-
榧树Torreya grandis是红豆杉科Taxaceae榧属Torreya中国特有的植物,呈片状分布于浙江、福建、江西、湖南、安徽等省的丘陵地带[1]。榧树雌雄异株,稀同株,种内变异十分丰富[2]。香榧T. grandis ‘Merrillii’是雌性榧树的一个优良栽培类型,其结实所需花粉来自雄性榧树天然居群。然而雄株因不结实而往往遭受随意砍伐,数量大幅度减少[3]。近年来随着香榧产业的发展,人们认识到充分优质的授粉对雌株种实产量及品质影响极大,日益重视在香榧丰产造林中配置授粉雄株的作用。因此,对榧树雄株开展调查与选优是提高香榧产量及质量的重要因素之一。表型性状多样性是植物多样性最直观的反映,是衡量生物多样性的重要指标,也是了解遗传变异的重要线索[4]。在板栗Castanea mollissima[5]、樱桃Cerasus pseudocerasus[6]、椰枣Phoenix dactylifera[7]、李Prunus divaricata[8]、玉米Zea mays[9]等物种中都开展了表型性状多样性研究。榧树雌雄异株,天然杂交,且生长地理环境存在差异,使得天然群体内榧树变异十分丰富[10]。近年来对榧树的研究主要集中在病害[11]、生物学特性[12]、栽培管理技术[13]、分子标记[14]等方面。由于香榧具有极高的经济价值,研究多集中在雌性香榧上,而雄性榧树的研究鲜见报道。董雷鸣等[15]探讨榧树雄株若干性状的变异发现:叶片与雄球花各指标均有较大的变异系数,且不同来源的花粉对香榧幼果坐果率有显著影响,表明榧树雄株具有很大的选育潜力。本研究以来自5个雄性榧树天然居群的121个单株为研究材料,对叶片、雄球花等10个表型性状指标进行多样性分析,并在此基础上初选雄性榧树优株,为今后雄性榧树资源保护、授粉树配置及新品种选育提供科学依据。
HTML
-
浙江省与安徽省是榧树主要的天然分布区。榧树目前主要生产与利用的是其种子,野生雄株天然居群除采种外受到的人为干扰较少。本研究在基本呈野生状态的雄性榧树天然居群开展,分布点分别为浙江省淳安县半夏村(淳安)、杭州市临安区洪岭村(临安)、杭州市富阳区洞桥村(富阳)、浙江嵊州市榆树村(嵊州)及安徽省黄山市呈坎村(黄山)开展。在分布点的雄性榧树林中各选取10~30株生长旺盛,彼此间距不小于50 m,无病虫害,树龄50~200 a的实生雄性榧树作为研究对象,用于试验观测与采样,同时利用全球卫星定位系统(GPS)定位采样点(表1)。各单株采集新鲜叶片即时装入加硅胶的自封袋中,带回实验室置于−80 ℃冰箱保存。各单株采集20个带雄球花的1年生幼枝,枝条底部用湿纸巾包裹后置于采样袋,带回实验室进行水培。
居群 采样数量/株 样品编号 纬度(N) 经度(E) 海拔/m 淳安 17 CA01~CA17 29°55′20″ 119°12′06″ 320~430 临安 24 LA01~LA24 29°59′30″ 119°12′32″ 305~360 富阳 24 FY01~FY24 30°05′47″ 119°38′42″ 360~410 嵊州 24 SZ01~SZ24 29°42′28″ 120°33′22″ 280~420 黄山 32 HS01~HS32 29°57′52″ 118°14′18″ 350~500 Table 1. Geographic locations of five natural populations in T. grandis
-
各单株选取20个叶片,用游标卡尺测量(精确至0.01 mm)叶宽和叶长,并计算叶形指数(叶宽/叶长);用托盘天平(PB1502-L,瑞士)称取叶质量。
-
各单株随机选取30个雄球花,用游标卡尺测量雄球花横径、纵径,并计算花形指数(球花横径/纵径),同时用托盘天平称取雄球花质量。
-
各单株选取3个雄球花数量较多且长势良好的枝条插入水中,置于浙江农林大学校园,培养条件设置为温度20 ℃,湿度75%,光强400 μmol·m−2·s−1,二氧化碳摩尔分数520 μmol·mol−1,2 d更换1次水,每天定期观察并记录花枝散粉情况。当雄球花的小孢子叶松散,颜色变黄,花粉囊纵裂,部分花苞散出黄色花粉时记为始花期,直至完全散粉。大约3 d时间。
-
每个单株各选30个即将散粉(即各小孢子叶松散,花苞颜色变黄,花粉囊开始纵裂)的雄球花,置干燥玻璃培养皿中,放置在室内干燥桌面上,使雄球花自然散粉。待雄球花完全开放,抖落所有花粉,收集花粉称量,计算花粉得率。花粉得率=[花粉质量/(花粉质量+撒粉后球花质量)]×100%。
-
在全程避光的条件下,用荧光染料反应法(fluorescein diaectate reaction,二乙酸荧光素染色)测定花粉生活力[16]。随机观测3个不同视野,统计有活力花粉(呈绿色)数量和花粉总数(绿色和黑色)。花粉生活力=(有活力花粉数量/花粉总数)×100%。
-
运用Excel和SPSS 17.0对各指标进行数据统计和方差分析、t检验及相关性分析等。
1.1. 材料
1.2. 方法
1.2.1. 叶片性状测定
1.2.2. 雄球花表型性状测定
1.2.3. 雄球花水培及散粉时间观察
1.2.4. 花粉得率测定
1.2.5. 花粉生活力测定
1.3. 数据处理
-
方差分析表明:叶片性状的叶长在居群间有显著差异(P<0.05),雄球花性状的花粉得率在居群间呈极显著性差异(P<0.01),其他指标均无显著差异(表2)。t检验分析结果显示:在居群内单株间10个表型性状均差异极显著(P<0.01),表明雄性榧树表型性状的变异可能主要来源于居群内。
指标 平方和 自由度 均方 F P 指标 平方和 自由度 均方 F P 叶质量 0.000 4 0.000 0.434 0.784 球花横径 0.354 4 0.089 0.310 0.871 叶宽 0.298 4 0.075 0.792 0.533 球花纵径 2.138 4 0.543 0.421 0.793 叶长 51.915 4 12.979 2.498 0.046* 花形指数 0.010 4 0.003 0.240 0.915 叶形指数 0.002 4 0.001 0.820 0.515 花粉得率 0.170 4 0.043 4.980 0.001** 球花质量 0.001 4 0.000 0.686 0.603 花粉生活力 0.095 4 0.024 0.355 0.840 说明:*表示差异显著(P<0.05),**表示差异极显著(P<0.01) Table 2. ANOVA in leaf- and male flower-associated parameters among five natural populations in T. grandis
多重比较分析发现:叶长在5个居群间差异显著(P<0.05),5个居群中淳安居群的叶片平均最长(18.99 mm),显著长于其他居群;嵊州与黄山居群的叶片最短,且2个居群间无显著差异;临安与富阳居群的叶片长度居中,2个居群间无显著差异(图1A)。花粉得率在5个居群间差异极显著(P<0.01),嵊州和富阳居群的花粉得率较高,显著高于其他居群,且2个居群间无显著差异;而淳安与临安居群花粉得率最低且无显著差异(图1B)。
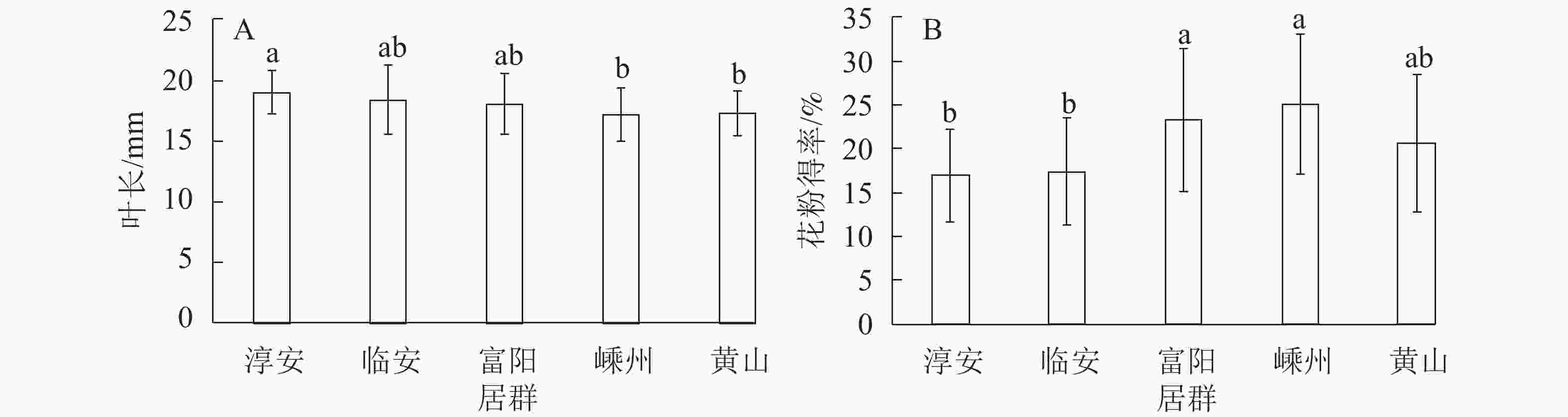
Figure 1. Varieties among five natural populations in the leaf length(A) and pollen yield(B) in T. grandis
变异系数与性状的离散程度呈正相关,变异系数越大表明个体间的差异越大,多样性越丰富,选择的潜力就越大[17]。本研究表明:同一性状在不同居群中变异系数不同;同一居群中不同性状的变异系数也存在差异。5个雄性榧树居群间叶片的变异程度由大到小依次为叶质量、叶长、叶形指数、叶宽;雄球花的变异程度由大到小依次为花粉生活力、花粉得率、球花质量、球花纵径、球花横径、花形指数。花粉生活力的变异最为丰富,5个居群的花粉生活力为51.70%~58.51%,变异系数为39.04%~45.40%,平均值为42.36%;其次是花粉得率(31.03%~37.77%),平均达34.13%;叶宽和球花横径变异系数较小,分别为10.82%和12.13%(表3)。
居群 叶质量 叶宽 最大值/g 最小值/g 平均值±标准差/g 变异系数/% 最大值/mm 最小值/mm 平均值±标准差/mm 变异系数/% 淳安 0.024 0.015 0.018±0.003 14.06 3.794 2.267 2.941±0.336 11.42 临安 0.025 0.012 0.018±0.003 19.44 3.775 2.400 2.901±0.345 11.90 富阳 0.023 0.013 0.017±0.003 15.36 3.422 2.344 2.850±0.281 9.86 嵊州 0.029 0.013 0.018±0.004 20.34 3.254 2.186 2.791±0.334 11.97 黄山 0.026 0.012 0.017±0.003 16.68 3.340 2.385 2.829±0.253 8.96 居群 叶长 叶形指数 最大值/mm 最小值/mm 平均值±标准差/mm 变异系数/% 最大值 最小值 平均值±标准差 变异系数/% 淳安 22.319 16.124 18.99±1.82 9.58 0.174 0.137 0.155±0.012 7.91 临安 25.475 13.650 18.41±2.84 15.45 0.228 0.107 0.161±0.029 18.27 富阳 25.139 15.360 18.05±2.51 13.93 0.205 0.130 0.159±0.015 9.68 嵊州 22.082 13.399 17.17±2.17 12.64 0.243 0.135 0.164±0.023 13.97 黄山 20.800 14.000 17.28±1.88 10.90 0.192 0.139 0.165±0.015 9.06 居群 球花质量 球花横径 最大值/g 最小值/g 平均值±标准差/g 变异系数/% 最大值/mm 最小值/mm 平均值±标准差/mm 变异系数/% 淳安 0.071 0.038 0.056±0.010 18.71 4.883 3.267 4.288±0.440 10.26 临安 0.078 0.032 0.058±0.014 24.51 5.344 2.947 4.369±0.594 13.61 富阳 0.089 0.034 0.054±0.014 25.96 6.283 3.714 4.452±0.551 12.37 嵊州 0.093 0.033 0.052±0.016 31.49 5.975 3.589 4.321±0.542 12.54 黄山 0.089 0.033 0.053±0.014 27.06 6.147 3.640 4.330±0.514 11.86 居群 球花纵径 花形指数 最大值/mm 最小值/mm 平均值±标准差/mm 变异系数/% 最大值 最小值 平均值±标准差 变异系数/% 淳安 6.937 4.425 5.671±0.718 12.66 0.893 0.656 0.760±0.060 7.84 临安 7.890 4.166 5.988±1.100 18.37 0.872 0.555 0.740±0.090 12.11 富阳 8.555 4.768 6.121±1.016 16.59 0.928 0.602 0.736±0.088 11.91 嵊州 10.884 4.458 5.900±1.423 24.12 0.938 0.434 0.753±0.110 14.57 黄山 9.889 4.516 5.907±1.146 19.40 0.854 0.588 0.745±0.083 11.04 居群 花粉得率 花粉生活力 最大值/% 最小值/% 平均值±标准差/% 变异系数/% 最大值/% 最小值/% 平均值±标准差/% 变异系数/% 淳安 26.38 5.82 17.00±5.28 31.03 92.71 21.61 58.51±25.05 42.80 临安 32.19 8.32 17.38±6.11 35.16 97.26 19.34 51.70±21.52 41.62 富阳 45.12 11.54 23.31±8.12 34.84 92.55 16.73 52.19±22.40 42.93 嵊州 41.39 10.23 25.08±7.99 31.85 92.08 11.09 56.70±25.74 45.40 黄山 36.56 8.72 20.67±7.81 37.77 94.30 23.90 56.56±22.08 39.04 Table 3. Extreme values and variation in the parameters of leaves and male flowers of five natural populations in T. grandis
居群差异体现了居群对当地环境的不同适应状况,数值大小反映了不同居群对不同环境的适应性[18]。基于叶片的表型指标分析发现:5个天然居群的变异系数临安(16.27%)最大,其次是嵊州(14.73%),淳安(10.74%)最小;而雄球花是嵊州(26.82%)和黄山(26.42%)的变异系数较高,淳安(19.49%)最小。本研究中榧树雄株的生殖器官花的变异系数大于营养器官叶。
-
榧树雄株的散粉时间不仅与遗传有关[15],也与地域气候、环境差异有关。榧树的雌雄花期不集中,且雄株花期比雌株早[19],散粉时间一般在4月中旬[20]。因此在新造林中要配置适当花期的雄性授粉树,满足雌花充分受粉的要求[15]。
经温室水培雄球花枝观测统计,雄球花散粉时间在4月7−17日,散粉周期约10 d。中花型所占比例最大(47株),占总样本数的38.84%,散粉时间在4月11−15日;晚花型比例最小(30株,24.79%),散粉时间在4月15−17日(表4)。5个居群中,淳安、临安、黄山这3个居群的中花型偏多,分别有11、13、13株,而富阳和嵊州的早花型略多,花期为4月7−11日(表4)。
居群 花期 花期类型 株数/株 群内比例/% 居群 花期 花期类型 株数/株 群内比例/% 淳安 4月9−17日 早 2 11.76 嵊州 4月7−15日 早 17 70.84 中 11 64.71 中 5 20.83 晚 4 23.53 晚 2 8.33 临安 4月9−17日 早 6 25.00 黄山 4月7−17日 早 7 21.87 中 13 54.17 中 13 40.63 晚 5 20.83 晚 12 37.50 富阳 4月7−15日 早 12 50.00 中 5 20.83 晚 7 29.17 Table 4. Pollen availability of male flowers in T. grandis
-
叶质量、叶长与叶形指数均呈极显著负相关(P<0.05),而其他指标间均为显著正相关(P<0.05)(表5)。这与董雷鸣等[15]的研究结果一致。
指标 叶质量 叶宽 叶长 叶形指数 叶质量 1 叶宽 0.381** 1 叶长 0.804** 0.476** 1 叶形指数 −0.626** 0.376** −0.513** 1 说明:**表示差异极显著(P<0.01) Table 5. Correlation coefficients among indexes of leaves in male T. grandis
球花质量与球花大小及花粉得率呈显著正相关(P<0.05),球花大小与花粉得率均显著正相关(P<0.05),花粉活力与花粉得率呈极显著正相关(P<0.01),而球花质量和球花纵径都与花形指数呈极显著负相关,其他指标间相关性不显著(表6)。
指标 球花
质量球花
横径球花
纵径花形
指数花粉
得率花粉
生活力球花质量 1 球花横径 0.735** 1 球花纵径 0.788** 0.693** 1 花形指数 −0.412** −0.020 −0.701** 1 花粉得率 0.209* 0.238* 0.296* −0.164 1 花粉生活力 0.103 0.010 0.035 −0.081 0.441** 1 说明:*表示差异显著(P<0.05),**表示差异极显著(P<0.01) Table 6. Correlation coefficients among indexes of male flowers in T. grandis
-
花粉会对植株坐果率高低、果实大小以及产量等产生直接影响[21−22]。筛选适宜的雄性优株可以提供香榧基地授粉雄株不足、香榧树体坐果率低的现状,从而促进香榧产业健康发展。营养器官叶片受环境影响较大,因而榧树可基于生殖器官的性状初选核心种质[23]。本研究主要依据榧树雄株散粉时间、花粉生活力、花粉量的多少等综合进行雄株优株的筛选。早花类型中表现较好的优株有富阳居群的雄9、嵊州居群的雄5以及黄山居群的雄23,花粉生活力分别高达92.55%、92.08%、90.01%,花粉得率也在20%以上;中花类型表现最佳的优株有临安居群的雄22、嵊州居群的雄1,花粉活力均在90%以上,花粉得率也不低;而晚花类型中,淳安居群的雄3、临安居群的雄18以及黄山居群的雄1花粉生活力分别为92.71%、92.61%、91.65%,共初选36个单株作为优良授粉树,占所有供试材料的29.75%(表7)。
居群 开花类型 编号 花粉生活力/% 花粉得率/% 花形指数 淳安 早花型 CA09 65.35 26.38 0.656 中花型 CA07 76.97 21.95 0.790 CA10 82.59 21.70 0.736 CA15 87.36 19.27 0.736 CA16 85.91 15.74 0.754 晚花型 CA01 86.00 22.19 0.743 CA03 92.71 19.98 0.826 CA17 82.05 22.16 0.735 临安 早花型 LA03 66.86 18.92 0.764 中花型 LA13 87.31 32.19 0.802 LA22 97.26 20.05 0.653 晚花型 LA17 87.00 20.98 0.818 LA18 92.61 23.91 0.850 富阳 早花型 FY03 70.56 34.28 0.782 FY06 81.58 32.29 0.822 FY09 92.55 23.02 0.605 FY14 85.04 30.23 0.660 FY19 69.44 33.37 0.602 晚花型 FY01 84.07 33.24 0.671 嵊州 早花型 SZ02 89.09 34.48 0.750 SZ05 92.08 38.23 0.725 SZ13 66.39 20.71 0.730 SZ16 86.05 30.28 0.799 SZ17 82.96 25.74 0.588 SZ24 88.63 25.67 0.766 中花型 SZ01 91.53 23.79 0.859 黄山 早花型 HS21 68.89 35.01 0.754 HS23 90.01 30.98 0.764 HS28 62.25 29.76 0.607 中花型 HS13 81.59 23.91 0.751 HS15 68.19 21.01 0.745 HS18 89.42 20.76 0.797 HS19 81.83 23.92 0.850 晚花型 HS02 91.65 25.67 0.705 HS04 87.61 21.98 0.595 HS29 87.16 24.81 0.801 Table 7. Screening of male elite trees in T. grandis
2.1. 雄性榧树叶片与雄球花表型性状的差异显著性分析
2.2. 雄球花散粉时间的变异
2.3. 雄性榧树叶片表型性状间的相关性分析
2.4. 雄性榧树优株的初选
-
本研究发现:在居群间,叶片与雄球花的10个表型指标,只有叶长和花粉得率差异显著,而居群内,单株间10个指标均差异极显著,表明居群内的变异比居群间更加丰富。该结果与刘浩凯等[24]研究雌性榧树居群遗传多样性的结果基本一致。因此,为配置香榧授粉树的榧树雄株选育可选择优良单株。
变异系数是衡量各观测性状变异程度的一个统计指标。本研究各指标的相对极差和变异系数的变化趋势完全不同,表明榧树雄株表型性状存在丰富的表型多样性。经过长期的自然选择,不同数量性状对环境形成了不同的适应能力。相关分析表明:雄性榧树的大部分表型性状相关性达显著或极显著水平,说明这些性状在环境适应中表现出相互调节的作用。
雄性榧株以提供花粉为主要利用目的,花粉得率和花粉生活力在居群间和个体间变异大,且比较稳定,不仅有利于选择,选择结果也相对可靠。本研究显示:雄球花各指标变异系数大,球花大小与花粉得率呈显著正相关,直接影响花粉得率。因此优株初选应从花粉生活力、花粉得率及球花大小等综合考虑。基于上述指标初步筛选出36个优良雄性单株,其中8个单株的花粉生活力在90%以上。在今后的育种工作中,这些雄性优株应是优先考虑的对象。本研究所有单株的花粉得率最高值为38.23%,与董雷鸣等[15]的研究结果类似。
居群内的变异主要受遗传因素的影响,而居群间的变异则与遗传和环境2个因素相关[25]。由于榧树雄株不结果,以往人为破坏严重,部分地区的雄株资源已濒临绝迹,雌雄比例严重失调。易官美等[26]对榧树资源分布进行调查发现:雌雄株比例在江西岩泉自然保护区为4∶1,在福建建瓯玉山镇居群为29∶1;在安徽居群为19∶1;在浙江诸暨赵家镇居群为99∶1。因此,加强雄性榧树资源保护刻不容缓,需加大种质资源调查与收集力度,建立雄性榧树优株资源库。
本研究仅对雄性榧树的表型多样性进行初步研究,后续的研究将扩大居群选择的范围及居群个数,并结合分子生物学从DNA水平深入揭示雄性榧树天然居群的遗传变异特点和规律。









 DownLoad:
DownLoad: