-
柿Diospyros kaki是柿科Ebenaceae柿属Diospyros落叶乔木,在中国分布范围较广,是一种重要木本粮食树种。柿果实富含黄酮、多酚和维生素C等活性成分,营养丰富,具有活血降压、止血凉血等保健功效及药用价值,可在产品加工、医疗美容等领域发挥重要作用[1]。柿开花特性复杂,大部分品种只开雌花,少数雌雄同株,只开雄花的品种十分罕见[2],雄性资源的严重缺乏限制了柿杂交育种工作的深入开展,这是造成中国柿产业发展缓慢的关键原因之一[3-4]。有研究指出,在花发育早期存在两性花器官原基,随着花芽的分化进程,在雌性或雄性花成熟之前,对应性器官发生了细胞程序性死亡,从而导致单性花的产生[5]。如玉米Zea mays[6],芦笋Asparagus officinalis[7]等单性花的产生都是由于两性花器官在花芽分化过程中,对应性器官的选择性败育导致的。目前,关于柿花芽分化的研究较少,SOBA-JIMA等[8]对柿‘平核无’‘Hiratanenashi’的雌花分化进程、YONEMORI等[9]对‘花御所’‘Hanagosho’等雌、雄花芽分化早期的形态学进行了报道。此外,本课题组对雌雄同株柿‘禅寺丸’ ‘Zenjimaru’的花芽发育进程进行了组织细胞学研究发现,在柿花发育早期同时具有雌、雄蕊原基,随着花芽分化的进行,到4月中旬时,柿雄花中的雌蕊原基有选择性的退化,雌花中的雄蕊原基也选择性的退化,最终形成了雌花或雄花等单性花[10]。本课题组对4月13日发育的柿‘禅寺丸’雌、雄花芽进行了RNA-Seq(转录组测序),从中筛选出了一些与柿花性别分化相关的关键基因,可为揭示柿花芽分化,尤其是对应性器官发生败育现象的分子机制提供一定的参考(此结果尚未发表)。目前,人们主要从激素测定、胚胎发育、显微观察等角度观察测定柿花芽分化的形成和发育过程[10-12],而对柿花芽不同分化时期的外部形态特点却没有比较详尽的描述。本研究以雌雄同株柿‘禅寺丸’为试材,对4月中旬(性器官发生败育)前后雌、雄花芽的显微结构与外部形态的相关性进行比较分析,以建立能够反映内部解剖结构的外部形态指标,同时利用转录组测序得到的与性别分化相关的关键基因进行实时定量聚合酶链式反应(qRT-PCR)测定,以进一步明确柿花芽分化的分子机制,从而为后期柿花性别的调控、柿雄性资源的培育以及杂交育种工作的深入开展提供理论依据。
HTML
-
试验所选材料为10年生的雌雄同株柿品种‘禅寺丸’。试验地点位于中国林业科学研究院经济林研究开发中心原阳试验基地(河南省原阳县)(34°55'30″~34°56'45″N, 113°46'24″~113°47'59″E)。选择树势基本一致、无病虫害并且结果正常的健康树体进行试验,园区进行统一常规管理。
-
根据李加茹等[10]关于‘禅寺丸’性别分化的石蜡切片观察可知,4月17日是柿花芽分化的第2个形态学关键时期,此时雌、雄花芽从外观上清晰可辨,并且雌、雄蕊原基已经发生败育。基于此,我们于2017年4月初(花芽处于萌动状态)至2017年4月中下旬(雌、雄蕊原基败育)进行试验材料的采集,隔3 d取样1次。具体采集日期为4月5日(阶段A),4月8日(阶段B),4月11日(阶段C),4月14日(阶段D)和4月17日(阶段E)。从每株树树冠外围中部东、南、西、北4个方向各随机抽取生长中庸的当年生枝条(分为开雄花的枝条和开雌花的枝条)各5个,采集每个枝条形态学上端的前3个饱满芽(分为雄花芽和雌花芽),用游标卡尺测量花芽和叶片的长度,用解剖针剥去芽外面的鳞片并拍照。每份样品采集后分成2部分,一部分投入甲醛冰醋酸乙醇(FAA)固定液中进行固定,另一部分于-80 ℃保存。
-
试验材料首先经过一系列梯度的乙醇(体积分数为70%乙醇、85%乙醇、95%乙醇、无水乙醇)进行脱水处理。材料彻底脱水后于二甲苯和无水乙醇配制的混合液以及纯二甲苯中进行透明。透明后的材料在1/2二甲苯+1/2石蜡中于38 ℃恒温箱中存放36 h后,15 min调高2~3 ℃进行变温处理,直至温度升为62 ℃,然后保持在此温度下对样品进行浸蜡处理。之后,对蜡块进行修整,并在切片机上制成纵切片,切片厚度为8~10 μm;将粘贴在载玻片上的蜡带置于烘箱中在38 ℃的条件下除去残余的黏贴剂,进行常规脱蜡(纯二甲苯和1/2二甲苯+1/2石蜡混合液),不同梯度乙醇复水,用苏木精伊红双重染色后,不同梯度乙醇脱水,1/2二甲苯+1/2石蜡配制的混合液和二甲苯透明,最后用中性树胶进行封片,烘干后在Olympus BX-51型光学显微镜下观察、拍照。
-
采用EZ-10 DNAaway RNA Mini-Preps试剂盒(Sangon)提取不同发育阶段各类型花芽的总核糖核酸(RNA),用Implen P330超微量分光光度计检测RNA的纯度和浓度,利用质量分数为1.0 %琼脂糖凝胶电泳法检测总RNA的完整性,样品检测合格后置于-80 ℃冰箱保存备用。采用TRUEscript 1st Strand cDNA Synthesis Kit(Kemix)将总RNA反转录成互补脱氧核糖核酸(cDNA)。之后用BIO-RAD CFX 96荧光定量聚合酶链式反应仪对各基因进行qRT-PCR测定。反应体系包括:1.0 μL primer mix(包括5 μmol·L-1上游forward引物和下游reverse引物),2.0 μL cDNA,10.0 μL SYBR Premix Ex TaqTM(2×)mix和7.0 μL水。反应条件为:94 ℃ 2 min(1个循环);94 ℃ 20 s,55~60 ℃ 20 s,72 ℃ 30 s(40个循环)。
从本课题组已有的转录组数据中选取6个与花芽性别分化相关的关键基因,分别为ANT(AINTEGUMENTA),LYK3(LysM containing receptor-like kinase 3),AP2(APETALA 2),ABCC10(ATP binding cassette subfamily C member 10),BI-1(Bax inhibiter 1)和RBOHB(Respiratory burst oxidase homolog B),通过对这些基因在花芽分化不同时期的表达模式进行分析,从而探索关键基因调控柿花芽分化的分子机制。用GAPDH作为内参以校正上样量,所用引物见表 1。
基因名称 引物序列(5′→3′) 退火温度/ ℃ 条带大小/bp GAPDH F: AGCTCTTCCACCTCTCCAGT 56.8 157 R: TGCTAGCTGCACAACCAACT 57.2 ANT F: TGCCATTATCCGCTCTAC 51.3 121 R: GACATTCAGCACCCAAGA 51.9 LYK3 F: CTTCCTCCCGAATCTGTC 51.6 134 R: TTCATTCTGGTGGGCTCT 52.7 AP2 F: GCAGTTCCTCCATTTTCA 49.9 153 R: CATCTATCTTGGGCTATTT 46.3 ABCC10 F: CCAGCCTAAACAGAGCCC 54.8 191 R: CAACCGTCCACCAGTCAA 54.6 BI-1 F: TCCTGTCTTCTGGGCTCT 54.0 188 R: GGCGTGCTTCACATAGTC 53.4 RBOHB F: ATGATTATTGGTGGTTTGT 46.9 233 R: CTTGGAAAGGTAGAGGAA 47.6 Table 1. Summary of primers used for floral sex differentiation-related genes in this study
-
相对基因表达量的计算采用2-△△Ct法,使用SPSS 20.0和Excel 2007等软件对试验数据进行处理,用Origin 6.0软件进行图表绘制等工作。
1.1. 试验材料
1.2. 取样方法
1.3. 石蜡切片观察
1.4. 性别分化相关基因qRT-PCR分析
1.5. 数据分析
-
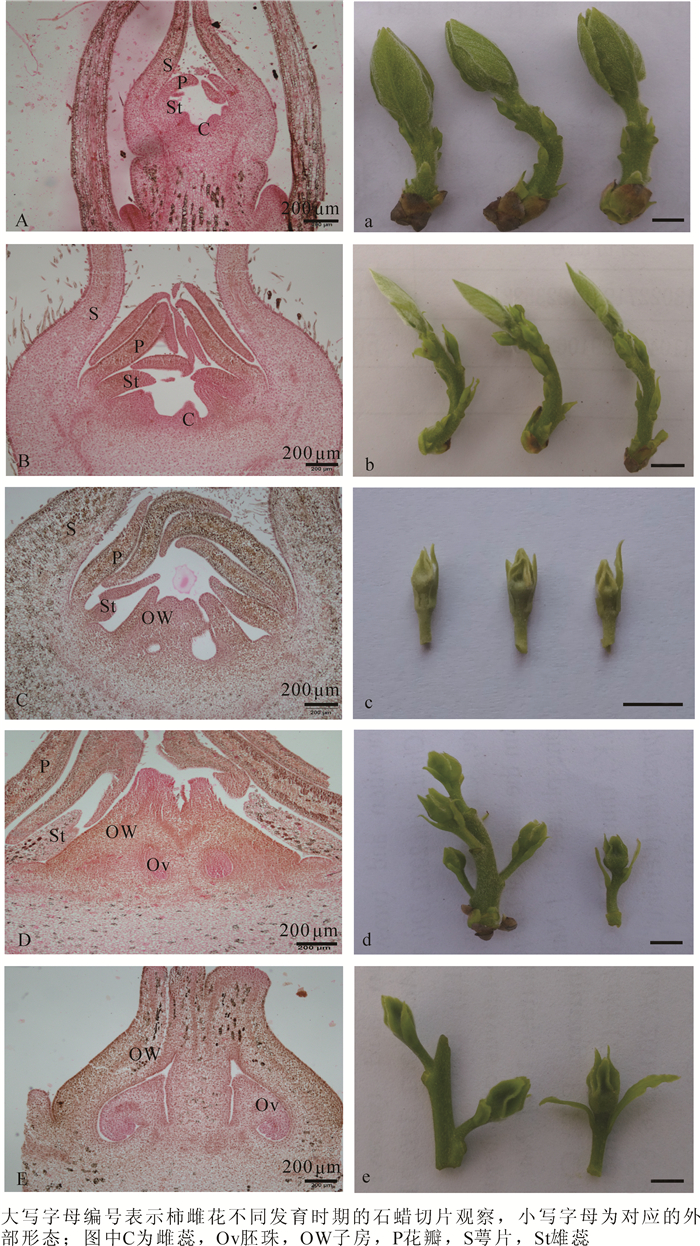
对柿雌、雄花芽不同分化时期外部形态变化和内部解剖特点进行比较,可以发现两者关系较为密切。随着温度的逐渐升高,树体在3月底开始萌动,顶芽露白并有所膨大,芽体外部包裹的鳞片由休眠期的锈黄色转变为嫩绿色,此时花芽开始进入分化前期。到阶段A,顶芽抽生出长为2.0~4.0 cm的嫩梢,肉眼能看到嫩梢上着生的不同类型的花芽,即能观察到雌花芽1朵单生花序,雄花芽3朵合生花序的形态变化,其中,雌花芽长度小于0.5 cm,雄花芽长度小于1.0 cm,幼叶数目不断增加,颜色由嫩绿色变为黄绿色(图 1a,图 2a);对切片进行显微观察发现,此时期雌、雄花芽表现一致,顶端均分化出了花瓣原基、雄蕊原基和心皮原基(图 1A,图 2A)。

Figure 1. Comparation between external morphological changes and internal anatomy structure of 'Zenjimaru' on female flower differentiation

Figure 2. Comparation between external morphological changes and internal anatomy structure of 'Zenjimaru' on male flower differentiation
在阶段B,由于降雨的原因致使温度有所降低,柿树发育进程相对较慢,此时前1年生的枝条上所有的绿芽逐渐开始抽生成新梢,芽体继续增大,其中,顶端嫩梢长至4.0~5.0 cm,雄花芽长约1.0 cm,雌花芽长为0.4~0.6 cm,叶片和花芽颜色仍为黄绿色(图 1b,图 2b);同时,各花器官原基进一步分化。观察发现,与雄花芽相比,雌花芽中的心皮原基发育速度较快,基部开始膨大(图 1B),而雄花芽中的雄蕊原基发育较雌花芽更加明显,雄蕊原基内部细胞分裂速度加快,顶端分生组织间或有幼嫩花药的生成(图 2B)。
由于春天温度快速升高,柿花各时期的分化进程也较快,在阶段C,嫩梢生长迅速,其中,顶端着生雌花的枝条长为4.0~5.0 cm,着生雄花的枝条生长相对较快,长为5.0~6.0 cm,叶片颜色逐渐加深。此时,雌花芽长为0.5~1.0 cm,雄花芽长为1.0~1.5 cm(图 1c,图 2c);随着各原基细胞的分裂分化,雌花芽内的心皮细胞上部逐渐融合形成子房,分化出花柱和柱头组织,雄蕊原基分化则相对缓慢,变化并不明显(图 1C),而雄花芽内的心皮细胞也开始融合,并有类似花柱和柱头组织的结构形成,雄蕊原基细胞分裂旺盛,基部缢缩形成花丝,花药数量逐渐增加,此时期雌、雄花芽内花柱基部两侧各长出小凸起,即为蜜腺组织(图 2C)。
阶段D顶端着生雌花的枝条长为5.0~6.0 cm,着生雄花的枝条长为6.0~8.0 cm,叶片颜色进一步加深。此时,雌花芽长约1.0 cm,雄花芽长为1.0~2.0 cm(图 1d,图 2d);石蜡切片观察可知,雌花芽子房内部已有胚珠的形成,并产生大孢子母细胞,雄蕊原基的生长停滞不前,发生败育(图 1D),而雄花芽内的心皮原基生长相对滞缓,雄蕊原基变化明显,整体不断伸长和膨大,其内部的花药数量显著增多,并有小孢子母细胞的产生(图 2D)。
在阶段E,枝条与花芽迅速伸长生长,枝条长10.0 cm左右,雌花芽长为1.0~1.5 cm,雄花芽长约2.0 cm,叶片颜色逐渐变为深绿色,枝条接近木质化(图 1e,图 2e);对不同花芽的内部解剖构造研究发现,雌花芽内子房形状已初步形成,大孢子母细胞之后将经过减数分裂形成功能大孢子(图 1E),而雄花芽内的心皮原基彻底发生败育,体积减小,雄蕊内的小孢子母细胞则开始经过减数分裂,逐渐形成花粉粒(图 2E)。
-
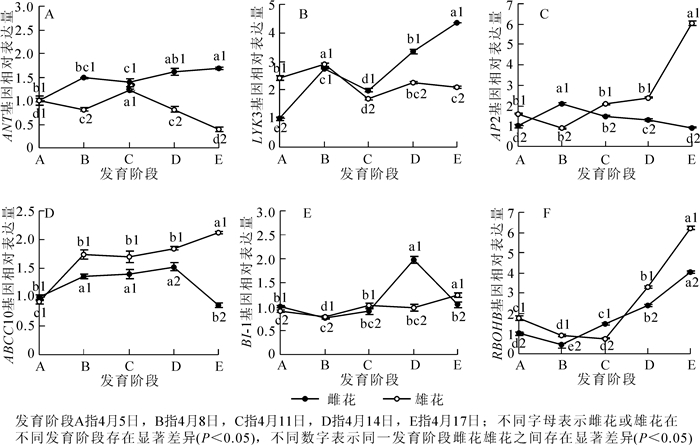
柿花花芽分化过程中与性别分化相关基因的表达模式分析见图 3。由图 3可以看出:除在阶段C处略有下降外,基因ANT的表达量在雌花中整体呈现上升的趋势,并且在阶段E处出现最高值。该阶段的基因表达量是阶段A的1.7倍;基因ANT在雄花中的表达模式与雌花完全相反,即除在阶段C处有显著提高(为阶段B的1.5倍)外,该基因在雄花中整体呈现下降的趋势,并且在阶段E处出现最低值,该阶段的基因表达量是阶段A的39.6%。对比ANT基因在雌、雄花中的表达模式发现,该基因的表达水平除在最初的阶段A无显著差异外,其他发育阶段均差异显著且在雌花芽中的表达明显高于雄花芽(P<0.05)(图 3A)。

Figure 3. qRT-PCR analyses for expression levels of sex differentiation-related genes in floral buds of 'Zenjimaru'during different developmental stages
柿雌花发育过程中,LYK3和ANT的表达模式基本一致,均从阶段B明显下降至阶段C后,急剧上升到阶段E。与ANT相似,虽然LYK3的表达量在阶段C略有下降,但该阶段的基因含量仍然较阶段A有所上升。整体来看,从阶段A到阶段E,该基因表达量增加了4.4倍。LYK3基因在整个雄花芽的发育过程中出现了2个峰值,即阶段B和阶段D。虽然在阶段D处有个峰值,但其值却明显低于阶段B,甚至是阶段A。由此可知,基因LYK3在阶段B处表达水平最高,为阶段A的1.2倍。此外,雄花芽中LYK3基因的表达水平在前2个发育阶段略高于雌花芽,其余各发育阶段在雌、雄花芽中的差异变化明显(P<0.05),并且在雌花芽中的表达量分别是雄花芽的1.2,1.5和2.1倍(图 3B)。
基因AP2在雌花不同发育过程中,从阶段A上升到阶段B(表达量是阶段A的2.1倍)后,其表达量呈直线下降趋势,并且该基因在阶段E(雌、雄花外部形态特征已完全呈现)的相对表达量略低于阶段A,是阶段A的92.6%;基因AP2在雌、雄花芽中的表达模式完全相反,该基因在雄花芽中的表达量从阶段A缓慢下降到阶段B,又开始大幅度上升至阶段E,最终该基因的表达量是阶段A的3.8倍。对比AP2基因在雌、雄花中的表达模式发现,除了阶段B雌花中的表达量显著高于雄花(P<0.05)(是雄花中的2.3倍)外,各发育阶段雄花芽中的表达水平总是高于雌花芽,且在阶段E处差异最大,该阶段内雄花芽的表达量是雌花芽的6.5倍(图 3C)。
基因ABCC10在雌花发育前期表达直线上升,到阶段D处达到最高点(是阶段A的1.5倍)后,又显著下降到阶段E。与阶段A相比,ABCC10的表达量在阶段E处降低了13.6%;ABCC10在雄花发育过程中的表达模式整体呈上升趋势,到阶段E时,该基因是阶段A的2.3倍。此外,雌、雄花芽中的ABCC10基因在阶段A处的差异不显著,而其余阶段在雄花芽中的表达量总是显著高于雌花芽(P<0.05),分别是雌花芽的1.3,1.2,1.2和2.5倍(图 3D)。
柿雌花发育过程中,基因BI-1的表达量从阶段A缓慢下降到阶段B后,又开始小幅上升至阶段D,最后下降到与阶段A表达量基本持平的阶段E(为阶段A的1.0倍)。总体来看,整个雌花性别分化的过程中,该基因在阶段D的表达量最高,与阶段A相比,提高了98.1%;在雄花中,BI-1基因的表达水平总体呈上升趋势,与阶段A相比,其余各阶段的基因相对表达量基本处在0.9~1.4的水平,其中以阶段B的表达量最低,阶段E的表达量最高。此外,从图 3中我们可以看出:在阶段A和阶段D处,BI-1基因在雌花芽中的表达量分别是雄花芽的1.5倍和2.0倍,而在阶段B,阶段C和阶段E处,BI-1基因在雌花芽中的表达量分别是雄花芽的97.5%,89.2%和83.9%(图 3E)。
RBOHB在雌、雄花芽整个发育过程中的表达模式基本相同,均为先下降后上升的状态。该基因分别在雌、雄花芽中的阶段B和阶段C处表达量最低,分别为阶段A的43.8%和52.5%。之后,随着花器官的建成,RBOHB的表达呈现出整体的上升趋势,到阶段E处其值达到最大,分别为阶段A的4.1倍和6.2倍。整体来看,除在阶段C处雌花芽的表达量是雄花芽的2.0倍外,其余各阶段均为雄花芽高于雌花芽(图 3F)。
2.1. 柿雌、雄花芽外部形态变化与内部解剖构造之间的对应关系
2.2. 性别分化相关基因在柿花发育过程中的表达模式分析
-
目前,人们主要通过制作石蜡切片后,利用光学显微镜观察植物花芽分化过程中各个发育阶段的特征从而确定形态分化的关键时期[13]。然而,植物的花芽分化受到试验地的立地条件、温度等气候因素、品种自身遗传性能、果树生长中普遍存在的大小年现象以及树体生长发育过程中营养物质的积累等影响[14-16],致使不同研究中花芽分化关键时期的确定有所差异。此外,果农在果园进行栽培管理的过程中,如果只是通过显微观察花芽分化的内部解剖特点,则往往缺少相应的技术及设备条件,从而不能准确地确定花芽分化进程的具体时期,进一步阻碍了柿产业的发展。本研究针对以上问题,通过制作石蜡切片观察柿花芽分化进程中内部解剖特点的同时,测量观察不同分化时期柿花芽外部的形态变化,以建立柿花芽的外部形态特征与内部解剖特点之间的对应关系,根据外部形态指标来判断柿花花芽的分化程度,有利于生产实践中在花器官败育之前,采取人为手段调控柿花性别,具有一定的科学性和实用性。
已有研究表明:植物单性花的形成与对应性器官的败育有关,例如,在石榴Punica granatum[17],玉米[18],文冠果Xanthoceras sorbifolium[19]等植物中均发现了雌蕊败育现象,在白花蝇子草Silene pratensis[20],中华猕猴桃Actinidia chinensis[21]中发现了雄蕊败育现象。此外,山葡萄Vitis amurensis雄花中的雌蕊败育与珠心组织细胞程序性死亡有关[22],白花山碧桃Prunus davidiana ‘Albo-plena’雄花中的雌蕊败育发生在子房室形成后,与胚珠退化有关[23],黄瓜Cucumis sativus在发育成雌花和雄花的过程中,对应性器官原基的败育发生在大孢子母细胞时期和小孢子母细胞时期[24]。在柿花芽分化早期,‘禅寺丸’雌、雄花芽内对应的雄蕊原基和雌蕊原基发育正常,在阶段D,即大、小孢子发生期,雌花芽产生大孢子母细胞,对应的雄蕊原基生长停滞不前,发生败育,而雄花芽内有小孢子母细胞的产生,对应的雌蕊原基生长相对缓慢。到阶段E时,雌蕊原基和雄蕊原基彻底败育,发生退化。此外,对柿雌、雄花芽的外部形态观察发现,在大、小孢子发生期,顶端着生雌花的枝条长为5.0~6.0 cm,着生雄花的枝条长为6.0~8.0 cm,雌花芽长约1.0 cm,雄花芽长为1.0~2.0 cm。因此,在生产实践中,果农可通过观察花芽的外部生长发育情况,在大、小孢子发生期,即雌、雄花原基发生败育之前,采取相应措施人为调控柿树的花芽分化,有目的地诱导雄花的产生,从而培育优良雄性种质资源。
ANT是一种类AP2乙烯响应转录因子,它在生殖器官发育过程中占据着重要地位。该基因的强突变体能够使雌配子体缺失,而弱突变体虽然能产生雌配子体,但该配子体无活力[25]。WILSON等[26]发现,ANT基因在拟南芥Arabidopsis thaliana心皮发育过程中的相关植物激素运输方面起到重要作用。LYK3是一种LysM类受体蛋白激酶,在植物的生长发育和抗病抗逆等方面具有非常重要的作用[27]。一般在逆境条件下,植物体内的脱落酸(ABA)含量增加,而拟南芥中AtLYK3基因的表达受到ABA的正调控[28],这表明LYK3可能是一种胁迫诱导基因。对雌、雄花芽中不同阶段性别相关基因的表达模式进行分析发现,ANT基因与LYK3基因具有相同的功能,即均与雌性花器官的产生关系密切。
AP2基因属于植物花器官发育的分子机制ABCDE模型中的A类功能基因,其在植物萼片和花瓣形成过程中起着重要的调控作用[29-30]。对拟南芥突变体的研究表明,弱AP2突变体可使花瓣转化成雄蕊的结构[31-32]。ABCC蛋白家族是ABC转运蛋白超级家族中的一个亚家族,其在植物生长发育中发挥重要作用,该基因家族成员的主要功能是参与重金属的转运过程以及色素在液泡中的积累过程等[33-34]。本研究结果表明,AP2基因和ABCC10基因在雄花芽中一直保持较高的表达水平,表明该基因可能促进雄性花器官的发育。
作为一种细胞凋亡抑制因子,BI-1基因广泛存在于动物、植物以及微生物中,其在细胞程序性死亡信号转导下游发挥作用[35]。西红柿Lycopersicon esculentum感染黄瓜花叶病病毒(CMV)以及大麦Hordeum vulgare感染白粉霉菌所致的细胞程序性死亡与感染部位的BI-1基因的高表达密切相关,表明该基因很可能对植物细胞死亡起抑制作用[36-37]。BI-1基因在雄花芽发育的整个阶段呈直线上升状态,表明该基因的积累有利于雄花芽的产生。此外,雌花芽中的BI-1基因仅仅在性器官败育关键时期(阶段D)的表达量最高,而其余阶段间该基因的表达基本一致。可以看出:该基因与雄性器官的细胞程序性死亡密切相关。结合BI-1基因在雌、雄花器官中的表达模式及其解剖结果,推测该基因作为一种雄性器官细胞凋亡抑制基因,间接促进柿雄花芽的发育。然而,关于BI-1基因抑制凋亡的作用机制还不是很清楚,因此,在以后的研究中可利用转基因等分子生物学手段,着重于该基因在植物细胞程序性死亡,尤其是花芽分化过程中的作用进行深一步的探讨。
RBOHB是一种呼吸爆发氧化酶(NADPH氧化酶)基因,这种酶又是活性氧(ROS)的主要来源之一,而ROS可引起细胞凋亡[38]。有报道指出,水稻中的RBOHB基因主要在绒毡层和小孢子中表达,通过产生ROS使生物体内出现细胞程序性死亡现象,进而影响花药的发育[39]。整个花芽发育过程中,除在阶段B和阶段C处略有下降外,花发育后期RBOHB表达量快速上升。此外,根据本研究解剖学观察结果,雌花芽在阶段E处子房形状已初步形成,而雄花芽内的心皮原基彻底发生败育,推测该基因诱导的细胞程序性死亡主要发生在花发育后期雌花芽雄蕊的败育和雄花芽雌蕊的败育,具体仍需进一步的试验验证。
-
本研究将柿雌、雄花芽分化过程中花芽的外部形态特征和内部解剖构造进行比较分析,外部形态(花芽、叶片和枝条等的变化)可以间接反映其内部结构的变化,通过建立能够反映内部显微变化的外部形态指标来判断柿雌、雄花芽的分化时期,具有一定的科学性和实用性。柿花器官在大、小孢子发生期已经发生败育,此时顶端着生雌花的枝条长为5.0~6.0 cm,着生雄花的枝条长为6.0~8.0 cm,雌花芽长约1.0 cm,雄花芽长为1.0~2.0 cm,可根据柿花外部形态等指标在花器官败育之前人为调控柿花的性别。性别分化相关基因的表达模式分析中,高表达量的ANT和LYK3基因对雌花的分化具有一定的促进作用,高表达量的AP2和ABCC10基因有利于雄花的分化。此外,我们推测BI-1基因作为一种雄性器官细胞凋亡抑制基因,间接促进柿雄花芽的发育。因此,建议对树体进行正常的修剪、施肥等常规处理的同时,在4月中旬花器官原基发生败育之前对树体进行相应激素的喷施,以调控柿花性别、培育雄性种质。









 DownLoad:
DownLoad: