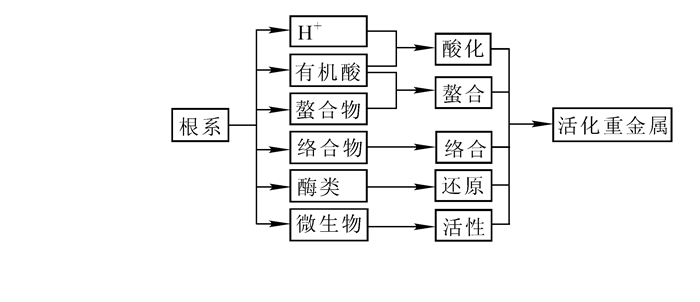
| [1] |
樊霆, 叶文玲, 陈海燕.
农田土壤重金属污染状况及修复技术研究[J]. 生态环境学报, 2013, 22(10): 1727-1736.
doi: 10.3969/j.issn.1674-5906.2013.10.015 |
FAN Ting, YE Wenling, CHEN Haiyan.
Review on contamination and remediation technology of heavy metal in agricultural soil[J]. Ecol Environ Sci, 2013, 22(10): 1727-1736.
doi: 10.3969/j.issn.1674-5906.2013.10.015 |
| [2] |
环境保护部, 国土资源部. 全国土壤污染状况调查公报[R/OL]//http://www.gov.cn/foot/site1/20140417/782bcb88840814ba158d01.pdf. |
| [3] |
邢艳帅, 乔冬梅, 朱桂芬.
土壤重金属污染及植物修复技术研究进展[J]. 中国农学通报, 2014, 30(17): 208-214.
doi: 10.11924/j.issn.1000-6850.2013-2867 |
XING Yanshuai, QIAO Dongmei, ZHU guifen.
Research progress of heavy pollution in soil and phytoremediation technology[J]. Chin Agric Sci Bull, 2014, 30(17): 208-214.
doi: 10.11924/j.issn.1000-6850.2013-2867 |
| [4] |
陈靖, 林振景, 孟媛媛.
土壤重金属污染的植物修复及超富集植物的研究进展[J]. 中国环境管理干部学院学报, 2011, 21(1): 69-71.
|
CHEN Jing, LIN Zhenjing, MENG Yuanyuan.
Research progress on phytoremediation for heavy metal contamination in soil and hyperaccumulators[J]. J Environ Manage Coll China, 2011, 21(1): 69-71.
|
| [5] |
BAKER A J M, MCGRATH S P, SIDOLI C M D.
The possibility of in situ heavy metal decontamination of polluted soils using crops of metal-accumulating plants[J]. Resour Conserv Recycl, 1994, 11(1/4): 41-49.
|
| [6] |
张知贵, 王明贤, 杨华.
植物根系分泌物研究进展[J]. 广东农业科学, 2013, (2): 219-222.
|
ZHANG Zhigui, WANG Mingxian, YANG Hua.
The research status of plant root exudates[J]. Guangdong Agric Sci, 2013, (2): 219-222.
|
| [7] |
PILON-SMITS E.
Phytoremediation[J]. Annu Rev Plant Biol, 2005, 56(1): 15-39.
doi: 10.1146/annurev.arplant.56.032604.144214 |
| [8] |
DUSHENKOV V, KUMAR P B, MOTTO H.
Rhizofiltration:the use of plants to remove heavy metals from aqueous stream[J]. Environ Sci Technol, 1995, 29(5): 1239-1245.
doi: 10.1021/es00005a015 |
| [9] |
SOLODUCHO J, CABAJ J.
Phenolic compounds hybrid detectors[J]. J Biomater Nanobiotechnol, 2013, 4(3): 17-27.
doi: 10.4236/jbnb.2013.43A003 |
| [10] |
钟斌, 陈俊任, 彭丹莉.
速生林木对重金属污染土壤植物修复技术研究进展[J]. 浙江农林大学学报, 2016, 33(5): 899-909.
doi: 10.11833/j.issn.2095-0756.2016.05.024 |
ZHONG Bin, CHEN Junren, PENG Danli.
Research progress of heavy metal phyteremediation technology of fast-growing forest tree in soil[J]. J Zhejiang A & F Univ, 2016, 33(5): 899-909.
doi: 10.11833/j.issn.2095-0756.2016.05.024 |
| [11] |
HARRISON S J, LEPP N W, PHIPPS D A.
Uptake of copper by excised roots:(Ⅳ) copper uptake from complexed sources[J]. Z Pflanzenphysiol, 1984, 113(5): 445-450.
doi: 10.1016/S0044-328X(84)80100-2 |
| [12] |
李汛, 段增强.
植物根系分泌物的研究方法[J]. 基因组学与应用生物学, 2013, 32(4): 540-547.
|
LI Xun, DUAN Zengqiang.
Progress on the research methods for root exudates[J]. Genom Appl Biol, 2013, 32(4): 540-547.
|
| [13] |
郭晓方, 卫泽斌, 许田芬.
不同pH值混合螯合剂对土壤重金属淋洗及植物提取的影响[J]. 农业工程学报, 2011, 27(7): 96-100.
|
GUO Xiaofang, WEI Zebin, XU Tianfen.
Effects of mixture of chelating agents with different pH values on phytoextraction and heavy metals removal[J]. Trans Chin Soc Agric Eng, 2011, 27(7): 96-100.
|
| [14] |
HARTMANN A, ROTHBALLER M, SCHMID M.
Lorenz Hiltner, a pioneer in rhizosphere microbial ecology and soil bacteriology research[J]. Plant Soil, 2008, 312(1): 7-14.
|
| [15] |
朱丽霞, 章家恩, 刘文高.
根系分泌物与根际微生物相互作用研究综述[J]. 生态环境, 2003, 12(1): 102-105.
|
ZHU Lixia, ZHANG Jiaen, LIU Wengao.
Review of studies on interactions between root exudates and rhizopheric microorganisms[J]. Ecol Environ, 2003, 12(1): 102-105.
|
| [16] |
ROVIRA A D, FOSTER R C, MARTIN J K. Note on terminology:origin, nature and nomenclature of the organic material in the rhizosphere[G]//HARLEY J L, RUSSELL R S. The Soil-Root Interface. London:Academic Press, 1979:1-4. |
| [17] |
GUO Wei, ZHANG Huayong, HUO Shouliang.
Organochlorine pesticides in aquatic hydrophyte tissues and surrounding sediments in Baiyangdian wetland, China[J]. Ecol Eng, 2014, 67(2): 150-155.
|
| [18] |
徐卫红, 王宏信, 刘怀.
Zn, Cd单一及复合污染对黑麦草根分泌物及根际Zn, Cd形态的影响[J]. 环境科学, 2007, 28(9): 2089-2095.
|
XU Weihong, WANG Hongxin, LIU Huai.
Effects of individual and combined pollution of Cd and Zn on root exudates and rhizosphere Zn and Cd fractions in ryegrass (Loliurn perenne L.)[J]. Environ Sci, 2007, 28(9): 2089-2095.
|
| [19] |
姜海燕, 袁秀英, 樊石磊.
胡杨根际土壤化感物质成分分析[J]. 内蒙古农业大学学报(自然科学版), 2011, 32(2): 48-51.
|
JIANG Haiyan, YUAN Xiuying, FAN Shilei.
Composition analysis of soil allelochemicals in Populus euphratica rhizosphere[J]. J Inner Mongolia Agric Univ Nat Sci Ed, 2011, 32(2): 48-51.
|
| [20] |
BESSONBARD A, GRAVOT A, RICHAUD P.
Nitric oxide contributes to cadmium toxicity in Arabidopsis by promoting cadmium accumulation in roots and by up-regulating genes related to iron uptake[J]. Plant Physiol, 2009, 149(3): 1302-1315.
doi: 10.1104/pp.108.133348 |
| [21] |
ZENG Fanrong, CHEN Song, MIAO Ying.
Changes of organic acid exudation and rhizosphere pH in rice plants under chromium stress[J]. Environ Poll, 2008, 155(2): 284-289.
doi: 10.1016/j.envpol.2007.11.019 |
| [22] |
CARBALLEIRA T, RUIZ I, SOTO M.
Effect of plants and surface loading rate on the treatment efficiency of shallow subsurface constructed wetlands[J]. Ecol Eng, 2016, 90(1): 203-214.
|
| [23] |
XIE Xiangyu, WEISS D J, WENG Bosen.
The short-term effect of cadmium on low molecular weight organic acid and amino acid exudation from mangrove (Kandelia obovata Yong) roots[J]. Environ Sci Poll Res, 2013, 20(2): 997-1008.
doi: 10.1007/s11356-012-1031-9 |
| [24] |
潘声旺, 袁馨, 刘灿.
苯并[α]芘对不同修复潜力羊茅属植物的根系分泌物中几种低分子量有机物的影响[J]. 植物生态学报, 2016, 40(6): 604-614.
doi: 10.17521/cjpe.2015.0426 |
PAN Shengwang, YUAN Xin, LIU Can.
Effects of benzo[α] pyrene on the organic compounds of low molecule weight excreted by root systems in five Festuca species with different remediation potentials[J]. Chin J Plant Ecol, 2016, 40(6): 604-614.
doi: 10.17521/cjpe.2015.0426 |
| [25] |
NAIDU R, HARTER R D.
Effect of different organic ligands on cadmium sorption by and extractability from soils[J]. Soil Sci Soc Am J, 1998, 62(3): 644-650.
doi: 10.2136/sssaj1998.03615995006200030014x |
| [26] |
LU Lingli, TIAN Shengke, YANG Xiao'e.
Improved cadmium uptake and accumulation in the hyperaccumulator Sedum alfredii:the impact of citric acid and tartaric acid[J]. J Zhejiang Univ Sci B BiomBiotechnol, 2013, 14(2): 106-114.
doi: 10.1631/jzus.B1200211 |
| [27] |
KRUMINS J A, GOODEY N M, FGALLAGHER F.
Plant soil interactions in metal contaminated soils[J]. Soil Biol Biochem, 2015, 80(): 224-231.
doi: 10.1016/j.soilbio.2014.10.011 |
| [28] |
LI Xiaopang, DING Changfeng, HUA Ke.
Soil sickness of peanuts is attributable to modifications in soil microbes induced by peanut root exudates rather than to direct allelopathy[J]. Soil Biol Biochem, 2014, 78(): 149-159.
doi: 10.1016/j.soilbio.2014.07.019 |
| [29] |
BOEUF-TREMBLAY V, PLANTUREUX S, GUCKERT A.
Influence of mechanical impedance on root exudation of maize seedlings at two development stages[J]. Plant Soil, , 172(2): 279-287.
doi: 10.1007/BF00011330 |
| [30] |
KUDOYAROVA G R, MELENTⅡEV A I, MARTYNENKO E V.
Cytokinin producing bacteria stimulate amino acid deposition by wheat roots[J]. Plant Physiol Biochem, 2014, 83(): 285-291.
doi: 10.1016/j.plaphy.2014.08.015 |
| [31] |
DIEZ J A, DUSENBERY D B.
Repellent of root-knot nematodes from exudate of host roots[J]. J Chem Ecol, , 15(10): 2445-2455.
doi: 10.1007/BF01020375 |
| [32] |
CIMMINO A, FERNÁNDEZ-APARICIO M, AVOLIO F.
Ryecyanatines A and B and ryecarbonitrilines A and B, substituted cyanatophenol, cyanatobenzodioxple, and benzodiox-olecarbonitriles from rye(Secale cereale L.)root exudates:novel metabolites with allelopathic activity on Orobanche seed germination and radicle growth[J]. Phytochemistry, 2015, 109(): 57-65.
doi: 10.1016/j.phytochem.2014.10.034 |
| [33] |
杨瑞吉, 牛俊义.
磷胁迫对油菜根系分泌物的影响[J]. 西南农业大学学报(自然科学版), 2006, 6(6): 895-899.
|
YANG Ruiji, NIU Junyi.
Effects of phosphorus deficiency on root exudation of rape (Brassica campestris L.)[J]. J Southwest Agric Univ Nat Sci, 2006, 6(6): 895-899.
|
| [34] |
HORCHHANI F, GALLUSCI P, BALDET P.
Prolonged root hypoxia induces ammonium accumulation and decreases the nutritional quality of tomato fruits[J]. J Plant Physiol, 2008, 165(13): 1352-1359.
doi: 10.1016/j.jplph.2007.10.016 |
| [35] |
ZHANG Fusuo, TREEBY M, RÖMHELD V.
Mobilization of iron by phytosiderophore as affected by other micronutrients[J]. Plant Soil, 1991, 130(1/2): 173-178.
|
| [36] |
张文明, 邱慧珍, 张春红.
马铃薯根系分泌物成分鉴别及其对立枯丝核菌的影响[J]. 应用生态学报, 2015, 26(3): 859-866.
|
ZHANG Wenming, QIU Huizhen, ZHANG Chunhong.
Identification of chemicals in root exudates of potato and their effects on Rhizoctonia solani[J]. Chin J Appl Ecol, 2015, 26(3): 859-866.
|
| [37] |
LUO Yongming, CHRISTIE P.
Alkaline sewage sludge solidsaffectthe chemical speciation and bio availability of Cu and Zn in the rhizosphere soil solution[J]. Soil Scid Plant Nutr, 1997, 43(special issue): 1041-1046.
|
| [38] |
陶波, 赵微, 韩玉军.
假苍耳根系分泌物对土壤中微生物的影响[J]. 东北农业大学学报, 2010, 41(2): 15-19.
|
TAO Bo, ZHAO Wei, HAN Yujun.
Effect of Iva xanthifolia root secretion on soil microorganisms amount[J]. J Northeast Agric Univ, 2010, 41(2): 15-19.
|
| [39] |
孙海燕. 基于微萃取技术的根系分泌物分析及其化感作用研究[D]. 哈尔滨: 哈尔滨工业大学, 2014. |
SUN Haiyan. Research on Root Exudate Analysis Based on Microextraction and Allellopathic Effect[D]. Harbin:Harbin Institute of Technology, 2014. |
| [40] |
马凤鸣, 王安娜, 吴蕾.
大豆根系分泌物的鉴定及PAL1, PAL2, C4H的克隆[J]. 作物杂志, 2011, (2): 65-71.
|
MA Fengming, WANG Anna, WU Lei.
Identification of soybean root exudates and cloning of the PAL1, PAL2, C4H genes[J]. Crops, 2011, (2): 65-71.
|
| [41] |
杨先国, 刘塔斯, 陈斌.
丹参根际土壤浸提物的GC-MS分析[J]. 中国农学通报, 2013, 29(10): 173-177.
doi: 10.11924/j.issn.1000-6850.2012-3436 |
YANG Xianguo, LIU Tasi, CHEN Bin.
GC-MS analysis of rhizosphere-soil extract of Salvia miltiorrhiza Bge[J]. Chin Agric Sci Bull, 2013, 29(10): 173-177.
doi: 10.11924/j.issn.1000-6850.2012-3436 |
| [42] |
SOROABELL M R, HERNÁNDEZFERNÁNDEZ A, SOTOCARREÑO F.
Inoculation responses with beneficial microorganisms in gerbera taking into account rhizosphere characterization[J]. Rev Chap Ser Hortic, 2009, 15(2): 41-48.
|
| [43] |
谢明吉, 严重玲, 叶菁.
菲对黑麦草根系几种低分子量分泌物的影响[J]. 生态环境, 2008, 17(2): 576-579.
|
XIE Mingji, YAN Chongling, YE Jing.
Effect of phenanthrene on the secretion of low molecule weight organic compounds by ryegrass root[J]. Ecol Environ, 2008, 17(2): 576-579.
|
| [44] |
杜茜, 卢迪, 马琨.
马铃薯连作对土壤生物群落结构和功能的影响[J]. 生态环境学报, 2012, 21(7): 1252-1256.
|
DU Qian, LU Di, MA Kun.
Effect of potato continuous cropping on soil microbial community structure and function[J]. Ecol Environ Sci, 2012, 21(7): 1252-1256.
|
| [45] |
罗庆, 孙丽娜, 胡筱敏.
镉超富集植物东南景天根系分泌物的代谢组学研究[J]. 分析化学, 2015, 43(1): 7-12.
|
LUO Qing, SUN Lina, HU Xiaomin.
Metabonomics study on root exudates of Cd hyperaccumulator Sedum alfredii[J]. Chin J Analyt Chem, 2015, 43(1): 7-12.
|
| [46] |
DINKCI N, AKALIN A S, GÖNC S.
Isocratic reverse-phase HPLC for determination of organic acids in Kargí tulum cheese[J]. Chromatographia, 2007, 66(1): 45-49.
|
| [47] |
EHLING S, COLE S.
Analysis of organic acids in fruit juices by liquid chromatography-mass spectrometry:an enhanced tool for authenticity testing[J]. J Agric Food Chem, 2011, 59(6): 2229-2234.
doi: 10.1021/jf104527e |
| [48] |
胡蓉, 乙引, 伍庆.
RP-HPLC法测定植物根系中有机酸含量[J]. 贵州农业科学, 2007, 35(2): 20-21.
|
HU Rong, YI Yin, WU Qing.
Determination of organic acids with low molecular weight in plant roots by RP-HPLC[J]. Guizhou Agric Sci, 2007, 35(2): 20-21.
|
| [49] |
HAO Zhengping, WANG Qing, CHRISTIEP P.
Allelopathic potential of watermelon tissues and root exudates[J]. Sci Hortic, 2007, 112(3): 315-320.
doi: 10.1016/j.scienta.2006.12.030 |
| [50] |
CANVIN D T, BEEVER H.
Sucrose synthesis from ac-etate in the germinating castor bean kinetics and pathway[J]. J Biol Chem, 1998, 236(4): 988-995.
|
| [51] |
YU Jingquan, MATSUI Y.
Phytotoxic substances in root exu-dates of cucumber(Cucumis sativus L.)[J]. J Chem Ecol, 1994, 20(1): 21-31.
doi: 10.1007/BF02065988 |
| [52] |
BLARI A C, HANSON B D, BRUNK G R.
New techniques and findings in the study of a candidate allelochemical implicated in invasion success[J]. Ecol Lett, 2005, 8(10): 1039-1047.
doi: 10.1111/j.1461-0248.2005.00805.x |
| [53] |
涂书新, 吴佳.
植物根系分泌物研究方法评述[J]. 生态环境学报, 2010, 19(9): 2493-2500.
|
TU Shuxin, WU Jia.
A review on research methods of root exudates[J]. Ecol Environ Sci, 2010, 19(9): 2493-2500.
|
| [54] |
唐新莲, 韦进进, 李耀燕.
在铝胁迫下黑麦根系分泌的柠檬酸和苹果酸的解毒机制的研究[J]. 广西农业科学, 2006, 25(4): 325-340.
|
TANG Xinlian, WEI Jinjin, LI Yaoyan.
Studies on Al-detoxification mechanism of both citrate and malate secreted from roots of rye under Al stress[J]. J Guangxi Agric Biol Sci, 2006, 25(4): 325-340.
|
| [55] |
PIÑEROS M A, MAGALHAES J V, CARVALHO ALVES V M.
The physiology and biophysics of an aluminum tolerance mechanism based on root citrate exudation in maize[J]. Plant Physiol, 2002, 129(3): 1194-1206.
doi: 10.1104/pp.002295 |
| [56] |
LI Xiaofeng, MA Jianfeng, MATSUMOTO H.
Pattern of Al-induced secretion of organic acids differs between rye and wheat[J]. Plant Physiol, 2000, 123(4): 1537-1543.
doi: 10.1104/pp.123.4.1537 |
| [57] |
张汝民, 张丹, 陈宏伟.
梭梭幼苗根系分泌物提取方法的研究[J]. 干旱区资源与环境, 2007, 21(3): 153-157.
|
ZHANG Rumin, ZHANG Dan, CHEN Hongwei.
Study on the extracts methods of the root exudates from the seedling of Haloxylon ammodendron (C. A. Mey.) Bunge[J]. J Arid Land Resour Environ, 2007, 21(3): 153-157.
|
| [58] |
INSKEEP W P, COMFORT S D.
Thermodynamic prediction for the effect of root exudates on mental speciation in the rhizosphere[J]. Plant, 1986, 9(3): 567-586.
|
| [59] |
ZENG Fanrong, CHEN Song, MIAO Ying.
Changes of organic acid exudation and rhizosphere pH in rice plants under chromium stress[J]. Environ Poll, 2008, 155(2): 284-289.
doi: 10.1016/j.envpol.2007.11.019 |
| [60] |
陈英旭, 林琦, 陆芳.
有机酸对铅、镉植株危害的解毒作用研究[J]. 环境科学学报, 2000, 20(4): 467-472.
|
CHEN Yingxu, LIN Qi, LU Fang.
Study on detoxication of organic acid to raddish under the stress of Pb and Cd[J]. Acta Sci Circumst, 2000, 20(4): 467-472.
|
| [61] |
王紫娟, 刘万学, 蔡静萍.
紫茎泽兰根系分泌物对旱稻的化感作用[J]. 现代农业科技, 2007, (16): 71-72.
doi: 10.3969/j.issn.1007-5739.2007.16.050 |
WANG Zijuan, LIU Wanxue, CAI Jingping.
Eupatorium upland rice root exudates on Allelopathy[J]. Mod Agric Sci Technol, 2007, (16): 71-72.
doi: 10.3969/j.issn.1007-5739.2007.16.050 |
| [62] |
KRISHNAMURTI G S R, CIESLINSKI G, HUANG P M.
Kinetic of cadmium release from soils as influenced by organic acids:implication in cadmium avail-ability[J]. J Environ Qual, 1997, 26(): 271-277.
|
| [63] |
HUANG, BLAYLOCK, KAPULNIKY.
Phytoremediation of uranium-contaminated soils:role of organic acids in triggering uranium hyperaccumulation in plants[J]. Environ Sci Technol, 1998, 32(13): 2004-2008.
doi: 10.1021/es971027u |
| [64] |
SANDAMANN E R I C, LOOS M A.
Enumeration of 2, 4-D-Degrading microorganisms in soils and crop plant rhizospherws using indication media:high populations associated with sugarcane (Saccharum officinarum)[J]. Chemosphere, 1984, 13(9): 1073-1084.
doi: 10.1016/0045-6535(84)90066-3 |
| [65] |
MACEK T, MACKOVÁM J, KÁŠ J.
Exploitation of plantsforthe removal of organics in environmental remediation[J]. Biotechnol Adv, 2000, 18(1): 23-34.
doi: 10.1016/S0734-9750(99)00034-8 |
| [66] |
KANEKO M, YOSHIMURA E, NISHIZAWA N K.
Time course study of aluminum-induced callose formation roots as observed digital microscopy and low-vacuum scanning electron microscopy[J]. Soil Sci Plant Nutr, 2002, 45(3): 701-712.
|
| [67] |
旷远文, 温达志, 钟传文.
根系分泌物及其在植物修复中的作用[J]. 植物生态学报, 2003, 27(5): 709-717.
|
KUANG Yuanwen, WEN Dazhi, ZHONG Chuanwen.
Root exudates and their roles in phytoremediation[J]. Acta Phytoecol Sin, 2003, 27(5): 709-717.
|
| [68] |
TOYAMA T, FURUKAWA T, MAEDA N.
Accelerated biodegradation of pyrene and benzo[a] pyrene in the Phragmites australis rhizosphere by bacteria-root exudate interactions[J]. Water Res, 2012, 45(4): 1629-1638.
|
| [69] |
GAO Yanzheng, REN Lili, LING Wanting.
Desorption of phenanthrene and pyrene in soils by root exudates[J]. Bioresour Technol, 2010, 101(4): 1159-1165.
doi: 10.1016/j.biortech.2009.09.062 |
| [70] |
YOSHITOMI K J, SHANN J R.
Corn (Zea mays L.) root exudates and their impact on 14C-pyrene mineralization[J]. Soil Biol Biochem, 2001, 33(12): 1769-1776.
|
| [71] |
YAN Dazhong, MAO Lingqi, LI Cunzhi.
Biodegradation of hexachlorobenzene by a constructed microbial consortium[J]. World J Microbiol Biotechnol, 2015, 31(2): 371-377.
doi: 10.1007/s11274-014-1789-7 |
| [72] |
章云云, 朱端卫, 王晓.
植物根系分泌物的作用及其与药用植物连作障碍的关系[J]. 湖北农业科学, 2014, 53(6): 1241-1245.
|
ZHANG Yunyun, ZHU Duanwei, WANG xiao.
Properties of plant root exudates and its obstacles for continuous cropping of medicinal plants[J]. Hubei Agric Sci, 2014, 53(6): 1241-1245.
|
| [73] |
李振侠, 徐继忠, 高仪.
苹果砧木SH40和八棱海棠缺铁胁迫下根系有机酸分泌的差异[J]. 园艺学报, 2007, 34(2): 279-282.
|
LI Zhenxia, XU Jizhong, GAO Yi.
Difference of organic acid exudation from roots of SH40 and balenghaitang under iron-deficiency stress[J]. Acta Hortic Sin, 2007, 34(2): 279-282.
|
| [74] |
苗欣宇, 周启星.
污染土壤植物修复效率影响因素研究进展[J]. 生态学杂志, 2015, 34(3): 870-877.
|
MIAO Xinyu, ZHOU Qixing.
Some research progresses in influencing factors for the efficiency of contaminated soil phytoremediation[J]. Chin J Ecol, 2015, 34(3): 870-877.
|
| [75] |
李德华, 向春雷, 姜益泉.
低磷胁迫下水稻不同品种根系有机酸分泌的差异[J]. 中国农学通报, 2005, 21(11): 186-189.
|
LI Dehua, XIANG Chunlei, JIANG Yiquan.
Difference of organic acid secretion form roots of various rice varieties under the stress of low phosphorus[J]. Chin Agric Sci Bull, 2005, 21(11): 186-189.
|
| [76] |
章爱群, 贺立源, 赵会娥.
根分泌物对活化土壤中难溶性磷的作用[J]. 水土保持学报, 2008, 22(5): 102-105.
|
ZHANG Aiqun, HE Liyuan, ZHAO Huie.
Effects of root exudates on mobilizing insoluble phosphate in soils[J]. J Soil Water Conserv, 2008, 22(5): 102-105.
|
| [77] |
李廷轩, 马国瑞, 张锡洲.
富钾基因型籽粒苋主要根系分泌物及其对土壤矿物态钾的活化作用[J]. 应用生态学报, 2006, 17(2): 368-372.
|
LI Tingxuan, MA Guorui, ZHANG Xizhou.
Root exudates of potassium-enrichment genotype grain amaranth and their activation on soil mineral potassium[J]. Chin J Appl Ecol, 2006, 17(2): 368-372.
|
| [78] |
李廷轩, 马国瑞, 张锡洲.
籽粒苋不同富钾基因型根系分泌物中有机酸和氨基酸的变化特点[J]. 植物营养与肥料学报, 2005, 11(5): 647-652.
doi: 10.11674/zwyf.2005.0513 |
LI Tingxuan, MA Guorui, ZHANG Xizhou.
Change characteristics of organic acid and amino acid in root exudates in different grain amaranth genotypes[J]. Plant Nutr Fert Sci, 2005, 11(5): 647-652.
doi: 10.11674/zwyf.2005.0513 |
| [79] |
左元梅, 张福锁.
不同禾本科作物与花生混作对花生根系质外体铁的累积和还原力的影响[J]. 应用生态学报, 2004, 15(2): 221-225.
|
ZUO Yuanmei, ZHANG Fusuo.
Effects of peanut mixed cropping with different gramineous plants on apoplast iron accumulation and reducing capacity of peanut[J]. Chin J Appl Ecol, 2004, 15(2): 221-225.
|
| [80] |
BAETZ U, MARTINOIA E.
Root exudates:the hidden part of plant defense[J]. Trends Plant Sci, 2014, 19(2): 90-98.
doi: 10.1016/j.tplants.2013.11.006 |
| [81] |
常学秀, 段昌群, 王焕校.
根分泌作用与植物对金属毒害的抗性[J]. 应用生态学报, 2000, 11(2): 315-320.
|
CHANG Xuexiu, DUAN Changqun, WANG Huanxiao.
Root excretion and plant resistance to metal toxicity[J]. Chin J Appl Ecol, 2000, 11(2): 315-320.
|
| [82] |
贺永华, 沈东升, 朱荫湄.
根系分泌物及其根际效应[J]. 科技通报, 2006, 22(6): 761-766.
|
HE Yonghua, SHEN Dongsheng, ZHU Yinmei.
Root exudates and their rhizospheric effects[J]. Bull Sci Technol, 2006, 22(6): 761-766.
|
| [83] |
桑伟莲, 孔繁翔.
植物修复研究进展[J]. 环境科学进展, 1999, 7(3): 40-44.
|
SANG Weilian, KONG Fanxiang.
Progress of study on phytoremediation[J]. Adv Environ Sci, 1999, 7(3): 40-44.
|
| [84] |
VYMAZAL, BŘEZINOVÁT.
The use of constructed wetlands for removal of pesticides from agricultural runoff and drainage:a review[J]. Environ Int, 2015, 75(): 11-20.
doi: 10.1016/j.envint.2014.10.026 |
| [85] |
BAETZ U, MARTINOIA E.
Root exudates:the hidden part of plant defense[J]. Trends Plant Sci, 2014, 19(2): 90-98.
doi: 10.1016/j.tplants.2013.11.006 |
| [86] |
周紫球, 邱永华, 周建革.
毛竹笋对土壤重金属吸收能力初探[J]. 浙江林业科技, 2013, 33(6): 61-63.
|
ZHOU Ziqiu, QIU Yonghua, ZHOU Jiange.
Preliminary report on absorbing capacity of soil heavy metal by Phyllostachy pubescens shoot[J]. J Zhejiang For Technol, 2013, 33(6): 61-63.
|
| [87] |
GRATANI L, CRESCENTE M F, VARONE L.
Growth pattern and photosynthetic activity of different bamboo species growing in the botanical garden of Rome[J]. Flora-Morphol Distr Funct Ecol Plant, 2008, 203(1): 77-84.
|







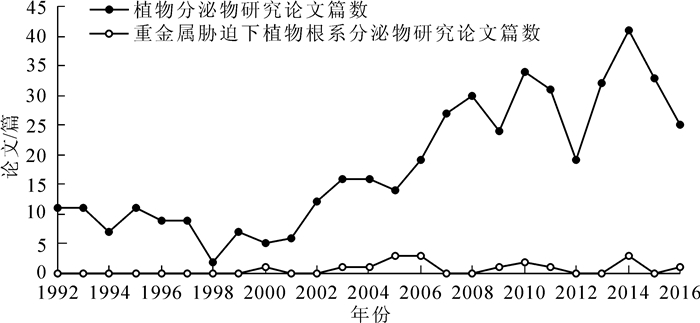





 DownLoad:
DownLoad: