-
扦插育苗是将离体植物营养器官如根、茎(枝)、叶等插入基质中,利用植物的再生能力,经过人工培育使之发育成完整新植株的繁育方法。扦插育苗方法简单,取材方便,能够保持母本优良性状并实现快速批量繁殖,是木本植物的重要繁育方法之一,但很多树种因生根困难而无法利用扦插进行繁殖[1]。以往扦插育苗主要应用于较易生根的树种,近年来针对难生根树种的生根机制和生根技术等方面开展了大量研究,发现通过插穗消毒处理、流水冲洗生根抑制物质、黄化处理及激素处理、调控环境温湿度等技术措施[2],能够大大提高难生根树种的扦插生根率。生物磁学(biomagnetism)是研究外磁场对生物体的影响以及生物磁性与生命活动关系的磁学和生物学相互渗透的新兴交叉学科。随着生物磁学在农业、医学、环保、食品以及生物工程等领域的广泛应用,生物磁学已经得到国内外各领域专家的重视,这为揭示生物磁学机制提供了有利的证据。研究发现磁化处理能够促进种子萌发[3]、提高作物产量[4]、促进植物细胞分裂[5-6]、对植物的生理生化反应也有一定的影响[7-11]。目前,磁化处理对于木本植物扦插生根的相关研究却很少见,在促进难生根树种生根、根系发育和扦插苗生长及生理特性方面,仍需做大量研究工作。绒毛白蜡Fraxinus velutina具有抗旱、耐涝、耐盐碱、速生、材性好、树姿美观等特点,广泛用于盐碱地造林和城乡园林绿化。绒毛白蜡优良无性系‘鲁蜡5号’Fraxinus velutina ‘Lula-5’耐旱性强、生长迅速、观赏价值高,然而由于其扦插繁殖困难,限制了该优良品种的快速繁育和大面积推广应用。本研究采用磁处理技术对绒毛白蜡新品种插穗进行处理,以探讨磁处理技术在难生根树种扦插育苗中的应用前景。
HTML
-
试验材料为绒毛白蜡优良无性系‘鲁蜡5号’,采自山东省济南市德林种苗有限公司3年生采穗圃。2015年8月下旬取采穗母树上无病虫害的当年生半木质化健壮新梢,剪取插穗长度12~15 cm,粗度1.0~1.2 cm,在芽上1 cm剪成平口,下切口剪成马耳形,保持切口平滑。保留2叶片并剪成半叶,将插穗在质量浓度为1.0 g·L-1高锰酸钾溶液中消毒0.5 h,然后将插穗基部浸泡在200 mg·L-1的吲哚丁酸(IBA)溶液中4 h。选用珍珠岩作为基质,扦插前5 d喷淋质量浓度为0.5%的高锰酸钾溶液消毒,用黑色塑料薄膜覆盖3 d后揭开薄膜,待药剂挥发后扦插。扦插棚为智能控制温湿度的联栋大棚。
-
试验设置磁化水喷淋(MW)+磁化毯(MR),磁化水喷淋(MW)和对照(ck,清水喷淋)3个处理,重复4次,扦插100株·小区-1。其中:① 喷淋处理:采用PP-25-ADS-600型磁化水处理器(Φ=25 mm,磁场强度为600 T,水流量为6 m3·h-1)处理的磁化水对插床进行喷淋处理,所用喷淋水为饮用自来水;② 磁化毯处理:将磁场强度为300 T的磁化毯铺设在扦插基质下方25 cm处,插穗下端高于磁化毯5 cm。设定棚内温度为25~27 ℃,相对湿度(85±5)%,自动控制喷淋。喷头采用十字雾化喷头(KL3073)。扦插后每7 d采样1次,随机抽样10株·处理-1,观察插穗生根进程及生长状况(在愈伤组织形成以后至生根以前,适当加大观测密度,以确定不定根产生的准确时间)。扦插90 d,调查各处理所有材料的生根率、根系特征及生长量、新梢及叶片生长量。
-
11月中旬,采用冲洗法将全部扦插苗整株取出,统计生根率,新梢高,茎、叶片生长量。随机抽样扦插苗10株·处理-1,去离子水冲洗根系表面残留物,将每个插穗构型完整的根系,按照PREGITZER等[12]的方法对根系进行分级,即将根系最外端的细根定为1级根,其母根为2级根,2级根的母根为3级根。将各级根序细根放入装有去离子水的培养皿中并进行编号,用根系分析仪Winrhizo(德国)测定各级根序细根长度、直径、表面积和体积,计算根系效果指数[13]。将各处理植株置于103 ℃烘箱中杀青10 min后80 ℃烘干至恒量,测定单株根系干质量。将烘干的根系及新梢分别研磨过100目筛,采用张志良等[14]的方法测定可溶性糖,凯氏定氮法测定总氮,采用燃烧氧化法用总碳分析仪(Elementar Vario,德国)测定总碳含量。
根系效果指数I=(L×M×R)/N。其中:L为插穗平均根长,M为插穗根系数量,R为插穗生根率,N为生根插穗数。
-
利用统计软件SAS 9.2,Excel 2016对数据进行分析,采用Duncan多重比较法(Duncan’s multiple-range)进行处理间差异性分析。生根率百分数经过反正弦转换。
1.1. 试验材料
1.2. 试验设计
1.3. 测定分析方法
1.4. 数据处理
-
由表 1可知:MW+MR处理的绒毛白蜡嫩枝扦插生根率最高,达到84.2%,MW为60.4%,分别比ck高60.7%和36.9%,差异极显著(P<0.01);扦插后第10天分别随机抽取10株发现,MW+MR处理有4株出现愈伤组织,MW和ck分别只有2株和1株出现愈伤组织;MW+MR处理在扦插后15 d出现不定根,MW不定根出现于19 d,ck则在扦插后25 d左右才出现不定根;MW+MR处理的插穗根系干质量相对较大(0.090 g),高于MW及ck,差异极显著(P<0.01);MW+MR处理的根系含水量比ck高0.516 g,MW比ck高0.284 g,差异极显著(P<0.01)。不同处理根系效果指数比较,MW+MR及MW处理根系效果指数均高于ck,而MW+MR比MW高0.577,说明磁化毯能够促进插穗生根。
处理 不定根出现期/d 生根率/% 根干质量/g 根含水量/g 根系效果指数 ck 25 23.5 ± 1.594 C 0.062 ± 0.002 C 0.510 ± 0.057 B 0.410 MW 19 60.4 ± 2.838 B 0.081 ± 0.005 B 0.782 ± 0.077 AB 0.987 MW+MR 15 84.2 ± 2.341 A 0.107 ± 0.005 A 0.996 ± 0.100 A 1.567 说明:数据为3次测定的平均值±标准误, 同列中不同大写字母表示处理间的差异达到极显著水平(P<0.01)。 Table 1. Comparison of different treatment on rooting date, rooting rate, root drought weight, root water contet and root effect index
-
由图 1可知:磁化处理能有效促进绒毛白蜡扦插苗的新梢和叶片生长。MW+MR,MW和ck等3个处理75 d龄扦插苗新梢平均基径分别为0.26,0.24和0.22 cm,MW+MR处理显著(P<0.05)高于MW及ck,MW显著(P<0.05)高于ck;MW+MR,MW和ck处理的新梢平均长度分别为4.95,4.07和2.83 cm,MW+MR显著(P<0.05)高于MW及ck,MW显著(P<0.05)高于ck。MW+MR处理的叶片数量及面积最高,分别为4.7片和24.15 cm2,其次为MW,分别为4.2片和17.41 cm2,ck最低,分别为2.8片和11.31 cm2,MW+MR和MW处理的叶片数量及面积显著(P<0.05)高于ck,MW+MR处理的叶片面积显著高于(P<0.05)MW,但MW+MR和MW处理的叶片数量差异性不显著(P>0.05)。说明磁化处理显著促进了插穗苗新梢和叶片的生长。
-
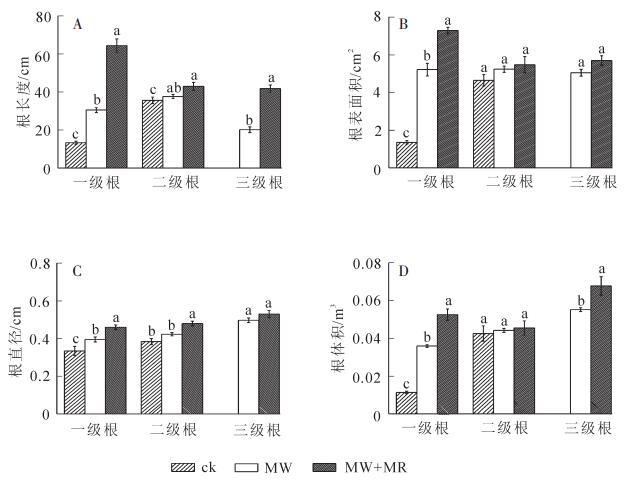
从图 2中看出:不同处理根系形态参数存在较大差异,MW+MR及MW处理的一级根序细根长度、直径、表面积、体积均显著(P<0.05)高于ck,虽然二级根系形态差异不显著(P>0.05),但是MW+MR及MW处理的根系分为3级,ck的根系只有2级,表明磁场有助于促进生根及根系发育,这与磁化处理高于ck的生根成活率是一致的。
-
由表 2可知:MW+MR处理的碳氮比极显著(P<0.01)低于MW及ck,MW+MR处理扦插苗氮质量分数最高,其次为MW,ck的氮质量分数最低,差异极显著(P<0.01)。MW+MR及MW处理钾、钙、镁、铁、锰、锌、铜质量分数均高于ck,差异极显著(P<0.01)。然而磁化处理后钠质量分数则减少,与ck相比,MW+MR及MW处理分别减少了22.8%和9.7%,差异极显著(P<0.01)。MW+MR处理的可溶性糖质量分数最高,MW次之,但高于ck,差异显著(P<0.05),因此磁化处理能够明显加快插穗可溶性糖的积累,促进不定根的形成,从而缩短生根周期。
处理 碳氮比 氮/(mg.g-1) 磷/(mg.g-1) 钾/(mg.g-1) 钙/(mg.g-1) 钠/(mg.g-1) ck 8.135±0.456 A 41.618±2.013 C 160.029±5.405 B 1.899±0.093 B 5, 566±0, 420 C 28, 732±1, 007 A MW 5.369±0.348 B 72, 326±2, 936 B 208.781±13.194 A 2.227±0.010 B 7, 499±0, 455 B 25.937±0.717 B MW+MR 3.966±0.315 C 103.797±8.508 A 226.402± 13.749 A 2.625±0.128 A 9.075±0.511 A 22.180±0.812 C 处理 镁/(mg.g-1) 铁/(mg.g-1) 锰/(mg.g-1) 锌/(mg.g-1) 铜/(mg.g-1) 可溶性糖/(mg.g-1) ck 0, 644±0, 021 A 0, 523±0, 098 B 0.128±0.017 A 0, 112±0, 008 C 0, 037±0, 001 C 142.954±1.175 C MW 0, 736±0, 008 B 1, 532±0, 257 B 0, 075±0, 003 B 0.198±0.016 B 0.228±0.013 B 154.554±2.544 B MW+MR 0.835±0.014 C 2, 963±0, 387 A 0.050±0.001 B 0.321±0.012 A 0.393±0.004 A 167.819±3.379 A 说明:数据为3次测定的平均值±标准误, 同列中不同大写字母表示处理间的差异达到极显著水平(P<0.01)。 Table 2. Comparison of different treatment on C/N and element
2.1. 磁化处理对扦插生根成活率和根系生长的影响
2.2. 磁化处理对新梢及叶片生长的影响
2.3. 不同处理插穗根系形态参数的比较
2.4. 磁化处理对扦插苗根系营养物质积累的影响
-
绒毛白蜡嫩枝插穗经过磁化处理对插穗形成愈伤组织、缩短生根时间以及对提升根系质量与扦插成活率有显著的促进作用。有研究认为:磁化处理能够影响酶的活性及内源激素的成分,从而促进植物愈伤组织与生根基因的表达,产生不定根[15]。磁化水水培小麦Triticum aestivum和水稻Oryza sativa能够促进次生根的分化,提高对矿质元素和水分的吸收效率[16]。也有研究认为:磁化处理后亚麻Linum sp.和小扁豆Polygala tatarinowii的细胞增殖活性减弱,增殖周期减缓[17]。本次研究发现,磁化处理后绒毛白蜡的一级根系形态参数明显高于对照组,表明在磁场环境下很可能激活了植物生长的某条代谢途径,或者生根关联酶活性,从而促进了绒毛白蜡生根及生长发育。
-
磁化处理技术在扦插繁育中起到有效的促进作用,不仅能够促进插穗生根,增加根系数量,而且提高了苗木繁育速度,大大缩短了育苗周期。本研究证明,磁化处理能够明显加快插穗可溶性糖的积累,促进插穗不定根的形成,从而缩短生根周期;能够促进扦插苗生物量积累,能够降低植物根系中钠质量分数,促进氮磷钾及微量元素的累积。有研究表明,磁化水通过影响水的渗透力、溶解力与缔合度产生的物理效应来促进插穗对水分的吸收[15],从而对植物生长发育、产量和生物量的提高具有显著影响。磁化处理提高了有效态养分的利用率,抑制了对钠离子(Na+)的吸收、积累,促进植株对钾离子(K+),钙离子(Ca2+),镁离子(Mg2+)等矿物养分离子的选择吸收能力[18]。磁化微咸水能够增强欧美杨-107 Populus × euramericanna ‘Neva’根系对水分和养分的吸收,能够极显著地提高其根、叶的生物量累积[19]。磁化处理能够显著提高鹰嘴豆Cicer arietinum的种实量、秸秆量及生物产量[7]。高矿化度水经过磁化处理能够促进绒毛白蜡植株的光合作用,从而促进其生长及生物量的累积[20]。
也有研究表明:不同磁场强度对植物影响不同,这可能与磁场环境改变了某些关联酶活性有关[21]。磁场对于植物的效应还与磁场的分布及频率有关,所以磁场作用于植物生长是由多种机制共同作用决定最终效应的[22]。磁化作用对于植物的最佳作用机制和技术条件还需进一步探究,以促进该技术在林业领域更广泛的应用。












 DownLoad:
DownLoad: