-
葫芦科Cucurbitaceae植物多为1年生爬藤植物,全球广泛分布,以热带和亚热带地区居多;葫芦科共有90属800余种,中国有26属130余种,以西南地区各省最为丰富[1]。葫芦科植物在农业生产中具有重要地位,其重要性仅次于禾本科Gramineae、豆科Leguminosae和茄科Solanaceae。葫芦科园艺作物为人类日常生活所需蔬菜和水果的重要来源,如黄瓜Cucumis sativus、南瓜Cucurbita moschata、丝瓜 Luffa cylindrica等重要蔬菜[2],西瓜Citrullus lanatus、甜瓜Cucumis melo等重要水果[3];此外,葫芦Lagenaria siceraria还与中国特殊文化息息相关。卷须是葫芦科植物重要的形态学特征,赋予其攀爬和附着能力,来获取更好的生存条件(如空间、光照等),但是,卷须与植物生长发育之间并没有直接的关联性。生产中,多为爬地或人工绑蔓栽培,卷须则成为消耗养分的多余器官。因此,卷须需人工摘除,一来减少养分消耗,防止卷须和果实之间的养分需求竞争;二来便于人工控制植物空间分布,防止因卷须造成的无序攀爬生长。卷须的摘除不仅增加劳动力成本,摘除后留下的伤口也为病菌滋生提供机会,因此,无卷须育种是满足设施园艺栽培需求的重要育种方向。卷须为植物特殊的触觉器官,适当的刺激即可导致渗透驱动的可逆缠绕,卷须缠绕可敏感地响应外界环境(光、温度)和内源信号(生长素、茉莉酸、钙离子)刺激,因此,卷须是研究特定条件刺激和植物快速应答的良好模型[4]。葫芦科植物卷须最近被确定为茎(侧枝)变态来源,且受关键基因TCP1调控[5],为植物卷须发生调控的标志性研究成果。伴随着葫芦科植物遗传转化体系和基因编辑技术的日趋成熟[6],实现无卷须精准编辑育种将成为可能。基于此,本研究对葫芦科园艺作物卷须变态来源、关键调控基因、卷须特异(或高量)表达基因、环境条件和内源激素调控卷须等方面的研究结果进行综述。
HTML
-
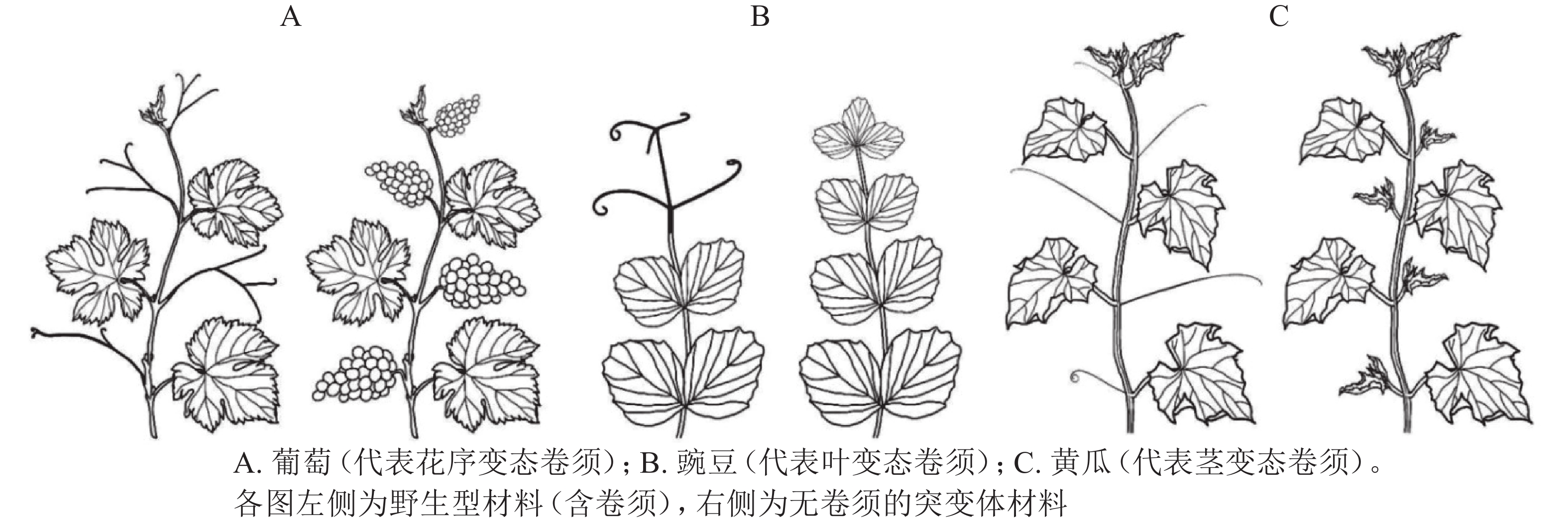
卷须是植物行使攀爬、吸附、机械支撑的特殊变态器官,有助于植物获取有利的光照条件和生存空间。不同科属植物卷须的变态来源不尽相同,可分为花序变态卷须、叶变态卷须和茎变态卷须(图1)[7]。不同变态来源的卷须,其发生过程和调控机制存在显著差异。
-
花序变态来源卷须的植物常见于葡萄科Vitaceae、西番莲科Passifloraceae、花须藤科Petermanniaceae、无患子科Sapindaceae、蓼科Polygonaceae、夹竹桃科Apocynaceae、车前草科Plantaginaceae和桔梗科Campanulaceae[7]。该类型植物卷须发生的典型特征为:植物早期无卷须,进入生殖期后才发生卷须。其中,以葡萄科和西番莲科报道居多[8]。
葡萄Vitis vinifera为葡萄科葡萄属Vitis木质藤本植物,其卷须与花序为同源器官(图1A)。BOSS等[9]基于一份矮化葡萄无卷须突变体材料,发现其卷须被替换为花序,证明葡萄卷须为花序的变态器官。研究发现:矮化无卷须葡萄为VvGAI(编码DELLA蛋白)的赤霉素不敏感型突变,VvGAI与小麦Triticum aestivum绿色革命基因Reduced height-1和拟南芥Arabidopsis thaliana insensitive(GAI)基因同源,证明赤霉素可抑制葡萄花序,促进卷须发生[9]。
-
叶变态来源卷须的植物常见于豆科Fabaceae、菊科Asteraceae、紫葳科Bignoniaceae、罂粟科Papaveraceae、毛茛科Ranunculaceae和花荵科Polemoniaceae[7],其中,以豆科的豌豆Pisum sativum被研究得最为清楚[7]。豌豆叶片属于复叶,复叶末端的小叶变态形成卷须。因此,较容易判断其卷须为叶片来源的变态器官(图1B)。
豌豆中存在丰富的卷须缺陷材料,如uni、tl、lath等[10]。HOFER等[10]基于tendril-less(tl)研究发现:HD-ZIP Ⅰ为豌豆卷须发生的关键调控子,HD-ZIP Ⅰ通过抑制叶片舒展从而变态发育形成卷须。此外,UNIFOLIATA/LEAFY、LATH可通过HD-ZIP Ⅰ调控豌豆卷须发生。进化树分析显示:该HD-ZIP Ⅰ属于一个特殊小分支家族基因,推测蝶形花亚科Papilionoideae野豌豆Vicia sepium的卷须进化产生与HD-ZIP Ⅰ基因的获得相关[10]。
-
茎变态是被子植物卷须最为常见的形式,植物茎变态卷须分多次独立进化产生。茎变态卷须植物常见于:木兰目Magnoliales的番荔枝科Annonaceae;超菊类植物,如铁青树科Olacaceae、茶茱萸科Icacinaceae、夹竹桃科Apocynaceae和马钱科Loganiaceae;蔷薇类植物,如卫矛目Celastrales、酢浆草目Oxalidales、金虎尾目Malpighiales、豆目Fabales、蔷薇目Rosales、葫芦目Cucurbitales和无患子目Sapindales[7]。有趣的是,蔷薇类植物下属的各个目几乎都含有茎变态卷须的代表植物。相较于花序变态和叶变态卷须,茎变态卷须研究积累较少,且仅限于葫芦科植物[7]。
-
卷须是葫芦科植物重要的形态学特征,除少数转变为刺(如Momordica spinosa)或者丢失(如Citrullus ecirrhosus,Melancium campestre)之外,葫芦科植物普遍具有卷须[7]。葫芦科植物卷须发生于叶腋处。该位置同时存在侧分枝、叶片、花序,因此,卷须变态来源一直存在争议。曾经流行3种不同的观点:花变态[11]、茎变态[12]、茎-叶变态[5]。GERRATH等[12]对刺囊瓜Echinocystis lobata叶腋复合体(axillary bud complex)产生雌雄花序、侧分枝、卷须的次序进行详细观察,提出葫芦科植物卷须为侧分枝变态器官。
解剖观察发现:葫芦科植物卷须的结构与茎的结构存在相似性。SENSARMA[13]发现:葫芦科植物卷须内部的维管结构与茎(或者茎-叶)的维管结构相似,暗示其卷须与茎为同源器官。谭敦炎等[14]解剖发现:西瓜卷须的结构与茎的结构基本相似。何金铃等[15]解剖发现:黄瓜卷须和茎的横切面均包含表皮、皮层与维管柱3个部分;卷须解剖结构由基部向末梢越来越简单,主要表现为皮层中的厚角组织和厚壁细胞层数逐渐减少,维管束结构逐渐简化。此外,黄瓜卷须基部维管柱内含有维管束5~9束(通常为5束),排成1圈,远轴面3束,较大;近轴面2束,较小;卷须的维管束结构与茎相同,为典型的双韧维管束[15]。解剖结构的相似性,也提示葫芦科植物卷须的茎变态来源。
瓜类蔬菜遗传协会(Cucurbit Genetics Cooperative, http://cuke.hort.ncsu.edu/cgc/)记录的葫芦科植物卷须缺陷突变体,包括黄瓜的tendrilless(td)[16]、西瓜的tendrilless/branchless(tl/bl)[17]、西葫芦Cucurbita pepo的tendrilless(td)[18],多为卷须发育缺陷、退化的突变体,而非卷须发生缺陷突变体,且相应功能基因未被克隆。
OIZUMI等[19]于2005年报道一份甜瓜无卷须(Tendril-less,TL)材料“Chiba TL”。“Chiba TL”源自甜瓜品种‘Fuyukei 1’的自发突变(收集于1991年),为单基因隐性突变。MIZUNO等[5]将“Chiba TL”(简写为ctl)与“Fuyukei 1”(Wildtype,简写为CTL)杂交获得CTL/ctl杂合子,详细记录CTL、CTL/ctl、ctl的卷须发生。CTL的第5节位及之后节位均发生1个侧分枝和1个卷须;ctl的每个节位则发生2个侧分枝,侧分枝1和侧分枝2(与CTL的卷须同源);CTL/ctl的第10节位及之后节位发生1个侧分枝和1个卷须(与野生型CTL第5节位及之后的表型一致),第10之前的节位产生一种特殊的茎-卷须过渡结构,基部为茎变态结构,末梢处为叶变态结构。由此确定,甜瓜卷须为侧分枝的茎叶变态结构(modified stem-leaf complex)[5]。同年,WANG等[16]在3 342份黄瓜材料中发现1份无卷须材料(Tendril-less line,CG9192 line,命名为ten),其无攀爬能力,卷须之外的其他器官均正常;表型观察发现:ten的缺陷卷须由茎、雄花、1片或几片叶(叶柄卷曲)构成,证明黄瓜卷须为侧分枝的变态器官[16]。基于上述甜瓜(ctl)和黄瓜(ten)无卷须材料,证明葫芦科植物卷须为茎变态(侧分枝)来源(图1C)[16]。
1.1. 花序变态来源的卷须
1.2. 叶变态来源的卷须
1.3. 茎变态来源的卷须
1.4. 葫芦科植物卷须的茎变态来源
-
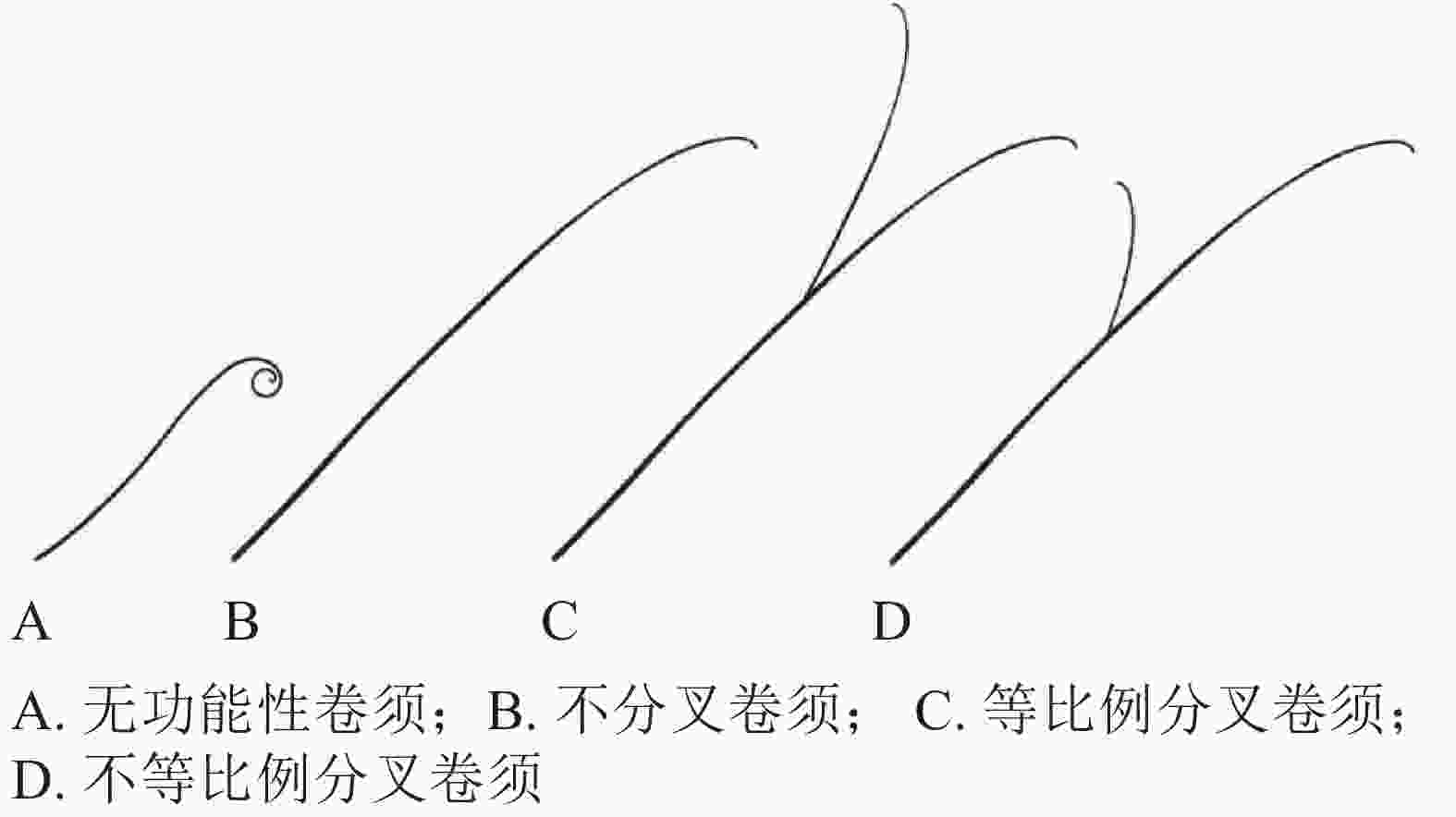
卷须初始发生是葫芦科植物由幼年期进入抽蔓期的形态标志,有助于葫芦科园艺作物熟性育种研究。以黄瓜为例,通常3~5节位初始发生卷须,前面节位发生的卷须较细、较短,颜色较白,为不具备卷曲缠绕能力的“无功能性”卷须(图2A);后面节位发生的卷须较粗,长度一般10~20 cm,颜色淡绿,顶部弯曲,与主茎夹角小于90°,具有卷曲缠绕能力(图2B)[15]。
依据末梢是否分叉和分叉比例,可分为不分叉、等比例分叉和不等比例分叉3种不同类型卷须(图2C和图2D)。黄瓜、甜瓜的卷须多为不分叉类型,西瓜的卷须多为等比例分叉类型,南瓜、丝瓜、葫芦的卷须多为不等比例分叉类型[18]。苦瓜的卷须存在不分叉和等比例分叉2种类型,前者更为普遍[20]。钟建等[20]将‘佛沥734’(卷须分叉,栽培苦瓜Momordica charantia自交系,作母本)和‘AVBG1602’(卷须不分叉,半野生苦瓜自交系,作父本)进行杂交,发现苦瓜卷须分叉/不分叉受1对显性基因控制,卷须分叉为显性性状。
-
基于甜瓜ctl和黄瓜ten无卷须突变体,证明葫芦科植物卷须的茎变态来源,并确定卷须发生的关键调控基因CTL和TEN,两者为同源基因,编码葫芦科植物特有的TCP1转录因子[5]。TCP(TEOSINTE BRANCHED 1/CYCLOIDEA/PCF)为植物特有的转录因子家族,含有1个TCP结构域,负责核定位和DNA识别结合[21]。植物TCPs可分为Ⅰ类型和Ⅱ类型,其中Ⅱ类型又可细分为CIN类型和CYC/TB1类型。CIN类型的TCPs多为miR319
的靶基因;CYC/TB1类型的TCPs与植物侧分枝发育相关,如CsBRC1通过抑制CsPIN3基因表达,进而通过生长素的积累来抑制黄瓜侧枝的发育[22]。Ⅰ类型TCPs可识别结合GGNCCCAC序列,Ⅱ类型TCPs则可识别结合G(T/C)GGNCCC序列,两者在识别DNA序列上的存在重叠[23]。TCPs转录因子可行使转录激活或者抑制的功能,这可能与相应的互作蛋白相关[24]。TCPs已被证明参与植物多个生长发育和逆境胁迫响应调控。随着研究的深入,植物TCPs转录因子的功能将得到进一步丰富。 MIZUNO等[5]通过图位克隆将甜瓜ctl突变位点定位在Ⅸ连锁群1个约200 kb(第9连锁群的1238~1451 kb)的区间,区间内含有17个注释基因。二代测序显示“Chiba TL”和“Fuyukei 1”仅在MELO3C022091基因第1外显子内存在1个单碱基插入/缺失差异。MELO3C022091属于CYC/TB1类型的TCP基因,将其命名为CmTCP1。该基因在甜瓜卷须特异表达。CmTCP1Fuyukei 1可编码1个完整TCP蛋白,含TCP结构域和C端的R domain(Ⅱ类型TCP蛋白特有,长度为18~20 个氨基酸,富含精氨酸);CmTCP1Chiba TL编码的TCP蛋白则缺失包含R domain的C端序列[5]。
氨基酸序列比对分析发现:TCP结构域的Helix I(属于bHLH结构域,负责DNA结合)区域内有1个高度保守的丙氨酸(Ala),在葫芦科植物TCP1中被特异性替换为丝氨酸(Ser)[5]。有趣的是,野豌豆族植物卷须的关键调控子HD-ZIP Ⅰ也存在类似现象,HD-ZIP Ⅰ的Helix 3(属于Homeodomain结构域,负责DNA结合)区域内有1个高度保守的丙氨酸,在野豌豆族植物HD-ZIP Ⅰ中被特异性替换为苏氨酸(Thr)[10]。丙氨酸和丝氨酸/苏氨酸在亲水性和极性上存在差异,且丝氨酸/苏氨酸可被丝氨酸/苏氨酸蛋白激酶(Ser/Thr protein kinases)修饰。葫芦科植物和野豌豆族植物上述相似的关键氨基酸替换和卷须的进化出现,暗示不同植物卷须独立进化发生可能存在机制上的相似性。
WANG等[16]通过基因组重测序和基因表达分析,确定ten突变基因为黄瓜第5连锁群上的Csa5G644520
,TEN与CmTCP1为同源基因。喷瓜Ecballium elaterium为葫芦科植物中卷须缺失的物种,序列分析显示喷瓜TCP1基因至少存在2个提前终止突变,喷瓜TCP1为无功能基因,该序列分析结果与喷瓜无卷须表型相吻合[16]。黄瓜ten无卷须突变体中,CsTCP1(TEN)第338位天冬酰胺替换为酪氨酸(N338Y),该突变氨基酸位于TCP结构域和R结构域之外[16]。蛋白序列比对分析显示:葫芦科植物TCP1在337~343处存在7个保守的氨基酸序列(CNNFYFP,下划线为ten的突变氨基酸位置);有趣的是,该序列在所有已知物种TCPs蛋白序列中均不存在(包括rosid Ⅰ下属的与葫芦科植物相近物种)。由此,WANG等[16]提出该特有氨基酸序列对于葫芦科植物进化产生卷须具有重要贡献。 -
双分子荧光检测系统证明:CsTCP1为一个转录激活子,CsTCP1ten的转录激活能力显著低于CsTCP1TEN(wild type),表明葫芦科植物特有的CNNFYFP序列可能是1个转录激活功能域(transcriptional activation domain,TAD)[16]。转录组分析显示:黄瓜ten的缺陷卷须中,1 271个基因显著上调,433个基因显著下调[16]。
1 271个上调基因的基因注释(GO)分析显示:上调基因主要参与调控植物形态建成,如器官极性形态、叶片形态和花形态[25]。以CsYAB1(Csa5G160210)为例,该基因属于YABBY基因家族,在ten突变体中上调约80倍。拟南芥中,YABBYs基因缺失突变体的叶片呈现针状形态(needle-like leaf),类似于黄瓜卷须的形态。EMSA检测显示:CsTCP1不直接结合CsYAB1基因启动子,提示CsTCP1通过间接抑制CsYAB1表达以确保卷须正常发育[16]。
433个下调基因的GO分析显示:下调基因主要参与趋向性、生长素极性运输、钙离子转运、谷氨酸代谢、木质素代谢。这些过程已知与卷须发生、发育、卷曲缠绕密切相关[25]。组织特异性表达分析显示:433个下调基因中,71个基因在卷须中特异表达,这些基因主要编码钙离子和钾离子转运体、水通道蛋白、生长素响应相关蛋白、果胶酯酶、合成酶等,与细胞响应刺激、细胞膨胀、信息传递相关[25]。CsTCP1被证明是转录激活子,下调基因可能为CsTCP1的直接下游靶基因。上述转录组分析结果可为CsTCP1介导黄瓜卷须发生、发育、卷曲缠绕的分子网络解析提供重要参考。
-
CHEN等[26]通过EMS诱变筛选获得1份黄瓜无卷须突变体td-1(tendril-less mutant),为隐性突变。td-1同时具有多样性缺陷表型,包括矮化、表皮毛减少、叶片褶皱、根系短小。图位克隆和BSA测序确定突变基因为CsGCN5
(Csa6G527060),为错义突变,造成第82位天冬氨酸突变为天冬酰胺。组织表达检测显示:CsGCN5在黄瓜根、茎、叶、卷须等组织均有较高表达量,与td-1具有多样性缺陷表型相吻合[26]。CsGCN5编码黄瓜1个组蛋白乙酰转移酶(histone acetyltransferase),已知组蛋白乙酰转移酶调控基因活化表达,提示表观修饰对黄瓜卷须发生具有重要调控作用[27]。但是,CsGCN5调控黄瓜卷须发生的具体分子机制、CsGCN5与卷须发生关键基因CsTCP1是否存在关系等问题仍然未知。 近期,国内相继报道的黄瓜圆叶突变体(round-leaf mutants,rl)均为Csa1G537400基因的等位突变体,Csa1G537400基因编码CsPID[28]。PID为丝氨酸/苏氨酸激酶,通过磷酸化修饰PIN而调控生长素的极性运输过程[29]。rl突变体除叶片形态缺陷表型外,其卷须发生、花器官、胚珠也存在缺陷[29],提示生长素极性运输及生长素信号在黄瓜卷须发生中具有重要的未知调控功能。
Aux/IAA为生长素响应早期关键基因。庄丹等[30]对黄瓜29个IAA基因(CsaIAA1~CsaIAA29)表达检测发现:大部分CsaIAA不具备组织表达特异性,而CsaIAA29表现为卷须特异性表达,提示其可能在卷须响应生长素信号途径中具有重要作用。
3.1. 葫芦科园艺作物卷须发生的关键基因TCP1
3.2. CsTCP1调控的下游基因
3.3. 葫芦科园艺作物卷须发生的其他基因
-
生长素与黄瓜卷须发生调控方面:WANG等[16]鉴定得到5个卷须特异表达的生长素响应基因(Auxin-responsive SAUR、AUX/IAA),并且这5个基因在黄瓜ten无卷须突变体中均显著下调。此外,庄丹等[30]也鉴定发现:CsaIAA29为黄瓜卷须特异表达基因。黄瓜rl圆叶突变体(具有卷须发生缺陷表型)的突变基因为CsPID。CsPID通过磷酸化修饰PIN来调控生长素信号途径,检测发现rl圆叶突变体特定组织中生长素(IAA)水平降低[31]。生长素调控黄瓜卷须发生的具体分子机制未知,但从上述研究报道可推测:黄瓜卷须的发生或生长发育过程涉及生长素信号途径。
赤霉素与黄瓜卷须发生调控方面:AMEHA等[11]通过体外添加方法,研究激素对黄瓜花芽和卷须发生的影响,发现体外添加GA4+7可导致黄瓜节间伸长,抑制节间花芽发生和促进卷须发生。
-
卷须的生长发育快速,且对外界刺激响应敏感,因此,不利外界环境下,葫芦科园艺作物卷须最先出现异常。以黄瓜为例,正常生长条件下,新生卷须通常比较粗壮,颜色为淡绿色,与主茎呈<90°夹角,有弹性且易折断。植株根系缺水、营养不足或出现衰老时,黄瓜新生卷须细短、末梢卷曲;植株体内糖含量降低时,黄瓜新生卷须颜色发黄;外界环境温度过低时,黄瓜卷须发生数量将增加;环境温度高湿度大时,黄瓜卷须出现直立生长状态。因此,生产中,可通过卷须的形态特征指示葫芦科园艺作物生长状况,但是,外界环境条件如何调控卷须的相关研究积累很少。
LÓPEZ-JUEZ等[32]报道1份黄瓜长下胚轴(long-hypocotyl mutant,lh)突变体,表现为光形态建成缺陷和过度避光反应(extreme shade avoidance)。lh突变体具有节间伸长、叶片变薄、卷须发生增强的表型。野生型黄瓜用远红光(模拟遮光反应)处理后,出现节间伸长、叶片变薄、卷须发生增强,类似于lh突变体表型。以上研究结果表明:遮光可促进黄瓜卷须的发生,推测卷须发生增多,利于其向上攀爬,从而躲避光照不足,但是这种调控的分子机制未知。
-
综上所述,基于甜瓜(ctl)和黄瓜(ten)的无卷须突变体,证明葫芦科植物卷须为茎(侧分枝)变态,研究揭示卷须发生的关键基因为TCP1,并鉴定获得一些卷须特异表达基因[5]。总之,葫芦科园艺作物卷须发生的研究已经取得一些关键性成果。
TCPs为植物保守存在的转录因子家族,那么TCP1为何能作为葫芦科植物卷须发生的关键调控子?基于甜瓜ctl无卷须突变体,MIZUNO等[5]研究认为:TCP结构域内Helix Ⅰ区域内1个高度保守丙氨酸(Ala),在葫芦科植物TCP1中被特异性替换为丝氨酸(Ser),此氨基酸替换对于TCP1调控卷须发生具有关键作用。基于黄瓜ten无卷须突变体,WANG等[16]则研究认为:葫芦科植物卷须进化发生与TCP1的CNNFYFP序列(位于TCP结构域之后)获得相关。将来,对葫芦科植物TCP1特有氨基酸或氨基酸序列的分析和功能研究,将有望回答葫芦科植物卷须如何进化产生的科学问题。
植物卷须可为花序变态、叶变态和茎变态,分多次独立进化而成,为研究趋同进化(convergent evolution)的理想器官[16]。茎变态为植物最常见的卷须来源,涉及的科属物种也最为丰富,但是其研究积累少且仅限于葫芦科植物。因此,葫芦科植物卷须发生调控分子机制的解析,可为其他茎变态卷须植物的研究提供借鉴。甜瓜(茎变态卷须)卷须被认为与关键调控子TCP1的Helix Ⅰ区域1个Ala替换为Ser相关[5],豌豆(叶变态卷须)卷须被认为与关键调控子HD-ZIP Ⅰ的Helix 3区域1个Ala替换Thr相关[10]。此外,赤霉素可促进黄瓜(茎变态卷须)卷须发生[11];有趣的是,在葡萄(花序变态卷须)中,赤霉素通过抑制花序进而产生卷须[9]。上述研究结果提示:植物卷须在趋同进化过程中,可能存在相似的作用机制。今后,随着以葫芦科植物为代表的茎变态卷须发生机制的不断解析,有望回答植物卷须趋同进化的更多生物学问题。
设施栽培(或爬地栽培)中,卷须为耗能的多余器官。OIZUMI等[19]利用甜瓜无卷须材料ctl,将其杂交导入商用品种“Takami”,成功育成甜瓜无卷须商用种“TL Takami”。田间测试发现:“TL Takami”甜瓜的藤蔓管理劳动成本降低20%~40%,证明无卷须在葫芦科园艺作物育种上具有重要应用价值。今后,随着葫芦科植物卷须发生分子调控网络的不断解析,各葫芦科园艺作物基因组信息的不断完善,结合日趋成熟的遗传转化和基因编辑体系,可望实现基于基因精准编辑育成无卷须葫芦科园艺作物品种。









 DownLoad:
DownLoad:
