(qP)和非光化学猝灭系数(qN),PSⅡ的实际光化学量子产量(Yyield),表观光合电子传递速率(RET)等的变化可反映逆境胁迫对PSⅡ的损伤程度[11-12],已经广泛应用于光抑制、水分、高温、低温等逆境生理研究[13-14]。竹子是集经济、生态和社会效益于一体的优良林种,是区域农村经济社会发展的重要资源和生态环境保护的重要屏障。水分、温度、光照等环境条件的变化直接影响着竹子的生长发育和分布。随着全球气候的变化,水资源不均匀分布造成近年来极端干旱和洪涝灾害频发,水分胁迫已经成为影响竹子生长发育的主要逆境因子之一,研究竹子对水分胁迫的适应能力越来越受到关注[15]。目前,国内外相关研究主要集中在短期干旱或水淹对竹子生长和生理生态的影响[16-21],而对于长期处于浸渍环境中的竹子生理生态响应及其机制研究甚少[22]。河竹Phyllostachys rivalis隶属禾本科Gramineae倭竹族Shibataeeae刚竹属Phyllostachys,主要分布于浙江、福建等地,生于溪涧边、山沟旁,性喜水湿,鞭根系统极为发达,竹鞭韧皮部密生一圈肉眼可见的气孔,具有耐淹植物的特征。我们前期的研究表明,河竹鞭根系统可以通过抗氧化系统平衡调节、生物量合理分配和异速生长调节等来适应长期淹水环境,维持生长和更新[23-24],而长期水淹胁迫下河竹叶绿素荧光变化特征、能量耗散过程及其与河竹耐受水淹的关系尚不清楚。为此,本研究以2年生河竹盆栽苗为试材,设置不同的水淹深度处理,测定分析不同水淹时间下叶片荧光参数和能量耗散的变化规律,探讨持续淹水对河竹光能的吸收和转化、能量的传递与分配、反应中心的活性、过剩能量的耗散以及光合作用的光抑制和光破坏等的影响,并从光合系统“内在性”揭示河竹对持续淹水的响应与适应机制。
HTML
-
试验地位于浙江省临安市太湖源观赏竹种园内。该地属中亚热带湿润季风气候区,年平均气温为15.4 ℃,极端低温-10.3 ℃,极端高温44.5 ℃,年日照时数为1 850~1 950 h,日均高于10 ℃活动积温为5 100 ℃,年平均无霜期为235 d,年降水量为1 250~1 600 mm,年平均空气相对湿度80%以上。
2012年2月在河竹种苗林中挖取2年生小丛状竹苗,竹苗地径(1.0 ± 0.2)cm,全高(1.0 ± 0.4)m,保留5~6盘枝,选择规格基本一致的竹苗移栽到加仑盆中(上端直径32.0 cm,下端直径23.0 cm,高度27.0 cm),以V(红壤):V(细沙)=3:1为培养基质,填充基质约15.0 kg·盆-1,栽植竹苗10株·盆-1。移栽后正常喷灌和清除竹笋、杂草等管理。
2013年4月15日选择生长状况一致的河竹盆栽苗进行淹水处理。设3个梯度,即对照(ck),处理Ⅰ和处理Ⅱ。对照实行正常浇水,使盆栽基质相对含水率保持85.0%±5.0%;处理Ⅰ淹水水位高于土壤表面5.0 cm;处理Ⅱ淹水水位高于土壤表面10.0 cm。试验盆栽苗置于长度4.3 m,宽度3.3 m和深度0.5 m的方形水泥池中进行淹水处理,试验期间保持设定水位。设置重复10个·处理-1,即盆栽苗10盆。
-
根据已有报道,自然消落带淹水时间平均3个月左右,最长时间可达6个月[8]。而前期试验表明,淹水360 d后,河竹仍能正常生长,且表现出一定的更新能力。为了解河竹叶片荧光参数和能量耗散等方面对持续淹水的响应,本研究设置淹水处理时间分别为30,90,180,270和360 d。在设定时间,随机选取3盆·处理-1河竹盆栽苗,在9:00-10:00,采用PAM-2500便携式脉冲调制叶绿素荧光仪(德国Walz公司)测定叶片叶绿素荧光参数。对河竹顶部倒数第3~4盘枝选择4~7片成熟叶片,先将测定植株叶片用黑色布袋子罩住,暗适应30 min,使得待测叶片所处光环境一致,全部使用仪器提供的测量光、光化光及饱和脉冲光测定叶片的初始荧光(Fo)和最大荧光(Fm)。作用光打开后测定光下最小荧光(Fo′)和光下最大荧光(Fm′),以荧光慢诱导模式测定光系统Ⅱ(PSⅡ)的Fv/Fm,Fv′/Fm′,qP,qN,Yyield和RET。PSⅡ吸收光能分配百分率参照Demmig Adams和Adams公式计算[25]:光化学反应的能量(P)= Fv′/Fm′×qP;非光化学反应耗散的能量(E)=(1-qP)×Fv′/Fm′;天线色素耗散的能量(D)=1-Fv′/Fm′。
-
采用SPSS 20.0统计软件进行单因素方差分析(One-way ANOVA),用Duncan方法进行多重比较,用Excel 2010绘制图表。
1.1. 试验材料与处理
1.2. 叶绿素荧光测定方法
1.3. 数据处理与分析
-
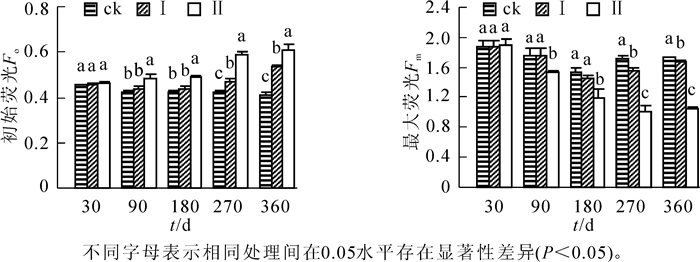
由图 1可知:随淹水时间的延长,淹水处理的河竹叶片Fo总体呈升高趋势,而Fm总体呈下降趋势。短期淹水处理(30 d)对河竹叶片Fo和Fm并无明显影响,但淹水时间进一步延长,处理间差异增大,水深效应也日趋明显,至淹水90 d和180 d时,处理Ⅱ的河竹叶片Fo显著高于处理Ⅰ和ck(P<0.05),而Fm显著低于处理Ⅰ和ck(P<0.05),且后两者Fo和Fm均无显著差异(P>0.05),其后至淹水处理结束,淹水处理的河竹叶片Fo持续升高,Fm总体上持续下降,且水位效应更加明显,处理间差异均达显著水平(P<0.05)。
-
随着淹水时间的延长和淹水深度的增大,河竹叶片Fv/Fm呈下降趋势,Fv′/Fm′呈先升高后降低的趋势(图 2)。相对ck,短期淹水(30 d),河竹叶片Fv/Fm降低,但处理间差异并不显著(P>0.05),而Fv′/Fm′则升高,且处理Ⅱ显著高于ck(P<0.05);至淹水90 d和180 d时,淹水处理河竹叶片Fv/Fm明显下降,处理Ⅱ显著低于处理Ⅰ和ck(P<0.05),而后两者间无显著差异(P>0.05);其后至淹水处理结束,淹水处理河竹叶片Fv/Fm持续下降,处理间差异达显著水平(P<0.05),水位效应明显,但处理Ⅰ和处理Ⅱ仍有ck的89.4%和55.4%。河竹叶片Fv′/Fm′较Fv/Fm对淹水胁迫更敏感,短期淹水即会引起Fv′/Fm′的明显升高,淹水处理30 d时,处理Ⅱ就显著高于ck(P<0.05),处理90 d时,处理Ⅰ和处理Ⅱ均显著高于ck(P<0.05),但至处理180 d时各处理的Fv′/Fm′均明显下降,且处理间并无显著差异(P>0.05),其后淹水处理的Fv′/Fm′显著下降,水位效应较为明显。
-
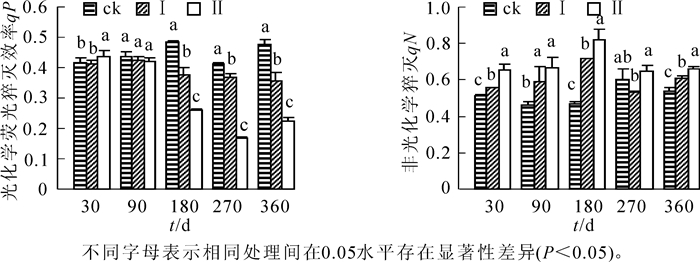
由图 3可知:随着淹水时间的延长,不同淹水处理下的河竹叶片qP总体呈下降趋势,qN呈升高趋势,不同处理间变化幅度不同。相对ck,淹水30 d时,处理Ⅱ的qP显著升高(P<0.05);淹水90 d时,河竹叶片qP开始降低,但处理Ⅰ和处理Ⅱ与ck差异不显著(P>0.05);淹水180 d时至处理结束,河竹叶片qP为ck>处理Ⅰ>处理Ⅱ,各处理间均有显著差异(P<0.05),水位效应明显。整个淹水过程中,河竹叶片qN基本上为ck<处理Ⅰ<处理Ⅱ,总体上淹水处理显著高于ck(P<0.05),水位效应也较为明显。
-
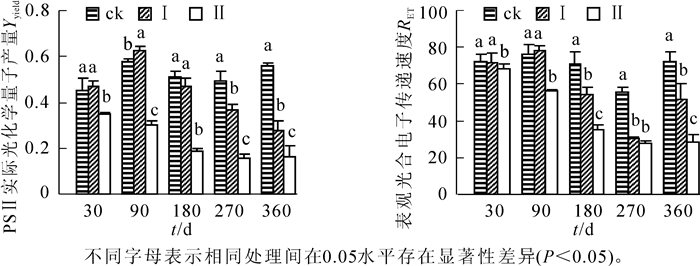
由图 4可知:不同淹水处理的河竹叶片Yyield和RET的变化不同。随着淹水时间的延长,处理Ⅰ的Yyield先升高后降低,RET先升高后降低再升高,在淹水90 d时均达最高值。在处理180 d后,各处理间河竹叶片Yyield和RET总体上差异显著(P<0.05);处理Ⅱ的Yyield和RET均随着淹水时间的延长而逐渐降低,各处理时间点上均显著低于ck(P<0.05)。至淹水180 d后,河竹叶片的Yyield和RET均随着淹水深度的增大而显著降低(P<0.05),存在明显的水位效应。
-
由表 1可知:随着淹水时间的延长,处理Ⅰ和处理Ⅱ的河竹叶片光化学反应能量(P)均呈先升高后下降的变化趋势。淹水30 d和90 d时,处理Ⅰ和处理Ⅱ的P较ck升高,且处理Ⅱ与ck差异显著(P<0.05);淹水180,270和360 d时,处理Ⅰ和处理Ⅱ的P均显著低于ck(P<0.05),且处理Ⅰ和处理Ⅱ间差异显著(P<0.05),水位效应明显。天线色素耗散能量(D)随着淹水时间的延长呈先降低后升高的变化趋势。淹水30 d和90 d时,处理Ⅰ和处理Ⅱ的D均显著低于ck(P<0.05);淹水180 d时,处理Ⅰ和处理Ⅱ的D仍低于ck,但未达显著差异水平(P>0.05);淹水270 d和360 d时,处理Ⅰ和处理Ⅱ的D均显著高于ck(P<0.05)。整个淹水处理过程中,河竹叶片天线色素耗散能量(D)的水位效应总体上并不明显。在淹水30,90和180 d时,处理Ⅰ和处理Ⅱ的非光化学反应耗散能量(E)较ck显著升高(P<0.05),但处理Ⅰ和处理Ⅱ之间差异并不显著(P>0.05);淹水270 d时,处理Ⅰ的E较ck显著降低(P<0.05);淹水360 d时,处理Ⅰ和处理Ⅱ的E较对照降低(P>0.05),河竹叶片非光化学反应耗散能量(E)的水位效应总体上也并不明显。
参数 处理 不同淹水时间河竹叶叶片吸收光能/% 30 90 180 270 360 d 光化学反应能量(P) ck 22.8 ± 0.7 b 24.6 ± 0.6 b 23.5 ± 1.1 a 26.3 ± 0.3 a 28.1 ± 3.4 a Ⅰ 23.8 ± 0.7 b 26.1 ± 0.2 a 20.0 ± 1.2 b 19.5 ± 0.8 b 15.1 ± 0.7 b Ⅱ 25.2 ± 0.6 a 26.4 ± 0.2 a 12.6 ± 1.0 c 7.6 ± 0.3 c 8.5 ± 0.7 c 天线色素耗散能量(D) ck 45.1 ± 1.3 a 43.7 ± 3.0 a 51.3 ± 2.1 a 36.5 ± 1.1 c 41.2 ± 5.7 b Ⅰ 42.6 ± 1.6 b 38.4 ± 0.9 b 46.8 ± 3.5 a 47.3 ± 2.0 b 57.3 ± 2.5 a Ⅱ 42.2 ± 0.5 b 37.0 ± 0.5 b 51.5 ± 3.3 a 55.1 ± 2.4 a 61.7 ± 0.9 a 非光化学反应耗散能量(E) ck 32.1 ± 0.7 b 31.8 ± 2.6 b 25.2 ± 1.0 b 37.2 ± 0.8 a 30.7 ± 2.3 a Ⅰ 33.7 ± 0.9 a 35.6 ± 0.7 a 33.3 ± 2.3 a 33.3 ± 1.2 b 27.5 ± 2.8 a Ⅱ 32.5 ± 0.2 ab 36.6 ± 0.3 a 35.9 ± 2.4 a 37.3 ± 2.1 a 29.8 ± 0.6 a 说明:同列不同字母表示在0.05水平存在显著性差异。 Table 1. Effects of long-term flooding on characteristics fractions of absorbed light utilized in leaves of Phyllostachys rivalis
2.1. 持续淹水下河竹叶片初始荧光(Fo)和最大荧光(Fm)的响应
2.2. 持续淹水下河竹叶片PSⅡ最大光化学效率(Fv/Fm)和实际光化学效率(Fv′/Fm′)的响应
2.3. 持续淹水下河竹叶片光化学荧光猝灭系数(qP)和非光化学猝灭系数(qN)的响应
2.4. 持续淹水对叶片PSⅡ实际光化学量子产量(Yyield)和表观光合电子传递速率(RET)的影响
2.5. 持续淹水对河竹叶片吸收光能分配的影响
-
环境胁迫影响植物的光合作用过程,造成光化学转换效率和电子传递速率与能量分配之间产生矛盾,从而影响光合碳同化能力,PSⅡ反应中心生理功能的稳定性是植物抵抗逆境胁迫的能力体现[26-28]。叶绿素荧光参数从能量代谢与转换的角度反映光合机构受逆境胁迫伤害的程度[29-31],Fm,Fv/Fm降低表明植物叶片发生光抑制[10],而Fv/Fm下降的同时Fo上升,表明PSⅡ反应中心受到损伤[29]。本研究中,处理Ⅰ在淹水30 d至180 d时,Fo,Fm和Fv/Fm变化不显著,说明淹水深度5 cm持续淹水180 d,河竹叶片PSⅡ的活性一直维持在正常水平,具有良好的适应能力,至淹水270 d时,处理Ⅰ的Fo显著升高,Fm和Fv/Fm降低,说明此时河竹叶片PSⅡ反应中心的內禀光能转化效率和活性随淹水时间持续而降低,光合作用的原初反应受到抑制,不利于河竹叶片捕获光能的转化。而处理Ⅱ在淹水90 d时,Fo显著上升,Fm和Fv/Fm下降,说明淹水深度10 cm持续淹水90 d,河竹叶片PSⅡ开始受到损伤。淹水360 d,相对对照,处理Ⅰ和处理Ⅱ河竹叶片的Fv/Fm显著降低,但仍具有相对较高的水平,这与长期水淹后枫杨Pterocarya stenoptera幼苗[8]的研究结果一致。说明长期淹水影响河竹叶片PSⅡ反应中心的活性,但对PSⅡ的功能反应中心影响较小。
荧光猝灭是植物内光合量子效率调节的一个方面,分为光化学荧光猝灭(qP)和非光化学猝灭(qN)2类[32]。qP值的大小反映PSⅡ反应中心开放部分的比例及电子传递速率[33-34]。本研究中,淹水30 d时,处理Ⅰ维持相稳定对的qP,Yyield和RET值,有效地避免或减轻因PSⅡ吸收而引起的光抑制和光氧化,保护了光合机构正常运转。处理Ⅱ的qP值上升,说明初期水淹有利于河竹叶片PSⅡ反应中心电子传递能力的提高,促进捕获的光能更高效地用于光合作用。淹水180 d后,处理Ⅰ和处理Ⅱ的qP均显著下降,说明PSⅡ反应中心电子传递受阻,在一定程度上降低了河竹叶片PSⅡ的活性。非光化学猝灭(qN)常用来评价植物耗散过剩激发能的能力[4, 35]。本研究中,淹水处理Ⅰ和处理Ⅱ条件下河竹叶片的qN随着淹水深度及持续时间而增高,热耗散保护性作用增强。这可能是河竹适应淹水环境而形成的自我保护机制。
通过叶片的化学反应的能量(P),天线色素耗散的能量(D)和非光化学反应耗散的能量(E)及其占总吸收光能的比例,可以了解植物逆境环境下的光能利用能力[36]。本研究表明,淹水30 d和90 d时,处理Ⅰ和处理Ⅱ的P,E显著升高,D降低,说明淹水初期不同水位处理均提高了光合作用碳同化电子需求,E的增加可能会引起光合机构的可逆失活甚至破坏[10]。淹水360 d时,处理Ⅰ和处理Ⅱ的P明显减少,D显著升高,而E恢复对照水平,减轻了PSⅡ的激发压,从而能及时的使单线态叶绿素(1Chl)返回三线态叶绿素(3Chl)[37],降低了形成单线态氧(1O2)的机会,表明长期淹水后河竹叶片PSⅡ能力恢复,天线色素耗散的能量(D)上升,可减少PSⅡ和电子传递的过分还原,从而防止过剩光能对光合机构的破坏。持续淹水环境下,河竹吸收光强主要以天线色素耗散(D)为主要光能分配途径,淹水后期PSⅡ反应中心的非光化学反应耗散(E)的恢复起着重要作用,这种变化充分反映了河竹对淹水的适应能力。
-
综上所述,淹水环境下河竹能通过维持相对较高的RET,qP和P值,增强qN来调节自身能量代谢,以热耗散形式散失过多的光能,有效地避免或减轻光抑制和光氧化,河竹吸收光强主要以天线色素耗散(D)为主要光能分配途径,淹水后期PSⅡ反应中心的非光化学反应耗散(E)的恢复起着重要作用。这些可能是河竹适应淹水环境的自我保护机制。













 DownLoad:
DownLoad: