-
随着全球二氧化碳浓度上升,温室效应的出现,生态环境不断恶化,干旱成为限制植物生长的重要因子[1]。干旱胁迫通过影响叶片光合作用、叶绿素合成和营养代谢等各种生理和生化功能来抑制植物的生长,包括离子的吸收和转运,呼吸和碳水化合物代谢[2-3]。干旱胁迫导致植物叶片含水量和水势降低,同时破坏光合膜的结构,阻止各种色素的合成,导致光合作用下降[4]。MANIVANNAN等[5]发现:干旱胁迫导致不同向日葵Helianthus annuus品种的叶绿素a(Chl a),叶绿素b(Chl b)和总叶绿素(Chl t)大幅下降,光合作用降低。活性氧(ROS)是植物代谢过程中的毒副产品,干旱胁迫会导致细胞内ROS大量生成,ROS产生和消除之间的平衡受到干扰。ROS的过量累积会导致细胞水平的氧化损伤,破坏细胞膜,并导致细胞中的酶失活,蛋白质降解和离子失衡[6]。另一方面,植物可以通过复杂的非酶促和酶促抗氧化系统消除细胞内产生的过量ROS,在一定程度上减缓由ROS诱导的细胞稳态的损伤和破坏[7]。例如通过调节超氧化物歧化酶(SOD),过氧化物酶(POD)和过氧化氢酶(CAT),维持体内氧化还原平衡。目前,已有众多报道,如四季桂Osmanthus fragrans,栓皮栎Quercus variabilis和狼尾草Pennisetum alopecuroides等保护酶活性和植物体抗旱能力有一定关系[8-10]。同时,干旱胁迫条件下植株通过迅速积累小分子渗透调节物质,如可溶性糖、游离脯氨酸和甜菜碱等,降低细胞水势,提高细胞的吸水和保水能力,从而维持细胞膨压,保证植物生理代谢功能的正常进行[11]。陈洪国[12]发现:桂花O. fragrans幼苗的可溶性糖和脯氨酸含量随土壤含水量的降低而增加,游离脯氨酸通过增加生物聚合体的亲水性而提高植物的抗逆境能力,但并未对甜菜碱进行研究。前人研究结果表明:植物自身存在着应对土壤水分变化的生理响应机制,对干旱逆境具有一定的耐性[13]。复水后胁迫解除,植物发生结构及生理方面的适应性改变,迅速弥补由干旱胁迫造成的损伤。研究发现:适宜的干旱不仅不会对作物造成负面的影响,在复水后还会对植物物产生积极的效应,这就是补偿效应[14-15]。对不同的高粱Sorghum bicolor品种研究发现:各品种高粱的相对含水量、水势和光合参数等随干旱胁迫加重均有所下降,复水后不同品种的补偿效果不同[16]。JIN等[17]研究发现,干旱胁迫下马齿苋Portulaca oleracea的脯氨酸、可溶性糖和保护酶均上升,而复水后上述指标下降,植物恢复正常生长。桂花O. fragrans为木犀科Oleaceae木犀属Osmanthus植物,在园林和庭院的绿化、美化、香化及装饰盆景上有特殊地位[18]。以往对桂花的研究主要侧重在遗传多样性[19]、挥发物成分[20]以及基因表达[21]等方面,关于桂花对干旱环境耐受能力的研究则相对较少。本研究以2年生‘波叶金桂’盆栽苗为材料,研究了不同干旱天数及复水对‘波叶金桂’O. fragrans ‘Boyejingui’叶片中水分含量、渗透调节物质、光合色素以及ROS的伤害和抗氧化酶活性的变化的影响,揭示‘波叶金桂’在抗旱防御反应系统中渗透调节物质和抗氧化酶的生理响应调控机制,以及‘波叶金桂’抗干旱的适应能力以及复水后的恢复能力。以期为‘波叶金桂’的广泛栽培,提高其适应性提供依据。
HTML
-
试验材料为2年生‘波叶金桂’实生苗,由浙江杭州画境种业有限公司提供,苗高约50 cm,幼苗于2017年4月中旬栽植于盛有培养土[m(泥炭):m(沙土):m(蛭石)=1:2:1,干质量1.5 kg]花盆中(φ=25 cm,h=30 cm),1株·盆-1。于浙江农林大学大棚内缓苗培育1个月,进行正常栽培管理。实验期间大棚环境的昼/夜温度为28 ℃/20 ℃, 空气相对湿度为75%,光照条件为自然光。
-
2017年5月20日,选取生长良好、无病虫害且大小相近的‘波叶金桂’85盆,随机分成45盆对照处理,40盆干旱处理。对照组的45盆幼苗随机分为9组,正常浇水,采用称重法控制土壤含水量,每天傍晚及时补充水分,土壤含水量保持在20%左右。干旱组随机分为8组,实行自然干旱处理。5月20日测定1组对照,随后间隔4 d测定对照、干旱处理各1组,于4 d(5月24日),8 d(5月28日),12 d(6月1日),16 d(6月5日),20 d(6月9日)测定各项指标。测定完后对干旱组剩余3组进行复水处理,复水期间土壤含水量保持在20%左右。间隔2 d测定对照、干旱处理各1组,于22 d(6月11日),24 d(6月13日),26 d(6月15日)测定各项指标。
-
土壤含水量和叶片相对含水量测定采用称重法[22];叶水势测定采用美国WES-COR公司产的PSYPRO露点水势仪进行。
-
取0.5 g叶片,剪碎后置于带盖的试管中,加体积分数为80%的丙酮溶液5 mL,室温下遮光萃取48 h,至叶片完全变白,将浸提液稀释10倍,分别测定663,645和470 nm的光密度值(D)。然后根据LICHTENTHALER[23]的公式计算光合色素质量分数。
-
采用蒽酮比色法[24]测定可溶性糖质量分数;游离脯氨酸质量分数测定采用磺基水杨酸提取茚三酮显色法[24];甜菜碱质量分数采用饱和雷氏盐比色法[25]测定。
-
伤害率采用电导仪法测定。取‘波叶金桂’枝条中上部成熟叶片,放入去离子水中洗净, 用打孔器打孔20个小圆片放入盛有20 mL去离子水的小烧杯中,室温下放置30 min(每5 min搅拌1次)后测定电导率。然后,加热煮沸10 min后再次测定电导率。伤害率计算公式:
O2·-质量摩尔浓度测定参照李忠光等[26]的方法;H2O2质量摩尔浓度测定参照CHANDRA等[27]的方法;MDA质量摩尔浓度测定参照HODGES等[28]的方法。
-
酶液提取方法:将0.5 g冷冻的成熟叶片,液氮磨碎,加入9 mL磷酸缓冲溶液(0.1 mol·L-1,pH 7.4)研磨成匀浆,10 000 × g离心15 min(4 ℃)。取上清液用于SOD,POD和CAT活性的测定。SOD活性测定参照南京建成生物科技有限公司生产的A001-1型SOD试剂盒进行测定和计算。POD和CAT活性测定参照KUMARI等[29]的方法。
-
所有数据均为5次重复的平均值±标准差,利用Origin 9.0软件进行数据处理、图表制作和统计分析,统计方法采用one-way ANOVA,进行Turkey多重比较(P<0.05)。
1.1. 试验材料
1.2. 试验处理
1.3. 试验方法
1.3.1. 土壤含水量、叶片相对含水量和叶水势测定
1.3.2. 光合色素的测定
1.3.3. 渗透调节物质质量分数的测定
1.3.4. ROS和膜质过氧化的测定
1.3.5. 抗氧化酶活性的测定
1.4. 数据处理
-
‘波叶金桂’的土壤含水量、叶片相对含水量和叶水势随着干旱时间的延长和胁迫程度的加剧呈下降趋势(图 1)。干旱4 d后,土壤含水量急剧下降,比对照降低了27.1%(P<0.05);干旱处理12 d时,叶片相对含水量和叶水势才开始大幅度下降,分别比对照降低了17.2%和52.6%(P<0.05);干旱20 d后,土壤含水量、叶片相对含水量和叶水势分别比对照降低了56.9%,28.0%和76.9%(P<0.05)。复水后土壤含水量、叶片相对含水量能快速恢复到对照水平,叶水势在复水后有个恢复的过程,直到复水6 d后才能完全恢复。此结果也说明了‘波叶金桂’有较强的保水能力和抗旱能力。
-
随着干旱时间的延长和强度的增加,可溶性糖、游离脯氨酸和甜菜碱质量分数呈上升趋势(图 2)。干旱12 d时,甜菜碱达到最高,比对照增加了70.4%(P<0.05);干旱16 d时,可溶性糖、游离脯氨酸和甜菜碱分别比对照增加了48.7%,2.72倍和53.5%(P<0.05)。此时可溶性糖质量分数达最大;在干旱20 d时,三者分别比对照增加了38.5%,3.44倍和57.1%(P<0.05),此时游离脯氨酸质量分数达到最大值。复水4 d后,甜菜碱下降到正常水平,可溶性糖和游离脯氨酸质量分数迅速下降,直到复水6 d后,可溶性糖和游离脯氨酸才恢复到对照水平。干旱条件造成渗透调节物质的积累,说明了渗透调节在植物抵御逆境胁迫中起着重要的作用。
-
干旱胁迫对‘波叶金桂’叶片的光合色素产生了一定的影响。Chl a,Chl b和Chl t随干旱时间的延长整体呈下降趋势(表 1)。干旱8 d时,Chl a,Chl b和Chl t缓慢上升但与对照差异不大;干旱12 d时,Chl a,Chl b和Chl t开始下降;当干旱20 d时,Chl a,Chl b和Chl t质量分数下降到最低,分别比对照下降了19.0%,16.2%和18.4%(P<0.05)。复水处理下,Chl a,Chl b和Chl t质量分数持续增加,复水6 d后比对照增加了6.3%,24.3%(P<0.05)和10.4%。
处理 t/d w/(mg·g-1) Chl a/b Chl a Chl b Chl t Car 干旱 0 1.26 ± 0.10 abc 0.37 ± 0.03 b 1.63 ± 0.08 bc 0.21 ± 0.02 e 3.41 ± 0.31 a 4 1.28 ± 0.04 ab 0.41 ± 0.02 ab 1.69 ± 0.05 abc 0.24 ± 0.02 de 3.12 ± 0.28 a 8 1.36 ± 0.12 a 0.46 ± 0.03 a 1.82 ± 0.14 a 0.29 ± 0.03 cd 2.96 ± 0.14 a 12 1.21 ± 0.08 bc 0.42 ± 0.02 ab 1.63 ± 0.08 bc 0.34 ± 0.02 bc 2.88 ± 0.18 b 16 1.17 ± 0.11 c 0.37 ± 0.04 b 1.54 ± 0.13 c 0.39 ± 0.04 ab 3.16 ± 0.21 ba 20 1.02 ± 0.06 d 0.31 ± 0.03 c 1.33 ± 0.09 d 0.43 ± 0.03 a 3.29 ± 0.22 a 复水 2 1.22 ± 0.03 bc 0.41 ± 0.03 ab 1.63 ± 0.06 bc 0.32 ± 0.03 c 2.98 ± 0.26 a 4 1.31 ± 0.12 ab 0.43 ± 0.01 a 1.74 ± 0.08 ab 0.28 ± 0.02 d 3.05 ± 0.21 a 6 1.34 ± 0.04 a 0.46 ± 0.03 a 1.80 ± 0.05 a 0.24 ± 0.02d e 2.91 ± 0.14 b 说明:不同小写字母表示不同处理间差异显著(P<0.05) Table 1. Effects of drought stress and rewatering on photosynthetic pigment content of Osmanthus fragrans 'Boyejingui'
类胡萝卜素(Car)质量分数随干旱时间延长呈显著上升趋势(表 1)。干旱8 d时显著高于对照,比对照增加了38.1%(P<0.05)。干旱20 d时,Car比对照增加了1.05倍(P<0.05)。复水后Car质量分数降低,干旱6 d后基本恢复到对照水平。
随着干旱时间的延长,Chl a/b呈先降低后升高的趋势(表 1)。当干旱12 d时,Chl a/b比对照降低了15.5%(P<0.05),干旱16 d后开始缓慢上升,干旱20 d后基本恢复对照水平。复水后Chl a/b趋向稳定,在3.00左右。
-
随着干旱时间的延长,O2·-和H2O2呈逐渐升高的趋势(表 2)。干旱0~8 d时,O2·-和H2O2各处理间与对照差异不显著;当干旱12 d时,O2·-和H2O2质量摩尔浓度显著高于对照,比对照增加了66.2%和28.1%(P<0.05);干旱处理16 d时,H2O2质量摩尔浓度达最大,比对照增加了37.6%(P<0.05);而干旱处理20 d时,O2·-质量摩尔浓度才达到最高,比对照增加了82.4%(P<0.05)。
处理 t/d 伤害率/% b超氧阴离子/(μmol·g-1) b过氧化氢/(μmol·g-1) b丙二醛/(nmol·g-1) 干旱 0 10.92 ± 0.99 g 0.74 ± 0.08 d 30.63 ± 2.48 e 1.73 ± 0.14 e 4 15.94 ± 1.55 f 0.84 ± 0.09 cd 32.42 ± 2.31 de 1.93 ± 0.15 e 8 25.29 ± 1.90 de 1.02 ± 0.09 bcd 33.42 ± 1.68 de 2.34 ± 0.69 d 12 31.29 ± 1.48 bc 1.23 ± 0.31 ab 39.23 ± 1.65 ab 2.71 ± 0.86 bc 16 33.65 ± 2.05 b 1.27 ± 0.30 ab 42.15 ± 2.28 a 3.08 ± 0.47 a 20 40.16 ± 2.66 a 1.35 ± 0.13 a 38.12 ± 2.71 bc 3.16 ± 0.69 a 复水 2 27.49 ± 2.77 cd 1.31 ± 0.08 ab 34.17 ± 1.57 d 2.89 ± 0.12 ab 4 26.02 ± 2.70 de 1.11 ± 0.05 abc 34.96 ± 2.16 cd 2.53 ± 0.20 cd 6 17.25 ± 0.91 f 0.87 ± 0.07 cd 33.45 ± 1.69 de 2.35 ± 0.20 d 说明:不同小写字母表示不同处理间差异显著(P<0.05) Table 2. Effects of drought stress and rewatering on ROS and membrane peroxidation of Osmanthus fragrans 'Boyejingui'
ROS的积累造成植物细胞膜脂过氧化,产生MDA从而引起细胞膜的损伤,而伤害率是判定植物细胞膜稳定性的一个重要指标。MDA和伤害率与ROS有类似的变化趋势。干旱8 d时,MDA质量摩尔浓度迅速提升,比对照增加了35.3%(P<0.05);干旱20 d时,MDA质量摩尔浓度和伤害率达最大,分别比对照增加了0.80和2.68倍(P<0.05)。复水处理后各指标下降,复水6 d后,MDA质量摩尔浓度和伤害率分别比对照增加了35.8%和58.0%(P<0.05)。这些结果表明:干旱处理使‘波叶金桂’叶片膜透性遭到破坏,复水后膜透性仍未完全恢复。干旱处理可对‘波叶金桂’造成一定的胁迫。
-
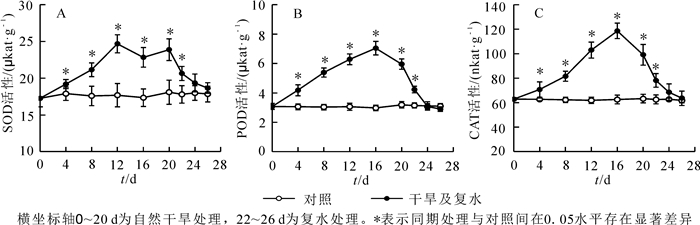
植物体内的SOD,POD和CAT主要作用是清除逆境下产生的活性氧,防止膜脂过氧化。随着干旱时间的增加,3种酶的活性呈先升高后降低的趋势(图 3)。当干旱4 d时,3种酶活性被显著激活;当干旱12 d时,SOD活性达到最大,比对照增加了39.7%(P<0.05),随后下降;干旱16 d时,而POD和CAT的活性达到最大,分别比对照增加了1.36和0.9倍(P<0.05),随后开始下降;干旱20 d时,酶活性下降,但仍显著高于同期对照。复水2 d后酶活性急剧下降,仍显著高于对照;复水6 d后酶活性与对照差异不显著,恢复到对照水平。这些结果说明‘波叶金桂’叶片的SOD,POD和CAT在干旱胁迫防御膜脂过氧化过程中起到重要作用。
2.1. 干旱胁迫和复水对土壤含水量、叶片相对含水量和叶水势的影响
2.2. 干旱胁迫和复水对渗透调节物质质量分数的影响
2.3. 干旱胁迫和复水对光合色素的影响
2.4. 干旱胁迫和复水对ROS和膜质过氧化的影响
2.5. 干旱胁迫和复水对抗氧化酶活性的影响
-
在土壤—植物—大气环流系统(SPAC)中,土壤含水量和叶水势可作为反映植物水分损失或水分条件的连续体。当土壤水势降低时,植物迅速降低叶片相对含水量和叶水势,调节幼苗水势和土壤水势梯度,从而增加植物的吸水能力[30]。研究发现:干旱胁迫下枇杷Eriobotrya japonica的土壤含水量与水势密切相关,植物水势随着土壤含水量的降低而降低;西鹃Rhododendron hybridiu和毛鹃Rhododendron pulchrum的土壤含水量和叶片相对含水量与干旱程度呈正相关[31-32]。本研究结果显示:随着干旱时间的延长,土壤含水量降低,导致‘波叶金桂’幼苗根系吸水困难,叶片相对含水量和叶水势随之下降。干旱前8 d,‘波叶金桂’通过减缓叶片相对含水量下降幅度,提高保水能力,从而保持光合作用系统的完整性,光合色素质量分数高,促使光合作用的正常进行。随着干旱胁迫程度加深,‘波叶金桂’叶片相对含水量和叶水势显著降低,光合色素合成受到抑制,‘波叶金桂’生长受到影响。复水后叶片相对含水量和叶水势随土壤含水量升高而升高,最后恢复到对照水平,体现了‘波叶金桂’较强的细胞持水力及恢复能力。
渗透调节被认为是植物对干旱的重要生理适应方式。植物体内渗透调节物质的积累是植物响应水分亏缺最常见的反应之一[33]。李州等[35]以大叶型和小叶型白三叶Trifolium repens为试验材料进行胁迫处理发现:干旱胁迫有助于2种白三叶可溶性糖和甜菜碱含量的积累,且渗透调节物质在白三叶响应干旱的过程中占据主要地位;干旱处理下的枸杞Lycium也被发现具有类似规律,可溶性糖与甜菜碱含量呈正相关[36]。本实验表明:可溶性糖、甜菜碱质量分数随干旱胁迫强度增加而升高。大量研究表明:可溶性糖和甜菜碱作为渗透调节物质,当植物细胞缺水时,它们可以迅速合成,增加细胞溶质势,降低细胞水势,从而吸水能力增加[37]。游离脯氨酸是蛋白质的一种结构成分,迅速生成可调节细胞渗透压,对能量吸收和还原力、氮储存化合物、羟自由基清除和保护酶具有保护和稳定功能的作用[38]。有研究认为:干旱胁迫下游离脯氨酸含量积累量与干旱程度呈正相关,小叶蚊母Distylium buxifolium,厚皮香Ternstroemia gymnanthera和轮叶蒲桃Syzygium grijsii等3种树种的游离脯氨酸随着干旱胁迫程度的增加快速上升[39]。安玉艳等[40]研究发现:干旱条件下,杠柳Periploca sepium游离脯氨酸的大量积累可能是其在胁迫条件下为生长储存碳源和氮源或能量的一种方式。本实验研究表明:游离脯氨酸质量分数在叶片中的积累需要一定的时间。当干旱20 d时,可溶性糖、甜菜碱合成受到抑制,而游离脯氨酸急剧增加,在胁迫下大量积累,其对渗透势的降低、膨压的维持贡献较大。‘波叶金桂’通过可溶性糖、甜菜碱和游离脯氨酸的积累来调节渗透势以提高抗旱能力。复水后,干旱胁迫解除,水势升高,幼苗体内调节物质逐渐向正常水平逆转,渗透调节能力逐步下降,可溶性糖、可溶性蛋白质及游离脯氨酸质量分数降低,植株开始正常生长。
叶绿素是光合作用的主要叶绿体成分之一,叶绿素参与光能的吸收、传递和转换等过程,与植物的抗旱性密切相关[41]。关于干旱胁迫下植株叶绿素含量的变化报道不一。李兰芳等[42]研究发现:干旱胁迫后白刺花Sophora davidii幼苗Chl a,Chl b,Chl t及Car随着干旱胁迫增强而呈上升趋势。柴胜丰等[43]认为:金花茶Camellia petelotii叶片Chl t,Chl a,Chl b含量均随干旱胁迫的增强而下降。本研究中,随着干旱胁迫的增强,‘波叶金桂’叶片Chl a,Chl b和Chl t质量分数整体呈下降趋势,Car质量分数持续上升,Chl a/b呈先下降后上升的趋势。可能是由于干旱0~8 d时,叶片相对含水量降低导致叶绿素浓缩,叶绿素质量分数稍有上升,该现象是幼苗在干旱胁迫下维持光合速率的生理适应机制[44]。但是植物色素的生物合成过程需要酶的参与,严重的干旱胁迫条件降低了叶绿体中一系列合成酶的活性[45]。叶绿素的生物合成减弱,同时植物体自身的活性氧积累,尤其是H2O2能穿透质膜,进入叶绿体中,与叶绿体内自生的H2O2共同引发叶绿体膜脂过氧化,其中间产物自由基和最终产物MDA都会严重损伤叶绿体膜结构和功能,进一步导致叶绿素的分解。复水后Chl a,Chl b和Chl t质量分数上升,说明干旱胁迫后复水对叶片Chl a,Chl b和Chl t等均具有一定程度的补偿效应。
干旱胁迫导致植物细胞ROS急剧上升,过量的ROS打破了植物体内的氧化还原平衡。ROS的大量积累引起膜脂过氧化加剧,对膜组分和结构造成氧化损伤,产生大量MDA,最终导致膜系统的破坏[46]。胡义等[47]研究发现:重度干旱胁迫(持续干旱10~16 d)下,樟树Cinnamomum camphora幼树叶片中的ROS和MDA大量积累;高晓宁等[48]研究发现:杜鹃花Rhododendron在干旱处理下产生过量的活性氧,但MDA变化不显著。本试验结果显示:随着干旱胁迫的加剧,叶片中O2·-,H2O2和MDA在前16 d显著上升,说明干旱胁迫造成ROS大量积累,产生了膜脂过氧化作用,对膜造成损害,从而MDA质量摩尔浓度上升,叶片伤害率也随之增加。因为MDA的积累可引起细胞膜透性增大,大量离子外渗,这是细胞膜受损的表现之一,这也印证了前面的观点。谢小玉等[49]对辣椒Capsicum annuum的研究得出了相同的结论。植物体内存在抗氧化防御系统, 可在一定程度上减缓ROS带来的伤害。SOD,CAT和POD是植物体内清除ROS的3种重要酶,在抵御干旱胁迫时发挥重要作用。SOD是抗氧化的第一道防线,它催化O2·-歧化生成H2O2和O2,CAT和POD进一步将有毒水平的内源性H2O2转化为H2O和O2。大量研究表明,植物在遭受干旱胁迫时,清除ROS的酶活性都增加,如四季桂Osmanthus fragrans,栓皮栎Quercus variabilis等。本研究也发现了类似的规律:‘波叶金桂’在干旱前16 d,SOD,POD和CAT活性逐渐增强,说明3种酶发挥协同作用,共同清除细胞内ROS。干旱20 d时,从图 3可看出SOD和POD活性仍然较大,继续保持清除细胞内大量的ROS,是抵御干旱胁迫的主要成员。复水后各指标均能得到恢复,可见‘波叶金桂’具有较强的耐旱性。‘波叶金桂’在干旱和复水条件下抗氧化酶系统积极发挥作用,清除ROS,使‘波叶金桂’能够在干旱条件下保持较好细胞膜稳定性和较低MDA质量摩尔浓度。
综上所述,‘波叶金桂’对于干旱胁迫及复水的响应是由多种内部生理生化反应共同完成的。短期的干旱胁迫下,叶片含水量和水势降低,ROS积累和膜透性增加,‘波叶金桂’通过增加渗透调节物质(可溶性糖、甜菜碱和游离脯氨酸)以及抗氧化酶(SOD,CAT和POD)的活性等减缓ROS给植物带来的伤害;长期的干旱胁迫下,叶片含水量和水势的急剧下降,ROS大量累积,抗氧化酶活性下降导致抗氧化系统清除ROS能力下降,光合色素合成受到抑制,而可溶性糖、甜菜碱质量分数和脯氨酸仍然保持高水平,通过渗透调节作用抵抗干旱胁迫,保证苗木代谢正常;胁迫解除后,幼苗表现出较强的旱后修复能力。因此,‘波叶金桂’在管理养护时,可制定科学合理的水分管理措施,对其进行短期的干旱胁迫(0~8 d),合理利用水资源,最大限度地发挥干旱胁迫的补偿效应,当胁迫超过20 d时需及时复水救苗,避免对‘波叶金桂’幼苗造成明显伤害并影响生长。













 DownLoad:
DownLoad: