-
十字花科Brassicaceae植物主产北温带,约375属3 200种。在中国主要集中于西南、西北、东北高山以及丘陵地区[1],其中萝卜属Raphanus与芸薹属Brassica植物是中国最重要的蔬菜与油料作物,该科部分种类还可作为药用、观赏用、染料用或食用[2]。植物表皮蜡质是覆盖在陆生植物地上部器官表面的脂质成分,也存在于木栓的基质、愈伤组织、花粉粒以及种皮中[3],其疏水结构在植物表面起极其重要的防卫功能,在植物与周围环境的相互作用中发挥着重要作用[4]。本研究从十字花科植物蜡质类型、结构、成分、含量、功能、遗传特性、合成与转运途径、分子机制等方面进行综述,为十字花科植物的蜡质代谢研究提供参考。
-
十字花科植物的蜡质呈片状、柱状和网状等26种形态类型,不同种类植物的蜡质形态不同[5]。徐秀萍等[6]采用扫描电镜(SEM)观察拟南芥Arabidopsis thaliana表皮蜡质,发现主要呈杆状,少量呈片状、管状、碟状和伞状。李红莲[7]发现:红菜薹Brassica campestris表皮蜡质为片状和网状构成的不规则三维结构。李帅等[8]发现:甘蓝型油菜‘中双11’Brassica napus‘Zhongshuang 11’叶表皮蜡质结构主要为杆状和颗粒状(小片状)。牟香丽等[9]发现:不同生长时期甘蓝Brassica oleracea var. capitata表皮蜡质呈现不同结构,苗期大多为颗粒状、片状和针状,结球期则较多为圆柱状和片状,成熟期以片状和针状为主。张曦[10]发现白菜Brassica pekinensis成熟叶片蜡质大多呈现出棒状且前端有圆形突起。
-
十字花科植物蜡质主要为超长链脂肪酸及其衍生物,包括烷烃、脂肪酸、醇、醛、酮的同系物,偶尔会出现环状化合物,如甾醇或三萜类化合物等[11]。蜡质通常使用三氯甲烷、正己烷等有机溶剂提取,采用气相色谱-质谱联用(GC-MS)技术鉴定成分[12]。植物表皮蜡质各成分占比不同。如白菜[10]成熟叶片蜡质的主要成分中酮类占1.02%,醇类占8.33%,烷烃占55.09%,酯类占26.34%。不同种类植物的表皮蜡质成分含量也存在差异[13]。如甘蓝型油菜‘中双11’叶[8]表皮蜡质成分中烷烃为8.24 μg·cm−2,次级醇为1.72 μg·cm−2,酮为1.62 μg·cm−2,初级醇为0.57 μg·cm−2,脂肪酸为0.10 μg·cm−2,醛为0.79 μg·cm−2,未知成分为9.33 μg·cm−2,总量为22.37 μg·cm−2;拟南芥[14]茎秆的表皮蜡质中初级醇为0.58 μg·cm−2,脂肪酸为0.10 μg·cm−2,醛为1.90 μg·cm−2,烷烃为13.13 μg·cm−2,次级醇为3.83 μg·cm−2,酮为5.95 μg·cm−2,总含量为28.99 μg·cm−2。而普通白菜自交不亲和系13S106[15]叶片的蜡质成分中烷烃为13.60 μg·cm−2,醛为0.90 μg·cm−2,醇为3.20 μg·cm−2,酮为8.30 μg·cm−2,脂肪酸为1.20 μg·cm−2,蜡酯为2.40 μg·cm−2。在上述不同十字花科物种中,蜡质主要成分均为烷烃,次要成分则有所不同。
-
十字花科植物表皮蜡质在维持水分平衡、反射紫外线、减少外来机械损伤、降低低温伤害、抵御细菌真菌入侵、防止昆虫侵食等抵抗生物与非生物胁迫中起着重要作用[16],同时兼具影响叶片和果实着色、防止果实开裂和植株育性等生理功能[17]。
-
低温胁迫易引起植物酶活性降低、细胞膜结构改变、细胞失水、代谢紊乱等,对植物生长发育造成多种负面影响[18]。倪郁等[19]发现:4 ℃低温胁迫下拟南芥生长发育缓慢、叶色变深,蜡质晶体的分布密度、大小、形态等发生改变。唐帅等[20]发现:4 ℃低温胁迫下拟南芥叶片表皮蜡质成分增加,烷烃、脂肪酸、醛、初级醇和酮相对含量分别增加54.34%、29.61%、54.40%、24.07%和137.80%;荧光定量聚合酶链式反应(PCR)检测显示蜡质相关基因的表达水平显著提高,说明拟南芥通过提高蜡质含量来缓解低温胁迫,预防低温对植物内部组织的伤害。
-
紫外线中UV-B波长为280~315 nm,可对植物表面造成损伤。PRUDNIKOVA等[21]发现:过量UV-B处理会造成植株干质量降低,叶面积减小,净光合速率下降及花期变短等。宋超[14]发现:UV-B胁迫下拟南芥蜡质相对含量明显增加,蜡质相关基因CER3、CER4、KCS1表达量显著提高,其中CER4基因的相对表达增加了13.80倍,CER1和WIN1表达量降低。此外,UV-B胁迫下拟南芥蜡质晶体结构发生熔融,晶体由杆状变成片状,蜡质覆盖面积增加,蒸腾作用减少,从而达到反射更多紫外线的效果。
-
植物蜡质作为疏水屏障,在限制非气孔水分散失中扮演重要角色[22-23]。柴凌燕[24]发现:拟南芥过量表达蜡质相关转录因子WIN1可提高蜡质合成量,调节表皮渗透性,增强植株耐旱性。LÜ等[25]发现:拟南芥CER9编码一种与植物抗旱性相关的决定因子,该因子缺失可以增加蜡质合成,阻塞更多的气孔,抑制蒸腾作用。周燕等[26]发现:甘蓝型油菜中BnWIN2C01的特异性表达影响了叶片蜡质合成,从而影响植株水分平衡。
-
花粉发育异常会导致植株减产或杂交不育,蜡质是花粉表面含油层的重要成分,在植物生殖发育方面发挥重要作用。KOCH等[27]发现:蜡质不仅影响植物叶片和果实的形态、发育,还影响植株花粉的发育情况。刘艳艳等[28]发现:FAX1基因缺失会抑制拟南芥营养生长,造成植株矮小、茎纤细、花粉稀少、角果短小等,同时还影响花粉壁的发育与花粉的育性,进而影响授粉过程。徐法青[29]发现:拟南芥CER3基因参与花粉脂质的合成或转运,该脂质的缺失会影响花粉与柱头的识别,阻断水合作用,最终导致雄性不育。
-
蜡质可以减少叶片表面水分,减少病菌停留和降低病菌入侵。JU等[30]发现:蜡质的晶体结构可促使水分形成水滴以便滑落,并带走叶片表面的灰尘、污染物和病菌等。SURVILA等[31]发现:相较于正常植株,拟南芥蜡质缺失植株表面细菌更多,更易发生病害。蜡质在植物抵抗虫害方面也起着重要作用,它可通过光的反射,改变植物表现出的颜色,影响昆虫视觉,减少昆虫取食和产卵。BOHINC等[32]以8种基因型甘蓝为对象进行田间试验,发现蜡质对甘蓝跳甲Phyllotreta spp.和菜椿Eurydema spp.的生存有抑制作用,且蜡质含量越高,植株上甘蓝跳甲和菜椿越少。
-
十字花科植物蜡质缺失表型明显,表面无蜡质覆盖的植株呈现叶色亮绿等性状,包括单基因隐性遗传、单基因显性遗传和双基因隐性遗传等3种遗传特性。
-
ANSTEY等[33]在青花菜Brassica oleracea var. italica中发现了十字花科植物中第1个符合单基因隐性遗传规律的蜡质缺失突变体。刘泽洲等[34]发现:甘蓝蜡粉缺失突变体10Q-961符合单基因隐性遗传规律。李红莲等[7]对无蜡质红菜薹与有蜡质红菜薹杂交建立6世代群体并进行研究,发现蜡质缺失突变体符合隐性遗传规律。王灿洁等[15]发现红菜薹自交系13S106蜡质缺失性状受隐性单基因控制。
-
蒲媛媛等[35]发现:甘蓝型油菜光叶突变体GL的蜡质缺失性状受单个显性基因控制。刘东明[36]发现:甘蓝10Q-974亮绿性状符合单基因显性遗传规律,突变基因 BoGL1位于8号染色体177 kb的区间内,与基因Bol018504的表达相关。
-
周熙荣等[37]发现:甘蓝型油菜杂交F2中出现少数蜡质缺失的植株,其无蜡质性状由2对隐性基因控制。莫鉴国等[38]对加拿大引进的无蜡质甘蓝型油菜种质材料‘Nilla’进行研究,发现其蜡质缺失性状也是受2对隐性基因控制。
-
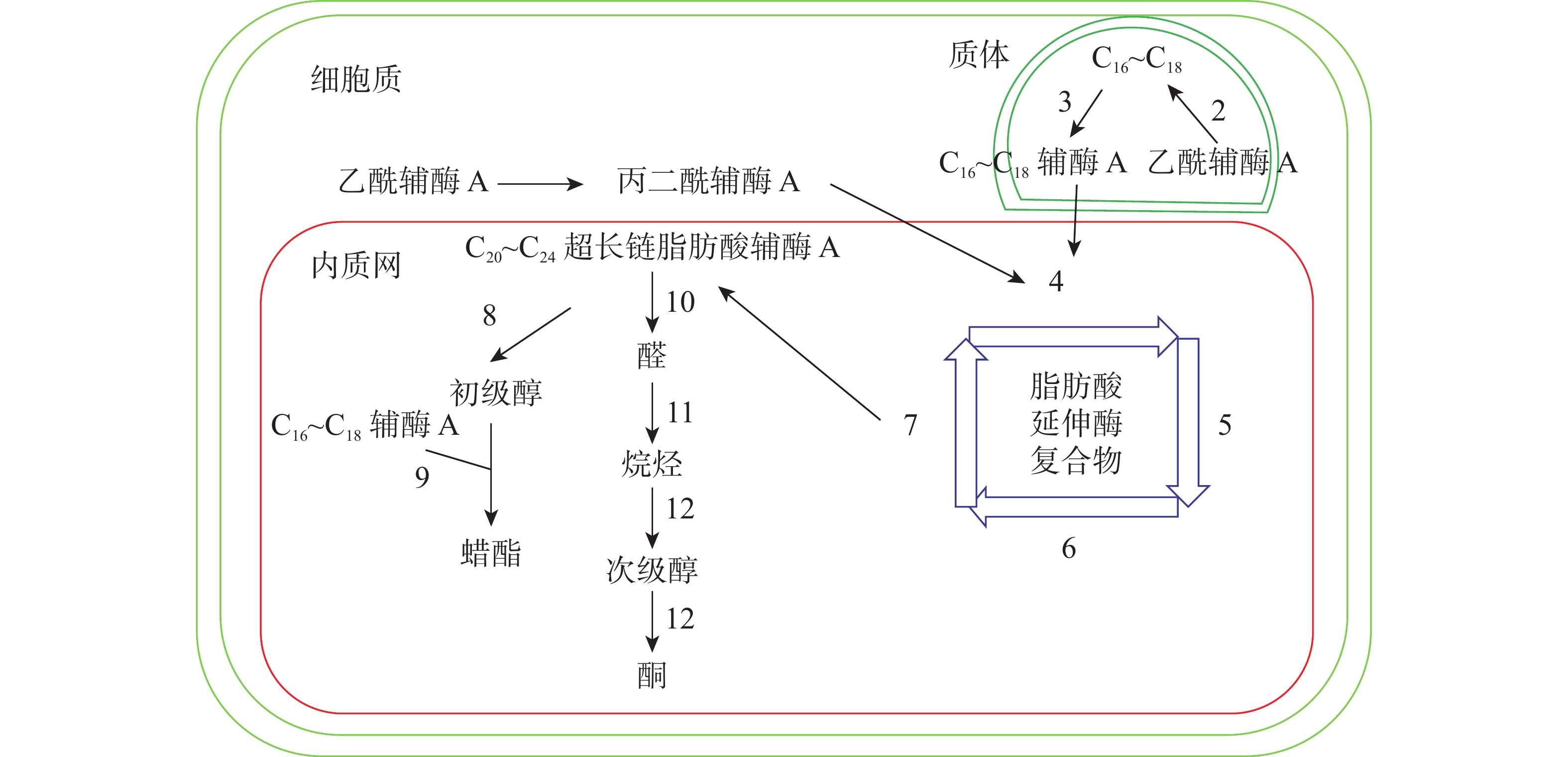
十字花科植物蜡质合成C16~C18脂肪酸合成、C20~C34超长链脂肪酸合成、超长链脂肪酸衍生物合成等3个途径(图1)。合成相关酶如表1所示。
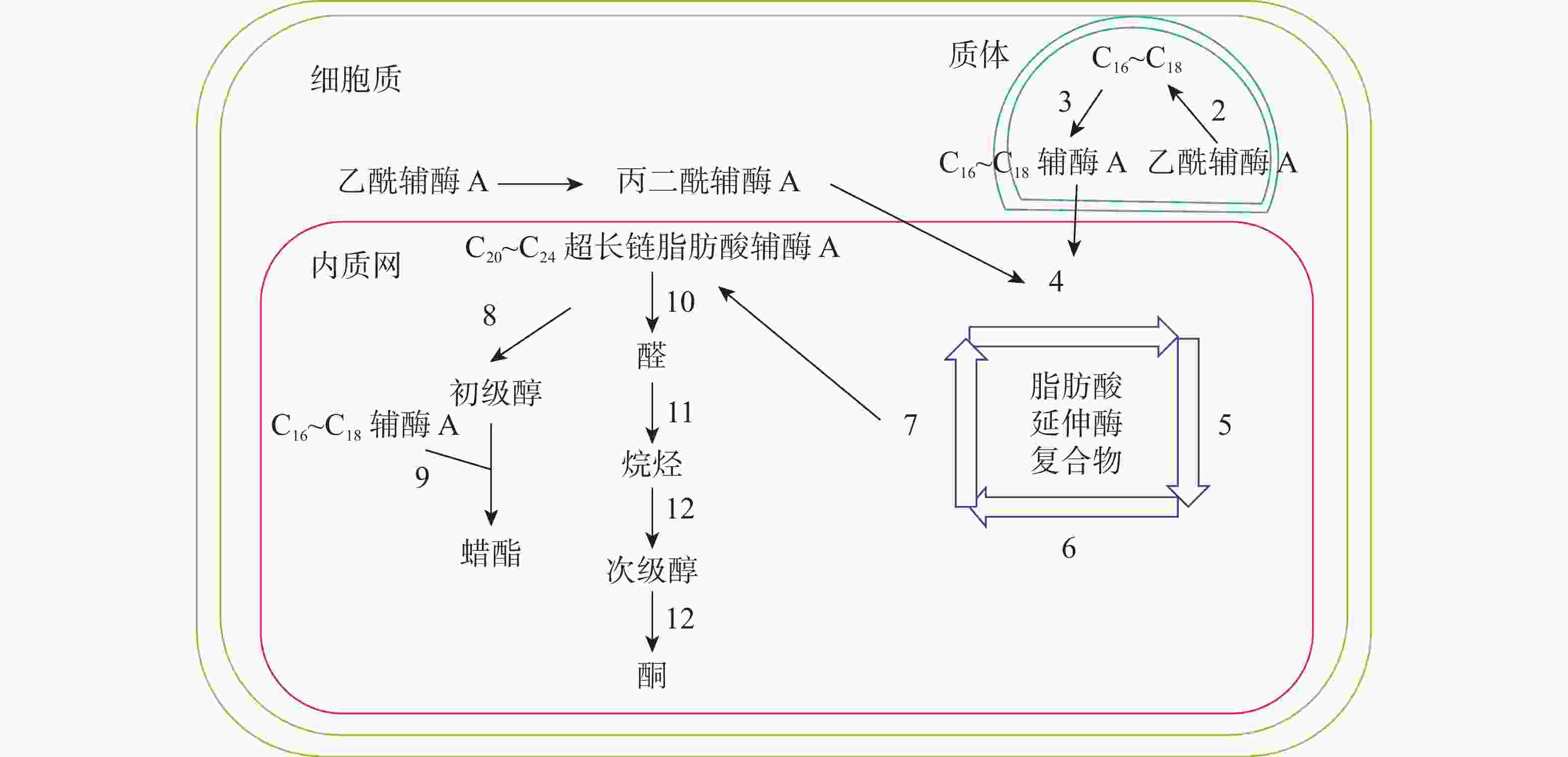
图 1 拟南芥蜡质生物合成途径[11]
Figure 1. Wax biosynthesis pathway in Arabidopsis thaliana
表 1 拟南芥参与蜡质生物合成的酶
Table 1. Enzymes involved in wax biosynthesis in Arabidopsis thaliana
序号 酶 缩略符 基因 参考文献 1 乙酰辅酶A羧化酶 ACC ACC1,ACC2 [39] 2 酰基载体蛋白硫解酶 FAT FATA,FATB [40] 3 长链酰基辅酶A合成酶 LACS LACS1,LACS2,LACS4 [41-44] 4 β-酮酰辅酶A合成酶 KCS FAE1,CER6,KCS1,FDH [45-46] 5 β-酮酰辅酶A还原酶 KCR KCR2 [11] 6 β-羟酰-酰基辅酶A脱水酶 HCD PAS1,PAS2 [47-48] 7 反式烯酰辅酶A还原酶 ECR CER10 [49] 8 脂肪酰辅酶A还原酶 FAR CER4 [50] 9 蜡酯合成酶 WS WSD1 [51] 10 脂肪酰辅酶A还原酶 FAR CER3 [52] 11 醛脱羰酶 AD CER1 [53-54] 12 中链烷烃羟化酶 MAH MAH1 [55] -
C16~C18脂肪酸在质体中合成,初始反应物乙酰辅酶A在乙酰辅酶A羧化酶(ACC)和脂肪酸合成酶的作用下,以每次增加2个碳原子的方式延长碳链,形成C16~C18的酰基载体蛋白[39],然后在酰基载体蛋白硫酯酶(FAT)作用下水解生成C16~C18脂肪酸[40]。脂肪酸经长链酰基碳烯A合成酶(LACS)催化后以脂肪酰辅酶A的形式进入内质网中[41],进行下一步反应。ZHAO等[42]发现:拟南芥的9个LACS基因中,LACS1和LACS2参与蜡质的合成。LÜ等[43]和JESSEN等[44]进一步发现:LACS1和LACS4参与花粉外被长链脂肪酸的合成。
-
C20~C34超长链脂肪酸合成场所是内质网。C16~C18脂肪酰辅酶A与丙二酰辅酶A通过脂肪酸延伸酶复合物(FAE)进行合成,每次循环增加2个碳原子,多次循环延伸碳链,最终形成C20~C34超长链脂肪酸,其中丙二酰辅酶A由乙酰辅酶A于细胞质中经过乙酰辅酶A羧化酶催化形成。该反应中的FAE属于多酶复合体,包括β-酮酰辅酶A合成酶(KCS),β-酮酰辅酶A还原酶(KCR),反式烯酰辅酶A还原酶(ECR)和β-羟酰-酰基辅酶A脱水酶(HCD)4种酶,其中KCS是该反应的关键酶,对反应底物具有特异性。QUIST等[45]发现:拟南芥KCS基因分为FAE1类和ELO类,前者包含FAE1、CER6、KCS1和FDH等4个亚组,而ELO类基因功能还未见报道。SUH等[46]发现:拟南芥与蜡质相关的基因有KCS1、KCS2、KCS13、KCS10、KCS20和CER6等;拟南芥中的KCR基因有KCR1和KCR2等2种,KCR1没有功能,KCR2参与超长链脂肪酸的合成。ZHAO等[47]发现:相比野生型,cer10突变体器官小,蜡质少;CER10基因在表皮和种子中有ECR功能活性,参与超长链脂肪酸合成。目前对HCD的研究较少,BACH等[48]发现:pas2-1突变体的蜡质含量明显少于野生型,PAS2基因功能完全丧失会最终导致胚死亡,推测PAS2在超长链脂肪酸合成和生物发育中起到非常关键的作用。ROUDIER等[49]发现:内质网中的PAS1和PAS2,KCR和ECR存在蛋白互作,并认为PAS1在多酶复合体中扮演分子构架的角色。
-
同位素示踪和气相色谱质谱技术已经验证了超长链脂肪酸通过酰基还原途径和脱羰基途径衍生出其他蜡质成分。酰基还原途径也叫醇合成途径,超长链脂肪酰辅酶A经脂肪酰辅酶A还原酶(FAR)还原产生初级醇,初级醇与C16~C18脂肪酸辅酶A经蜡酯合成酶(WS)缩合产生蜡酯。拟南芥通过酰基还原途径产生的蜡质相对含量约为20%。 CER4基因编码的酰基辅酶A还原酶在该途径中起到关键作用,主要将拟南芥表皮和根部脂肪酸还原成初级醇。ROWLAND等[50]发现:拟南芥cer4突变体茎中醇与蜡酯含量显著降低。LI等[51]发现:拟南芥wsd1突变体蜡酯含量明显少于野生型。脱羰基途径也叫烷烃合成途径,超长链脂肪酰辅酶A经脂肪酰辅酶A还原酶(FAR)还原产生的醛经醛脱羰酶脱羰产生烷烃,经中链烷烃羟化酶(MAH)1次羟化产生次级醇,再次羟化生成酮。拟南芥约80%的蜡质组分由该途径产生。BERNARD等[52]发现:拟南芥cer3突变体中醛含量减少,说明CER3基因在产生醛的过程起着重要作用。OSHIMA等[53]和刘秀林[54]发现:拟南芥cer1突变体茎表皮蜡质组分中烷烃含量减少,而醛含量增加,说明CER1编码的酶参与烷烃产生。GREER等[55]发现:MAH1是烷烃羟化酶,拟南芥mah1突变体中次级醇和酮的含量显著减少。
-
内质网上经过各种酶加工修饰合成的蜡质成分会先转运到细胞膜,再通过转运蛋白进行跨膜运输,最后经脂质转移蛋白跨细胞壁转运到角质层,转运途径及转运蛋白见图2和表2。
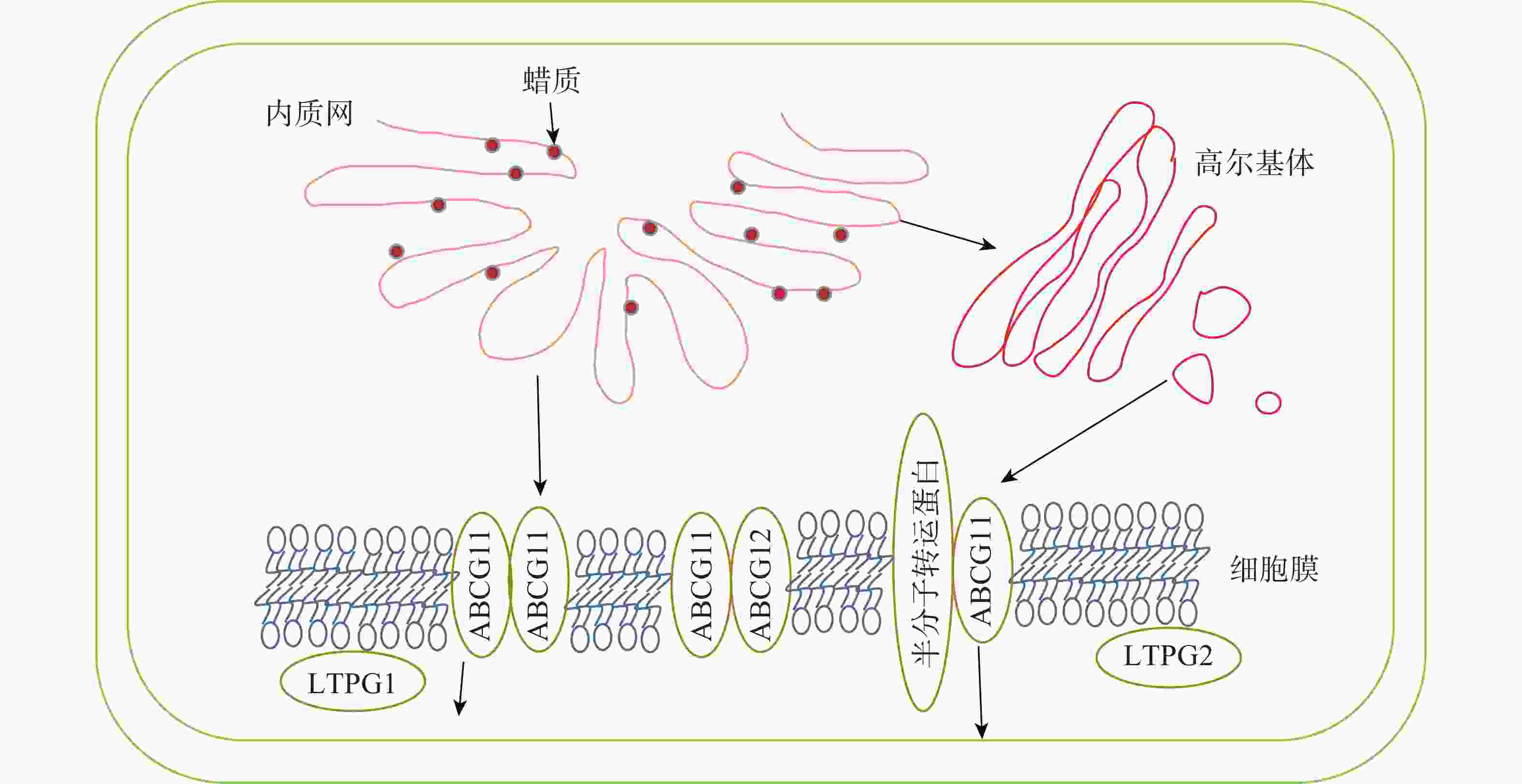
图 2 拟南芥蜡质转运途径[36]
Figure 2. Wax transport pathway in Arabidopsis thaliana
-
目前对蜡质从内质网转运到质膜有2种推测:①蜡质通过内质网与质膜内侧接触的部分直接进行运输;②蜡质先进入内质网分泌的囊泡,再经高尔基体转运到细胞膜内侧[56]。
-
蜡质到达质膜后利用相关转运蛋白进行跨膜运输。ABCG11和ABCG12是拟南芥中2个蜡质转运相关的半分子转运蛋白,LUO等[57]发现:ABCG11通过与另1个ABCG11结合形成同源二聚体或是与其他半分子转运蛋白结合形成异源二聚体来转运蜡质分子。BIRD等[58]发现:abcg11突变体生长速度减缓,表皮蜡质含量减少。QUILICHINI等[59]发现:ABCG12基因编码定位在质膜上的ABC转运蛋白,ABCG12基因的缺失导致拟南芥表皮部位的蜡质显著减少,而细胞内蜡质总含量并没有显著变化,说明该基因缺失只影响了质膜中的蜡质转运过程,而细胞内蜡质合成并没有受阻。
-
到达细胞膜外的蜡质由脂质转移蛋白(LTPs)转运到角质层。LTPG1是一种脂质转移蛋白,包含8个保守的半胱氨酸,形成疏水囊泡。DeBONO等[60]发现:ltpg1突变体的茎和角果表皮蜡质C29烷烃含量减少,但其他蜡质成分不存在显著差异,说明突变体缺失的蛋白可能对C29烷烃转移具有专一性。孙伟[61]发现:Th-nsLTP是一个非特异性脂转移蛋白,通过参与小盐芥表皮蜡质转移过程,使表皮蜡质含量减少,晶体结构从杆状转为柱状。
-
十字花科植物蜡质形成分子机制极其复杂,今后可从以下几个方面深入探究。①当前对蜡质形态结构与成分含量的研究都是独立的,若能寻找不同蜡质成分形成晶体过程中的空间折叠规律,将有助于探明蜡质成分与蜡质结构复杂多样性背后的具体对应关系。②目前在蜡质合成与转运方面研究较多,但在外界环境条件如低温、干旱或光照强度对蜡质合成影响方面的研究较少,需要更多的相关研究阐明环境影响蜡质合成的机制。③培育蜡质过量的新品种对于研究蜡质形成分子机制及抗病育种都具有重要意义。
Research review on features and molecular mechanism of wax formation in Brassicaceae
-
摘要: 十字花科Brassicaceae包含蔬菜、油料作物、药用植物、观赏植物和染料植物等,是一类1年生、2年生或多年生的植物,是中国最重要的蔬菜和油料作物之一。蜡质的主要成分是超长链脂肪酸及其衍生物,十字花科植物的蜡质是其适应外界环境变化而形成的保护结构,在维持水分平衡、反射紫外线、减少外来机械损伤、降低低温伤害、抵御细菌真菌入侵、防止果实开裂与昆虫侵食等抵抗生物与非生物胁迫中起着重要作用。对十字花科植物蜡质类型、生理功能、遗传特性、合成与转运途径等方面进行综述,可为十字花科植物的蜡质代谢研究提供参考。图2表2参61Abstract: Mainly composed of vegetables, oil crops, medicinal plants, ornamental plants and dyewood, Brassicaceae is a plant that can be annual, biennial or perennial, also as the most important family of vegetable and oil crops in China. Wax, comprised with very long chain fatty acids and corresponding derivatives, a layer of protective structure formed on plant surface to adapt different environmental conditions, plays an important role in resistance to biotic and abiotic stresses. It helps maintain water balance, reflect ultraviolet rays, reduce external mechanical damage, decrease low temperature damage, resist invasion of bacteria and fungi, prevent fruit cracking and fight off insect invasion. This paper, with a brief review of the type, physiological function, genetic characteristics, biosynthetic and transport pathways of wax, is intended to provide reference for the study of wax metabolism in Brassicaceae plants. [Ch, 2 fig. 2 tab. 61 ref.]
-
Key words:
- botany /
- Brassicaceae /
- wax /
- physiological function /
- biosynthetic pathway /
- molecular mechanism /
- review
-
图 1 拟南芥蜡质生物合成途径[11]
Figure 1 Wax biosynthesis pathway in Arabidopsis thaliana
图 2 拟南芥蜡质转运途径[36]
Figure 2 Wax transport pathway in Arabidopsis thaliana
表 1 拟南芥参与蜡质生物合成的酶
Table 1. Enzymes involved in wax biosynthesis in Arabidopsis thaliana
序号 酶 缩略符 基因 参考文献 1 乙酰辅酶A羧化酶 ACC ACC1,ACC2 [39] 2 酰基载体蛋白硫解酶 FAT FATA,FATB [40] 3 长链酰基辅酶A合成酶 LACS LACS1,LACS2,LACS4 [41-44] 4 β-酮酰辅酶A合成酶 KCS FAE1,CER6,KCS1,FDH [45-46] 5 β-酮酰辅酶A还原酶 KCR KCR2 [11] 6 β-羟酰-酰基辅酶A脱水酶 HCD PAS1,PAS2 [47-48] 7 反式烯酰辅酶A还原酶 ECR CER10 [49] 8 脂肪酰辅酶A还原酶 FAR CER4 [50] 9 蜡酯合成酶 WS WSD1 [51] 10 脂肪酰辅酶A还原酶 FAR CER3 [52] 11 醛脱羰酶 AD CER1 [53-54] 12 中链烷烃羟化酶 MAH MAH1 [55] -
[1] 王建林, 栾运芳, 大次卓嘎, 等. 中国十字花科(Cruciferae)的地理分布[J]. 植物资源与环境学报, 2006, 15(3): 7 − 11. WANG Jianlin, LUAN Yunfang, Dacizhuoga, et al. A study on geographical distribution of Cruciferae in China [J]. J Plant Resour Environ, 2006, 15(3): 7 − 11. [2] 王晓波. 十字花科共线性分析及数据库平台的构建[D]. 北京: 中国农业科学院, 2015. WANG Xiaobo. Analyses of Syntenic Relationship among Brassicaceae Species and Construction of Brassicaceae Database[D]. Beijing: Chinese Academy of Agricultural Sciences, 2015. [3] 王聪, 王嘉欢, 汪勇, 等. 大麦表皮蜡质的组分及晶体结构分析[J]. 麦类作物学报, 2018, 38(6): 693 − 700. WANG Cong, WANG Jiahuan, WANG Yong, et al. Analysis of cuticular wax components and crystal structure of barley [J]. J Triticeae Crops, 2018, 38(6): 693 − 700. [4] 陈伟, 刘德春, 杨莉, 等. 植物表皮蜡质及相关基因研究进展[J]. 植物生理学报, 2016, 52(8): 1117 − 1127. CHEN Wei, LIU Dechun, YANG Li, et al. Research progress of plant cuticular wax and related genes [J]. Plant Physiol J, 2016, 52(8): 1117 − 1127. [5] BARTHLOTT W, NEINHUIS C, CUTLER D, et al. Classification and terminology of plant epicuticular waxes [J]. Bot J Linnean Soc, 1998, 126(3): 237 − 260. [6] 徐秀苹, 谷丹, 冯旻. 适用于扫描电镜的拟南芥蜡质样品制备方法[J]. 电子显微学报, 2015, 34(1): 82 − 84. XU Xiuping, GU Dan, FENG Min. Comparison of sample preparation methods for scanning electron microscopy (SEM) of leaf epicuticular waxes in Arabidopsis [J]. J Chin Electron Microscopy Soc, 2015, 34(1): 82 − 84. [7] 李红莲. 红菜薹蜡粉性状遗传分析与基因初步定位[D]. 武汉: 华中农业大学, 2014. LI Honglian. Genetic Analysis and Preliminary Mapping on Wax in Purple-caitai[D]. Wuhan: Huazhong Agricultural University, 2014. [8] 李帅, 赵秋棱, 彭阳, 等. SA、MeJA和ACC处理对甘蓝型油菜叶角质层蜡质组分、结构及渗透性的影响[J]. 作物学报, 2016, 42(12): 1827 − 1833. LI Shuai, ZHAO Qiuling, PENG Yang, et al. Effects of SA, MeJA and ACC on leaf cuticular wax constituents, structure and permeability in Brassica napus [J]. Acta Agron Sin, 2016, 42(12): 1827 − 1833. [9] 牟香丽, 王超, 王帅. 甘蓝无蜡粉突变体叶表皮蜡质超微结构观察[J]. 中国蔬菜, 2013(4): 32 − 37. MU Xiangli, WANG Chao, WANG Shuai. Observation of ultra microstructure of wax-less mutant epicuticular wax on cabbage [J]. China Veg, 2013(4): 32 − 37. [10] 张曦. 大白菜蜡粉基因的精细定位及表达分析[D]. 沈阳: 沈阳农业大学, 2013. ZHANG Xi. Fine Mapping and Gene Expression of Wax Gene in Chinese Cabbage (Brassica rapa L. ssp. Pekinensis)[D]. Shenyang: Shenyang Agricultural University, 2013. [11] LEE S B, SUH M C. Cuticular wax biosynthesis is up-regulated by the myb94 transcription factor in Arabidopsis [J]. Plant Cell Physiol, 2015, 56 (1): 48 − 60. [12] HASSANZADEH-KHAYYAT M, AKABERI M, HAGHIGHI H M, et al. Distribution and variability of n-alkanes in waxes of conifers [J]. J For Res, 2019, 30(2): 429 − 433. [13] 王丽娜, 金勋, 杨柳, 等. 干旱胁迫下外源激素对大豆叶片表皮透性及蜡质微形态影响[J]. 大豆科学, 2018, 37(2): 246 − 250. WANG Lina, JIN Xun, YANG Liu, et al. Effects of exogenous hormones on cuticular permeability and wax micromorphology of soybean leaves under drought stress [J]. Soybean Sci, 2018, 37(2): 246 − 250. [14] 宋超. 菌核病菌、UV-B、低温胁迫条件下拟南芥表皮蜡质的响应机制研究[D]. 重庆: 西南大学, 2013. SONG Chao. Response Mechanism of the Arabidopsis thaliana Epicutlcular Wax under Sclerotinia Sclerotiorum, UV-B and Cold Stresses[D]. Chongqing: Southwest University, 2013. [15] 王灿洁. 白菜类蔬菜蜡质基因和红色基因的遗传克隆与分析[D]. 武汉: 华中农业大学, 2018. WANG Canjie. The Genetic Cloning and Analysis of Waxy Gene and Red Gene in Brassica rapa[D]. Wuhan: Huazhong Agricultural University, 2018. [16] SEGADO P, DOMÍNGUEZ E, HEREDIA A. Ultrastructure of the epidermal cell wall and cuticle of tomato fruit (Solanum lycopersicum L.) during development [J]. Plant Physiol, 2016, 170(2): 935 − 946. [17] BI Huihui, KOVALCHUK N, LANGRIDGE P, et al. The impact of drought on wheat leaf cuticle properties [J]. BMC Plant Biol, 2017, 17: 85. [18] 许英, 陈建华, 朱爱国, 等. 低温胁迫下植物响应机理的研究进展[J]. 中国麻叶科学, 2015, 37(1): 40 − 49. XU Ying, CHEN Jianhua, ZHU Aiguo, et al. Research progress on response mechanism of plant under low temperature stress [J]. Plant Fiber Sci China, 2015, 37(1): 40 − 49. [19] 倪郁, 宋超, 王小清. 低温胁迫下拟南芥表皮蜡质的响应机制[J]. 中国农业科学, 2014, 47(2): 252 − 261. NI Yu, SONG Chao, WANG Xiaoqing. Investigation on response mechanism of epicuticuloar wax on Arabidopsis thaliana under cold stress [J]. Sci Agric Sin, 2014, 47(2): 252 − 261. [20] 唐帅, 陈悦, 陈宁美, 等. 低温胁迫下盐芥和拟南芥蜡质组成及相关基因的表达差异[J]. 河南农业科学, 2018, 47(11): 37 − 44. TANG Shuai, CHEN Yue, CHEN Ningmei, et al. Comparison of wax composition and related gene expression in Thellungiella salsuginea and Arabidopsis thaliana under cold stress [J]. J Henan Agric Sci, 2018, 47(11): 37 − 44. [21] PRUDNIKOVA O N, RAKITINA T Y, KARYAGIN Y Y, et al. Adaptation to UV-B radiation in the ontogenesis of Arabidopsis thaliana plants: the participation of ethylene, ABA, and polyamines [J]. Russ J Dev Biol, 2019, 50(5): 250 − 256. [22] 曲玉莹, 曲波, 崔娜, 等. 水分胁迫对‘翠鸟’玉簪叶片表皮蜡质及生理特性的影响[J]. 园艺学报, 2019, 46(7): 1344 − 1350. QU Yuying, QU Bo, CUI Na, et al. Effects of water stress on the epicuticular wax and physiological characteristics of Hosta‘Halcyon’ leaves [J]. Acta Hortic Sin, 2019, 46(7): 1344 − 1350. [23] LI Dan, CHENG Yudou, GUAN Junfeng. Effects of 1-methylcyclopropene on surface wax and related gene expression in cold-stored ‘Hongxiangsu’ pears [J]. J Sci Food Agric, 2019, 99(5): 2438 − 2446. [24] 柴凌燕. 植物角质膜蜡质转录因子基因SHN1/WIN1的表达载体构建[D]. 郑州: 郑州大学, 2010. CAI Lingyan. Expression Vector Construction of Plant Cuticle Wax-related Transcription Factor Gene SHN1/WIN1[D]. Zhengzhou: Zhengzhou University, 2010. [25] LÜ Shiyou, ZHAO Huayan, Des MARAIS D L, et al. Arabidopsis ECERIFERUM9 involvement in cuticle formation and maintenance of plant water status [J]. Plant Physiol, 2012, 159(3): 930 − 944. [26] 周燕, 黄小虎, 许代香, 等. 甘蓝型油菜蜡质相关基因的克隆与表达分析[J]. 农业生物技术学报, 2017, 25(12): 1918 − 1929. ZHOU Yan, HUANG Xiaohu, XU Daixiang. Cloning and expression analysis of waxy-related genes in Brassica napus [J]. J Agric Biotechnol, 2017, 25(12): 1918 − 1929. [27] KOCH K, BHUSHAN B, BARTHLOTT W. Multifunctional surface structures of plant: an inspiration for biomimetic [J]. Prog Mater Sci, 2009, 54(2): 137 − 178. [28] 刘艳艳, 陈雨沁, 石垒, 等. 拟南芥脂肪酸外运蛋白FAX1影响雄性生殖发育的机制[J]. 植物生理学报, 2018, 54(1): 145 − 156. LIU Yanyan, CHEN Yuqin, SHI Lei, et al. Investigations into the mechanisms underlying the effects of Arabidopsis thaliana fatty acid export 1(FAX1) in male reproductive development [J]. Plant Physiol J, 2018, 54(1): 145 − 156. [29] 徐法青. CER3在拟南芥花粉表面结构形成及水合中的作用[D]. 上海: 上海师范大学, 2017. XU Faqing. The Role of CER3 in the Formation and Hydration of Arabidopsis Pollen Surface Structure[D]. Shanghai: Shanghai Normal University, 2017. [30] JU S, GO Y S, CHOI H J, et al. DEWAX transcription factor is involved in resistance to Botrytis cinerea in Arabidopsis thaliana and Camelina sativa [J]. Front Plant Sci, 2017, 8: 1210. [31] SURVILA M, DAVIDSSON P R, PENNANEN V, et al. Peroxidase-generated apoplastic ROS impaircuticle integrity and contribute to DAMP-elicited defenses [J]. Front Plant Sci, 2016, 7(5): 1945. [32] BOHINC T, MARKOVIČ D, TRDAN S. Leaf epicuticular wax as a factor of antixenotic resistance of cabbage to cabbage flea beetles and cabbage stink bugs attack [J]. Acta Agric Scandinavica, 2014, 64(6): 493 − 500. [33] ANSTEY T H, MOORE J F. Inheritance of glossy foliage and cream petals in green sprouting broccoli [J]. J Hered, 1954, 45(1): 39 − 41. [34] 刘泽洲, 杨丽梅, 方智远, 等. 结球甘蓝蜡粉缺失基因cgl1的精细定位[C]//中国园艺学会: 中国园艺学会2015年学术年会论文集. 北京: 中国园艺学会, 2015: 2677. [35] 蒲媛媛. 甘蓝型油菜显性光叶突变体BnaA. GL基因定位和表皮蜡质分析[D]. 武汉: 华中农业大学, 2013. PU Yuanyuan. Mapping of BanA. GL Gene in a Dominant Glossy Mutant and Cuticular Wax Analysis in Brassica napus L.[D]. Wuhan: Huazhong Agricultural University, 2013. [36] 刘东明. 甘蓝蜡质缺失基因BoGL4和BoGL1的克隆及功能分析[D]. 武汉: 华中农业大学, 2017. LIU Dongming. Cloning and Functional Analysis of BoGL4 and BoGL1 Involved In Cabbage Wax Reduction[D]. Wuhan: Huazhong Agricultural University, 2017. [37] 周熙荣, 周志疆, 李树林. 甘蓝型油菜无蜡质性状的遗传性[J]. 上海农业学报, 1995, 11(3): 87 − 89. ZHOU Xirong, ZHOU Zhijiang, LI Shulin. Inheritance of waxless character in rapeseed (B. napus L.) [J]. Acta Agric Shanghai, 1995, 11(3): 87 − 89. [38] 莫鉴国, 李万渠, 彭云强, 等. 甘蓝型油菜无蜡粉种质材料的改良以及在杂优育种上的应用[J]. 种子, 1999(5): 18 − 20. MO Jianguo, LI Wanqu, PENG Yunqiang. Improvement and application of waxless germplasm material (B. napus L.) in heterosis [J]. Seed, 1999(5): 18 − 20. [39] LÜ Shiyou, ZHAO Huayan, PARSONS E P, et al. The glossyhead1 allele of ACC1 reveals a principal role for multidomain acetyl-coenzyme a carboxylase in the biosynthesis of cuticular waxes by Arabidopsis [J]. Plant Physiol, 2011, 157(3): 1079 − 1092. [40] MORENO-PÉREZ A, CALERÓN-VENEGAS M, VAISTIJ F E, et al. Reduced expression of FatA thioesterases in Arabidopsis affects the oil content and fatty acid composition of the seeds [J]. Planta, 2012, 235(3): 629 − 639. [41] GACEK K, BAYER P E, BARTKOWIAK-BRODA I, et al. Genome-wide association study of genetic control of seed fatty acid biosynthesis in Brassica napus [J]. Front Plant Sci, 2017, 7(20): e2062. [42] ZHAO Lifang, HASLAM T M, SONNTAG A, et al. Functional overlap of long-chain acyl-CoA synthetases in Arabidopsis [J]. Plant Cell Physiol, 2019, 60(5): 1041 − 1054. [43] LÜ Shiyou, SONG Tao, KOSMA D K, et al. Arabidopsis CER8 encodes long-chain acyl-CoA synthetase 1 (LACS1) that has overlapping functions with LACS2 in plant wax and cutin synthesis [J]. Plant J, 2009, 59(4): 553 − 564. [44] JESSEN D, OLBRICH A, KNÜFER J, et al. Combined activity of LACS1 and LACS4 is required for proper pollen coat formation in Arabidopsis [J]. Plant J, 2011, 68(4): 715 − 726. [45] QUIST T M, SOKOICHIK I, SHI Huazhong, et al. HOS3, an ELO-like gene, inhibits effects of ABA and implicates a S-1-P/ceramide control system for abiotic stress responses in Arabidopsis thaliana [J]. Mol Plant, 2009, 2(1): 138 − 151. [46] SUH M C, SAMUELS A L, JETTER R, et al. Cuticular lipid composition, surface structure, and gene expression in Arabidopsis stem epidermis [J]. Plant Physiol, 2005, 139(4): 1649 − 1665. [47] BACH L, FAURE J D. Role of very-long-chain fatty acids in plant development, when chain length does matter [J]. Comptes Rendus Biol, 2010, 333(4): 361 − 370. [48] ROUDIER F, GISSOT L, BEAUDOIN F, et al. Very-long-chain fatty acids are involved in polar auxin transport and developmental patterning in Arabidopsis [J]. Plant Cell, 2010, 22(2): 364 − 375. [49] ZHAO Lifang, KATAVIC V, LI Fengling, et al. Insertional mutant analysis reveals that long-chain acyl-CoA synthetase 1 (LACS1), but not LACS8, functionally overlaps with LACS9 in Arabidopsis seed oil biosynthesis [J]. Plant J, 2010, 64(6): 1048 − 1058. [50] ROWLAND O, ZHENG Huanquan, HEPWORTH S R, et al. CER4 encodes an alcohol-forming fatty acyl-coenzyme a reductase involved in cuticular wax production in Arabidopsis [J]. Plant Physiol, 2006, 142(3): 866 − 877. [51] LI Fengling, WU Xuemin, LAM P, et al. Identification of the wax ester synthase/acyl-coenzyme A: diacylglycerol acyltransferase WSD1 required for stem wax ester biosynthesis in Arabidopsis [J]. Plant Physiol, 2008, 148(1): 97 − 107. [52] BERNARD A, DOMERGUE F, PASCAL S, et al. Reconstitution of plant alkane biosynthesis in yeast demonstrates that Arabidopsis ECERIFERUM1 and ECERIFERUM3 are core components of a very-long-chain alkane synthesis complex [J]. Plant Cell, 2012, 24(7): 3106 − 3118. [53] OSHIMA Y, SHITAKA M, KOYAMA T, et al. MIXTA-like transcription factors and WAX INDUCER1/SHINE1 coordinately regulate cuticle development in Arabidopsis and Torenia fournieri [J]. Plant Cell, 2013, 25(5): 1609 − 1624. [54] 刘秀林. 高浓度CO2调控表皮蜡质合成的研究[D]. 武汉: 中国科学院大学, 2017. LIU Xiulin. Study on the Syntesis and Regulation of Cuticular Wax at High Carbon Dioxide Concentration[D]. Wuhan: University of Chinese Academy of Science, 2017. [55] GREER S, WEN Miao, BIRD D, et al. The cytochrome P450 enzyme CYP96A15 is the midchain alkane hydroxylase responsible for formation of secondary alcohols and ketones in stem cuticular wax of Arabidopsis [J]. Plant Physiol, 2007, 145(3): 653 − 667. [56] KUNST L, SAMUELS A L. Biosynthesis and secretion of plant cuticular wax [J]. Progr Lipid Res, 2003, 42(1): 51 − 80. [57] LUO Bin, XUE Xueli, HU Wenli, et al. An ABC transporter gene of Arabidopsis thaliana, AtWBC11, is involved in cuticle development and prevention of organ fusion [J]. Plant Cell Physiol, 2007, 48(12): 1790 − 1802. [58] BIRD D, BEISSON A, BRIGHAM J. Characterization of Arabidopsis ABCG11/WBC11, an ATP binding cassette (ABC) transporter that is required for cuticular lipid secretion [J]. Plant J, 2007, 52(3): 485 − 498. [59] QUILICHINI T D, FRIEDMANN M C, SAMUELS A L, et al. ATP-binding cassette transporter G26 is required for male fertility and pollen exine formation in Arabidopsis [J]. Plant Physiol, 2010, 154(2): 678 − 690. [60] DeBONO A, YEATS T H, ROSE J K C, et al. Arabidopsis LTPG is a glycosylphosphatidylinositol-anchored lipid transfer protein required for export of lipids to the plant surface [J]. Plant Cell, 2009, 21(4): 1230 − 1238. [61] 孙伟. 非特异性脂转移蛋白与植物耐逆性的相关性研究[D]. 济南: 山东师范大学, 2003. SUN Wei. Nonspecific Lipid Transfer Proteins Relate to Plant Stress Tolerance[D]. Jinan: Shandong Normal University, 2003. -

-
链接本文:
https://zlxb.zafu.edu.cn/article/doi/10.11833/j.issn.2095-0756.20200138






 下载:
下载: