-
黄瓜Cucumis sativus在中国各地普遍栽培, 是夏季主要菜蔬之一, 常用冷藏法保鲜。黄瓜储藏难度较大, 当在低于7~10 ℃下储藏或流通时极易发生冷害[1], 症状为表面出现水渍状溃烂、暗斑和腐烂斑, 从而影响其食用品质和商业价值。有报道称外源一氧化氮(NO)[2], 茉莉酸甲酯(MeJA)[3], 壳聚糖-g-水杨酸[4]和6-苄基腺嘌呤(6-BA)[5]均能减轻黄瓜冷害的发生, 但对环境或人体健康存在潜在威胁。采后热处理也是果蔬储藏中广泛使用的物理保鲜技术, 可有效减轻番茄Lycopersicon esculentum[6], 番木瓜Carica papaya[7]和哈密瓜Cucumis melo var.saccharinus[8]的储藏冷害。也有报道认为热处理会对果蔬品质造成一定的不良作用, 如果肉变红和絮败[9], 总酚含量降低等[10]。低剂量短波紫外线(UV-C)处理是一种无化学污染的物理处理方法, 广泛用于果蔬采后处理, 能够控制果蔬的采后腐烂, 延缓衰老和延长果蔬保鲜期[11-14]。目前, 未见将热处理和UV-C处理两者联合来抑制冷害的研究。本研究以黄瓜为试验材料, 筛选UV-C处理抑制黄瓜冷害的适宜剂量, 研究UV-C与热处理结合对低温下黄瓜冷害发生、抗氧化代谢和食用品质的影响以及UV-C联合热处理延缓黄瓜冷害发生的协同互补机制, 期望为减缓黄瓜储运冷害提供理论依据。
HTML
-
实验黄瓜品种‘戴多星’‘Deltasta’购于杭州市临安区蔬菜批发市场。选择大小均一(长10~12 cm, 直径2.0~2.5 cm), 无机械损伤, 无病虫害的黄瓜为试验材料。
-
将黄瓜置于紫外灯管下约30 cm处, 用紫外辐射照度计测得此距离的紫外辐照度为0.17 mW·cm-2。辐射能流按0, 2.5, 5.0, 7.5, 10.0 kJ·m-2分为5组, 15根·组-1。以不照射为对照。预处理完毕后用0.02 mm的聚乙烯袋包装, 置于4 ℃储藏, 隔2 d测得冷害指数(按方法1.2.1)。根据不同UV-C辐射能流筛选结果(图 1)和前人报道[15], 选择5.0 kJ·m-2辐射能流进行后续实验。
-
试验用黄瓜分为4组, 40根·组-1, 重复3次。①热处理(T1):将黄瓜置于37 ℃, 相对湿度90%的生物培养箱中处理12 h; ②UV-C处理(T2):黄瓜经5.0 kJ·m-2剂量的UV-C辐射处理; ③UV-C结合热处理(T3):黄瓜先进行5.0 kJ·m-2剂量的UV-C辐射处理, 然后置于37 ℃, 相对湿度90%的生物培养箱中处理12 h; ④对照处理(T0):不作上述处理。处理完后将黄瓜装入0.02 mm的聚乙烯袋(袋子上留有通气孔), 6~8根·袋-1, 挽口后置于4 ℃下储藏待测。
-
试验前随机取10根黄瓜测定各项指标作为初始值。冷藏第4天、第8天、第12天、第16天取完整黄瓜鲜样, 测定冷害指数、电导率、总抗氧化能力(TEAC)等指标。冷藏16 d后, 取5根黄瓜, 去皮, 将果肉切成约1 cm × 1 cm × 1 cm的小块, 立即用液氮速冻, 用粉粹机高速粉碎后在-80 ℃的超低温冰箱中储存并测定黄瓜其他储藏品质。
-
根据YANG等[2]的方法, 将冷害级别分为5级:0为果面无水渍状斑点或溃烂, 1为果面水渍状斑点或溃烂<25%, 2为果面水渍状斑点或溃烂在25%~50%, 3为果面水渍状斑点或溃烂在51%~75%, 4为果面水渍状斑点或溃烂>75%, 褐变斑块连成片。冷害指数(0~4)=∑(冷害级别×(该级别果数)/果实总数。冷害指数越大, 表明冷害程度越严重。
-
参考文献[15]的方法测定电导率, 用细胞被破坏前后2次测定比值的百分数表示相对电导率; 重复3次, 取平均值。参考文献[16]的方法测定丙二醛质量分数, 重复3次。
-
称取4.0 g黄瓜果肉样品, 置于经预冷的研钵中, 加入12 mL经4 ℃预冷的提取缓冲液, 冰浴研磨匀浆后, 于4 ℃, 12 000 r·min-1下离心30 min, 收集上清液即为酶提取液。参考文献[5]测定超氧化物歧化酶(SOD), 过氧化氢酶(CAT), 谷胱甘肽还原酶(GR)和抗坏血酸过氧化物酶(APX)活性。
-
参考文献[17]方法, 略有改动。总酚以1.0 kg黄瓜样品(鲜质量)中没食子酸当量(GAE)计。总抗氧化能力以清除2, 2-联氮-二(3-乙基-苯并噻唑-6-磺酸)二铵盐(ABTS)自由基能力计, 以溶于体积分数80%甲醇的水溶性维生素E(Trolox)溶液为标样, 以吸光值的减少值与Trolox质量浓度作标准曲线, 结果以1.0 kg黄瓜样品(鲜质量)中含有相当Trolox的质量表示。
-
采用TMS-PRO质构仪(美国FTC公司)测定黄瓜硬度, 单位为牛顿(N)。采用丙酮提取、比色法测定叶绿素质量分数。采用阿贝折光糖仪测定可溶性固形物(TSS)。用2, 6-二氯靛酚滴定法测定抗坏血酸质量分数。
-
实验数据统计分析采用SPSS 13.0统计软件, 结果用平均值±标准差表示, 采用ANOVA进行邓肯氏多重差异分析(P<0.05)。
1.1. 试验材料及处理
1.1.1. UV-C辐射剂量筛选
1.1.2. UV-C结合热处理方法
1.2. 指标测定与方法
1.2.1. 冷害指数(CI)
1.2.2. 相对电导率和丙二醛质量分数
1.2.3. 抗氧化酶活性
1.2.4. 总酚质量分数和总抗氧化能力(TEAC)
1.2.5. 其他储藏品质
1.3. 数据分析
-
如图 2所示:T0和T2处理组的黄瓜在储藏4 d表现出轻微冷害症状, 此后冷害程度继续加剧。4 d后T0组处理黄瓜的冷害指数显著高于T1, T2, T3处理的黄瓜(P<0.05)。8 d后T0处理黄瓜的冷害指数已经显著高于T1和T2处理的黄瓜(P<0.05)。16 d时T0和T2处理黄瓜的冷害指数分别达到3.13和2.85, 冷害程度严重, 而T3处理对冷害发生表现出更有效的抑制作用, 16 d时冷害指数分别为T0, T2和T1处理的48%, 53%和80%。表明UV-C和热处理能够协同抑制黄瓜冷害的发生。
-
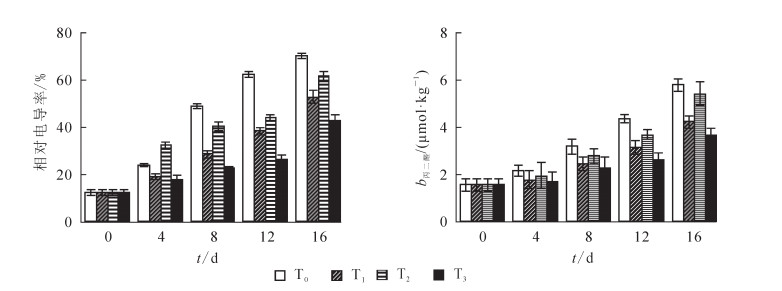
如图 3所示:黄瓜果实的相对电导率和丙二醛质量摩尔浓度随着冷藏进程不断上升, T2或T1处理均能抑制黄瓜相对电导率和丙二醛质量摩尔浓度的增加(图 3)。就单独比较而言, 热处理对相对电导率和丙二醛的抑制效应强于UV-C处理。冷藏16 d后T3处理黄瓜的相对电导率和丙二醛质量摩尔浓度均低于T0处理, 表明两者结合处理能更有效地抑制黄瓜相对电导率和丙二醛的增加(P<0.05)。
-
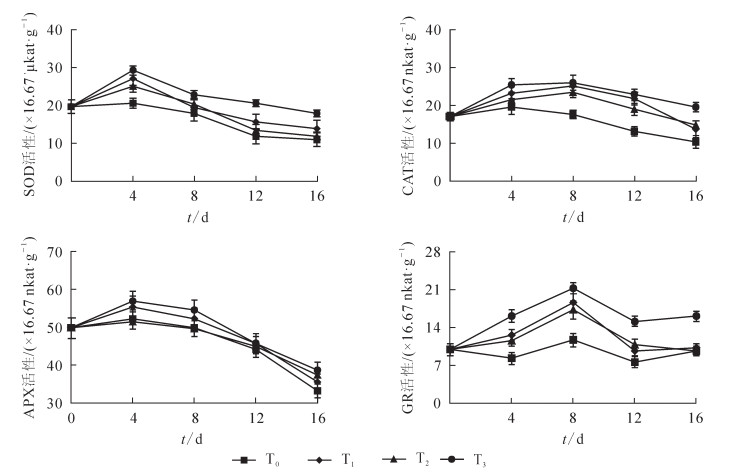
如图 4所示:对照组黄瓜SOD, CAT, APX和GR的活性在储藏期间基本呈现为先上升后下降, 说明冷藏开始抗氧化酶活性有一应激升高的过程, 这可能是储藏果蔬的一种保护性反应; 当低温胁迫持续时, 冷敏果蔬抗氧化保护系统的活性会下降或紊乱, 过量的活性氧将在果实组织内积累而发生脂质过氧化, 从而导致膜的完整性受到破坏, 组织内生理代谢紊乱, 最终引起冷害的发生。本研究发现:与对照相比, T1, T2和T3在储藏初期均能诱导黄瓜抗氧化酶活性短暂上升, 其中SOD和APX活性在储藏第4天达到最大值, CAT和GR在第8天达到最大值, 然后逐渐下降; 但T3处理下, 黄瓜SOD, CAT, APX和GR活性均显著高于T0, T1和T2处理(P<0.05)。
-
如图 5所示:在黄瓜储藏期间对照果实的总酚质量分数和总抗氧化能力先上升后下降, T1, T2和T3处理黄瓜的总酚和总抗氧化能力在第4天达到高峰, 然后逐渐下降。但T3处理黄瓜的总酚和总抗氧化能力始终高于其他3个处理。
-
果实硬度, 可溶性固形物(TSS), 叶绿素和抗坏血酸质量分数是表示果蔬储藏品质的主要指标。如表 1所示:经过16 d冷藏, 黄瓜果实的4个品质指标呈下降趋势。T2和T1处理均能抑制硬度、可溶性固形物以及叶绿素和抗坏血酸质量分数的降低, 经过T3处理的黄瓜叶绿素和抗坏血酸质量分数显著高于其他处理(P<0.05), 但硬度和可溶性固形物含量与T1和T2处理无显著差异(P>0.05)。
t/d 处理 硬度/N 可溶性固形物/D w叶绿素/(mg·kg-1) w抗坏血酸/(mg·kg-1) 0 22.84 ± 1.95 3.41 ± 0.12 41.12 ± 1.28 124.05 ± 3.61 16 T0 14.26 ± 1.11b 2.56 ± 0.15 b 28.38 ± 1.24 c 95.46 ± 1.61 c T1 19.10 ± 1.58 a 3.12 ± 0.17 a 32.04 ± 1.18 b 104.38 ± 2.42 b T2 15.74 ± 1.68 b 2.94 ± 0.11 a 31.58 ± 1.12 b 98.42 ± 1.76 b T3 19.79 ± 1.23 a 3.21 ± 0.13 a 35.68 ± 1.64 a 116.48 ± 2.58 a 说明:硬度为10次测定的平均值±标准差。可溶性固形物, 叶绿素和抗坏血酸均重复3次测定。处理间不同小写字母表示差异显著(P<0.05) Table 1. Effects of combined UV-C and heat treatment on quality parameters of cucumber fruit during storage at 4 ℃ for 16 days
2.1. UV-C结合热处理对黄瓜冷害发生程度的影响
2.2. UV-C结合热处理对黄瓜电导率和丙二醛的影响
2.3. UV-C结合热处理对黄瓜抗氧化酶活性的影响
2.4. UV-C结合热处理对黄瓜总酚和总抗氧化能力的影响
2.5. UV-C结合热处理对黄瓜储藏品质的影响
-
植物在代谢过程中会伴随产生活性氧(ROS), 如超氧阴离子(O2·-), 单线态氧, 过氧化氢(H2O2)和羟基自由基等。植物的抗氧化保护系统包括SOD, CAT, APX以及抗坏血酸、谷胱甘肽和酚类等抗氧化物; SOD能专一地歧化O2·-形成氧和过氧化氢, CAT能将过氧化氢转化为水和氧分子, 而APX通过AsA-GSH循环催化过氧化氢与抗坏血酸反应从而清除过氧化氢, 植物中SOD和APX活性取决于GR还原的谷胱甘肽水平[19]。在正常代谢过程中, 这些保护酶和抗氧化物相互协调作用能及时清除O2·-和过氧化氢, 维持细胞内活性氧在较低水平[20]。许多研究已经证明:抗氧化系统是植物克服低温胁迫的重要响应机制[21-23]。外源一氧化氮和水杨酸(SA)处理均能诱导SOD, CAT和APX等抗氧化酶活性提高黄瓜的抗冷性[2, 24], 与本研究所用的UV-C结合热处理得到的结果一致。
果蔬冷害的发生通常以组织电解质渗出率(电导率)和MDA含量作为标志的生理指标[25]。本实验中, 对照黄瓜组织的相对电导率和MDA质量摩尔浓度随着储藏进程不断增加, 单独的5.0 kJ·m-2 UV-C照射或37 ℃热处理对相对电导率和MDA的变化均表现出一定的抑制作用。与SHADMANIN等[7]和王静等[8]的结果一致。本研究发现:将5.0 kJ·m-2的UV-C照射与37 ℃热联合处理比单独处理更有效地抑制了相对电导率和丙二醛质量摩尔浓度增加, 从而更能有效地减轻4 ℃下黄瓜的冷害发生程度(P<0.05), 可以认为, UV-C和热处理在诱导黄瓜抵御冷害胁迫的过程中存在相互协同作用。究其原因, 一方面UV-C或热处理均能提高植物[12-13]的抗氧化酶活性, 另一方面, 酚类物质是果蔬中非酶促抗氧化系统的主要活性物质。本实验发现:热处理减少了黄瓜总酚质量分数, 而UV-C能提高黄瓜的总酚质量分数, 热处理抑制总酚合成与LEMOINE等[10]对西兰花Brassica oleracea var.italic的研究报道一致, 而UV-C处理也能提高一些果蔬[13, 27-28]的总酚质量分数。另外, UV-C照射与37 ℃热联合处理的黄瓜也保留了较高抗坏血酸质量分数, 因此, UV-C结合热处理黄瓜的总抗氧化能力也显著高于对照或单独处理。总之, UV-C与热联合处理能保持黄瓜中较高的酶促和非酶抗氧化系统活性, 有效降低活性氧积累造成的组织氧化胁迫, 从而减缓了冷害发生。
-
5.0 kJ·m-2UV-C照射结合37 ℃热处理能够诱导低温下黄瓜组织中SOD, CAT, APX和GR等抗氧化酶保持较高的活性以及较高的总酚和抗坏血酸质量分数, 维持黄瓜组织中较高的抗氧化能力, 延缓了低温胁迫下活性氧积累造成的膜质过氧化, 阻止了黄瓜相对电导率增加和丙二醛的积累, 显著降低了低温下黄瓜的冷害发生程度。同时, UV-C结合热处理也较好地保持了黄瓜的硬度、可溶性固形物和叶绿素质量分数。UV-C结合热处理作为一种无药剂污染的物理保鲜方法, 有助于减少黄瓜在低温储运过程中的冷害发生和保持品质。















 DownLoad:
DownLoad: